Introduction
Small ruminant production system in India is dependent mainly on community pastures that are highly degraded. Such pastures produce forages that are low in quality and are unable to provide a sustained supply of nutrients to animals. Utilisation of poor quality feeds by ruminants can be improved through concentrate supplementation, which increases the digestibility of nutrients (Santra et al., Reference Santra, Karim and Chaturvedi2002) through stimulating rumen fermentation (Sultan and Loerch, Reference Sultan and Loerch1992). Supplementation of maize, and soya-bean meal on wheat straw based diets improved digestible organic matter intake (Abebe et al., Reference Abebe, Merkel, Animut, Sahlu and Goetsch2004). Similarly, supplementation of peanut oil meal (2.5 g/kg live weight) resulted in higher average daily gain and was economic for growing kid production (Ott et al., Reference Ott, Muir, Brown and Wittie2004). A supplementation of 250 g concentrate in addition to grazing is now being recommended to raise sheep in semi-arid regions of India (Karim et al., Reference Karim, Santra and Singh2004). Such situations have increased the pressure on supplemental feed resources under tropical animal production systems (Shem et al., Reference Shem, Kakeni, Mtengeti and Otsyina2001). Therefore, for long-term sustenance, the existing degraded pastures/waste lands need to be revived. Development of grass-legume-based pastures is one of the recognised strategies in many countries for enhancing both the quality and quantity of feed resources that could play an important role in improving low input animal production systems (Shem et al., Reference Shem, Mtengeti, Luaga, Ichinohe and Fujihara2003). This can be done through discouraging rigorous grazing and suggesting stall-feeding. Locally available roughage-based complete feed blocks were found suitable for domestic ruminants (Jakhmola, Reference Jakhmola2005). Forage to concentrate ratio in these blocks is invariably kept at 60:40, which was required for greater intake and digestibility of dry matter (DM), organic matter (OM) and crude protein (CP) (Haddad, Reference Haddad2005). Adequate ruminal energy supply coupled with an appropriate amount of ruminally available nitrogen promotes microbial nitrogen synthesis and efficiency (Henning et al., Reference Henning, Steyn and Meissner1993). Thus, complete feed blocks that contain locally available feed materials can be useful in maintaining sheep productivity under stall-feeding.
In the present study two feeding systems, complete feed block under stall-feeding and grazing plus supplementation were assessed in terms of nutrient intake and utilisation, rumen fermentation pattern and performance of lambs.
Material and methods
Site and environmental conditions
The experiment was conducted at the Central Sheep and Wool Research Institute, Avikanagar (Rajasthan, India) located at 26° 17'N latitude and 75° 28'E longitude and 320 m above seal level. The climate is hot and semi-arid. The experiment was conducted for 90 days during the winter season from September to December 2002. During the experimental period, minimum and maximum ambient temperatures ranged from 5° to 12°C and 32° to 41°C, respectively. Relative humidity varied from 51 to 95%.
Selection and distribution of animals
Twenty-two Malpura lambs (231 ± 2.3 days of age and 22 ± 0.3 kg live weight (LW)) were selected from the institute farm. Animals were dewormed before the experiment using ‘Albendazole’ at 10 mg/kg LW (Wockhardt India Ltd, Bombay). Animals were randomly divided into two groups. One group (SF) was kept under stall-feeding and received complete feed blocks (CFB) as their sole feed daily each morning. The animals were fed individually and penned in a well ventilated shed, being allowed access to an open paddock (without vegetation) for 2 h in the morning. Another group was maintained on grazing and supplementation (GR). The GR animals were allowed to graze a rangeland that was dominated by Cenchrus ciliaris (sward height < 20 cm) for a period of 8 h. When returned to the shed at 1700 h, animals were fed individually a 250 g concentrate mixture. Water was offered ad libitum twice a day at 1000 and 1600 h. Lambs were weighed at weekly intervals to assess health status and growth performance. The wool yield was calculated by shearing an 8 × 8 cm area of left flank before and after the experiment following the procedure of Pierce (Reference Pierce1934) using sheep surface area in metres {(surface area)2 = 0.121(LW, kg)0.59}.
Diet preparation
The complete feed blocks had forage: concentrate ratio of 60:40. Different ingredients (composition in Table 1) were thoroughly mixed using a horizontal mixer. The mixed complete feed was then compressed at 4000 p.s.i. into CFB using a horizontal complete feed-block-making machine. The concentrate mixture given to GR animals (composition in Table 1) was in the mash form.
Table 1 Composition of complete feed block (CFB) supplement and grazing pasture

† Composition (per kg): calcium 320 g, phosphorus 62 g, manganese 2.7 g, zinc 2.6 g, iron 1 g, fluorine 900 mg, iodine 100 mg, copper 100 mg.
Digestibility trial
A digestibility trial was conducted after 45 days of initial feeding on six animals from each group that had comparable LW. Chromium III oxide (Cr2O3), an indigestible marker, was used as an indicator to estimate intake (Harris, Reference Harris1967). The animals on trial were dosed for 10 consecutive days with 1 g Cr2O3 in a paper capsule twice daily at 0800 h and 1700 h. The initial 5 days were allotted as adjustment period for uniform Cr2O3 excretion in the faeces and the later 5 days were used for faecal sample collection. The faecal samples were drawn manually from the rectum in the morning and evening hours. Samples of faeces collected over a 5-day period were pooled and representative samples were drawn. One set of samples was preserved at − 20°C pending nitrogen analysis by the Kjeldahl method (Association of Official Analytical Chemists (AOAC), 2000), while another set of samples was dried in an oven at 60 to 70°C to constant weight. The dried samples were subsequently ground to pass a 1-mm screen and stored for laboratory analysis. Dried faecal samples were analysed for Cr2O3 content and faecal output was determined (Harris, Reference Harris1967). The faecal out put was used to determine pasture intake and nutrient digestibility by lignin ratio (Wallace and Van Dyne, Reference Wallace and Van Dyne1970). In brief, the proportion of concentrate in total faecal output was estimated by the predetermined in-vitro DM digestibility of concentrate. Subtracting the faecal out put of concentrate from total faecal output, provided the faecal output from pasture intake. Pasture DM intake was then calculated {(lignin in faeces × pasture faecal output) / lignin of diet}.
Chemical analysis
Feeds and pasture samples were analysed for DM by drying at 100°C for 24 h. The samples of feed, pasture and faeces that were dried at 60 to 70°C and ground to pass a 1-mm sieve were used for chemical analysis. The OM was determined by ashing at 550°C for 4 h and nitrogen was determined by Kjeldahl technique (AOAC, 2000). Neutral-detergent fibre (NDF) and acid-detergent fibre (ADF) were determined by a procedure of Van Soest et al. (Reference Van Soest, Robertson and Lewis1991), sodium sulphite or alpha-amylase was not used for NDF determinations. Acid-detergent lignin (ADL) was determined according to the method described by Robertson and Van Soest (Reference Robertson and Van Soest1981). NDF and ADF were expressed with residual ash. Gross energy was estimated using a bomb calorimeter (Gallenkamp, Middlesborough, UK).
Collection and analysis of rumen liquor
During the middle part of the experiment, rumen liquor samples from each animal were drawn at 6 h post feeding on 3 consecutive days. About 100 ml of representative rumen liquor was collected from the rumen with a stomach tube using light suction. Rumen liquor pH was recorded immediately after collection using a digital pH meter (EC 5652, Electronic Corp. India Ltd). The rumen liquor was then strained through four layers of muslin cloth. The strained rumen liquor (SRL) samples were preserved after adding a few drop of saturated mercury II chloride solution and kept in labelled polypropylene bottles at − 20°C till further analysis. SRL samples were analysed for total nitrogen (total-N; micro-Kjeldahl), ammonia nitrogen (NH3-N; Conway, Reference Conway1962) and total volatile fatty acids (TVFA; Barnett and Reid, Reference Barnett and Reid1957). The activities of carboxymethylcellulase (CMCase), microcrystallinecellulase (MCCase), estaerase, xylanase and protease in the SRL (extra cellular, EC and cellular, C) were estimated according to Agarwal (Reference Agarwal, Chaudhary, Agarwal, Kamra and Agarwal2000) with slight modifications (Raghuvansi, Reference Raghuvansi2003). Ten millilitres of fresh rumen liquor was centrifuged at 14 000 r.p.m. for 20 min and the supernatant was used as source of enzyme for extracellular fraction. The pellet containing microbial biomass (Bacteria, protozoa and fungi) was suspended in 5 ml 0.1 mol/l phosphate buffer (pH 6.8) and in to it, 2 ml CCl4 and 2 ml lysozyme (4 g/l) was added. The suspension was then incubated for 3 h at 39°C and then centrifuged at 14 000 r.p.m. for 20 min. The supernatant was collected and used as an enzyme source for the cellular portion. The activities of each enzyme were determined separately for individual animals.
For the estimation of CMCase and xylanase, the reaction mixture contained 1 ml phosphate buffer (0.1 mol/l, pH 6.8), 0.5 ml SRL and 0.5 ml substrate (carboxymethyl cellulose; 10 mg/ml for CMCase or xylan; 2.5 mg/ml for xylanase), and was incubated at 39°C for 60 and 15 min, respectively. For determining MCCase, reaction mixture that contained 1 ml phosphate buffer (0.1 mol/l pH 6.8), 1.0 ml SRL and 1.0 ml avicel (10 mg/ml) was incubated at 39°C for 60 min. The reaction was stopped by the addition of dinitro-salicylic acid reagent. The glucose thus produced was estimated according to Miller (Reference Miller1959). The activities of CMCase and MCCase were then calculated considering that one unit of enzyme was able to produce 1μmol glucose per hour from degradation of respective substrates. In case of xylanase activity, one unit equalled 1 mol of xylose that was produced per min from xylan. For estimation of the protease activity (μg hydrolysed protein per h), the reaction mixture that contained 1 ml buffer, 0.25 ml SRL and 0.25 ml casein (2.5 mg/ml) was incubated for 2 h at 39°C. After stopping reaction by adding trichloroacetic acid (200 ml/l), the protein was estimated (Lowry et al., Reference Lowry, Rosebrough, Farr and Randall1951). The activity of esterase was determined by the method of Huggins and Lapides (Reference Huggins and Lapides1947). The assay mixture contained 0.1 ml SRL, 0.9 ml substrate (2 mmol/l p-nitrophenyl acetate in phosphate buffer of pH 6.0) and 2.0 ml phosphate buffer and incubated for 10 min at 39°C and absorbance was then recorded at 410 nm. The activity (U per ml) was expressed as nmol of p-nitrophenol released per min under the assay condition.
Data obtained were statistically analysed using the Statistical Packages for the Social Sciences (1997) for one-way analysis of variance to assess treatment effect.
Results and discussion
Chemical composition of diet
The CP content in the supplement and in the CFB was optimum to meet the requirements of sheep (Indian Council of Agricultural Research, 1998). There was no rain during this period so, pasture was dominated by dry stubble and had 77 g CP per kg DM. The values of CP and ADF content in the pasture resembled those reported by Shinde et al. (Reference Shinde, Karim, Sankhyan and Bhatta1998). The chemical composition of pasture is influenced by season (Ramirez et al., Reference Ramirez, Alonso, Hernandez and Ramirez1995; Shinde et al., Reference Shinde, Karim and Patnayak1996). The type of soil and its fertility, stocking density on the pastureland, type of grazing pasture and climate also contribute to the variability in chemical composition and nutritive value of pasture (Bryant et al., Reference Bryant, Kothmann and Merrill1979; Tripathi et al., Reference Tripathi, Mishra, Misra and Karim2001). The concentrate supplement was formulated to provide adequate protein nutrition thus had higher CP and lower fibre fractions.
Nutrient intake and digestibility
The intake of DM by SF animals was almost 1.5 of the GR animals and was significantly (P < 0.01) higher in the former. This reflected in an obvious effect on OM and CP intake, which were also higher in the SF group. Digestibility of OM, CP and energy was also higher (P < 0.01) in SF animals whereas, DM digestibility was not different (P < 0.05) between two groups (Table 2). Differences in DM intake between SF and GR groups might be due to the physical nature of the feed as well as post ingestion phenomenon. The SF sheep that were offered CFB were unable to make selection and it might have encouraged animals to eat more. Also, the even intake of concentrate and forage portions by SF animals would have improved microbial fermentation in their rumen that in turn would have increased intake. The GR animals on the other hand grazed pasture, and were supplemented with restricted quantity of concentrate mixture. It is well established that efficient microbial growth in the rumen require a balanced supply of nitrogen (amino acids and ammonia) and energy and diets containing less than 8% CP limits microbial growth in the rumen (Beever, Reference Beever, Forbes and France1993). The GR animals provided concentrate supplement once in the evening, so even supply of energy could not be maintained; this could have lowered intake and nutrient utilisation. Present findings corroborate the findings of Raghuvansi et al. (2006) and Samanta et al. (Reference Samanta, Singh, Das, Maity and Kundu2003) when complete feed blocks were fed to sheep/goats. Higher nutrient intake and digestion of the CFB diet resulted in a higher plane of nutrition of CFB fed animals.
Table 2 Nutrient intake and digestibility by SF and GR lambs (n=12)†
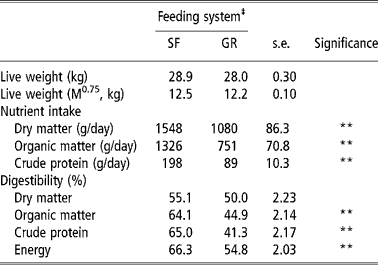
† Values based on digestibility trial.
‡ Feeding system: SF, complete feed block fed; GR, grazing plus supplementation.
Rumen fermentation and microbial enzymes
Total-N and NH3-N concentrations in the rumen were higher (P < 0.01) in SF animals than those of GR animals, but TVFA concentrations and pH were no different between the two groups (Table 3). The rumen fermentation parameters in the present study were within a normal range and comparable with those reported on complete feed mash or block (Santra et al., Reference Santra, Karim and Chaturvedi2002; Samanta et al., Reference Samanta, Singh, Das, Maity and Kundu2003; Mishra et al., Reference Mishra, Tripathi, Chaturvedi, Misra, Raghuvansi, Prasad and Jakhmola2005). The higher concentration of total-N and NH3-N could be because of higher CP intake and digestibility (Chaturvedi and Walli, Reference Chaturvedi and Walli2002). The total-N concentration in SRL was corroborated with the findings of Punia and Sharma (Reference Punia and Sharma1980).
Table 3 Rumen fermentation characteristics of SF and GR lambs
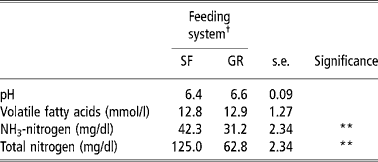
† Feeding system: SF, complete feed block fed; GR, grazing plus supplementation.
The rumen microbial enzyme activities are a qualitative reflection of rumen microbes involved in the digestion of feed. Carboxymethyl cellulase is involved in the degradation of amorphous cellulose, while xylanase is responsible for degradation of pentosans. The CMCase, xylanase and protease activities in extra cellular contents were higher in GR animals than in SF animals, while activities of MCCase, an enzyme that degrades crystalline cellulose and that of acetyl esterase were higher in the later (Table 4). Cellular microbial enzymes activities were higher in SF animals than in GR animals, except esterase and protease. It is known that amylolytic and cellulolytic bacteria rapidly colonize the soluble and easily degradable carbohydrates, and this facilitates the availability of specific substrate for the renewed growth of other fibrolytic bacteria (Costerton and Cheng, Reference Costerton, Cheng, Burns and Slater1982). The greater activities of CMCase, MCCase and xylanase in cellular fraction than liquid fraction in SF fed animals shows that the higher numbers of anaerobic microbes were attached to the solid fractions of the feed. A higher number of solid fraction associated microbes are known to increase fibrolytic enzyme activities and mainly contribute to fibre digestion (Cheng et al., 1983/Reference Cheng, Stewart, Dinsdate and Cosertor1984; Cheng and McAllister, Reference Cheng, McAllister, Hobson and Stewart1997).
Table 4 Rumen microbial enzymes activity (Unit) of SF and GR lambs
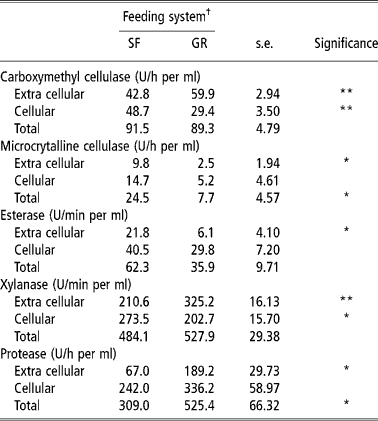
† Feeding system: SF, complete feed block fed; GR, grazing plus supplementation.
Total (cellular plus extra cellular) CMCase, esterase and xylanase activities were similar in two groups. However, total MCCase activity was higher (P < 0.05) and total protease activity was lower (P < 0.05) in SF than in GR. It indicated that a higher amount of fibrous residue was degraded in the rumen of SF animals so more amount of energy was available which saved proteins from under going degradation for gluconeogenic purposes. Thus it is quite likely that response in microbial growth and fibrolytic micro-organism population would have been better in SF animals because of improved overall balance of energy and nitrogen supply in rumen, which is known to improve intake, nutrient utilisation and microbial growth (Sultan and Loerch, Reference Sultan and Loerch1992; Arroquy et al., Reference Arroquy, Cochran, Villarreal, Wickersham, Llelwellyn, Titgemeyer, Nagaraja, Johnoson and Gnad2004).
Wool yield and growth performance
Total wool yield was similar in both groups, whereas SF resulted in a 0.29 proportionate increase in average daily gain (P < 0.01) in comparison with GR (Table 5). Owing to higher nutrient intake and favourable rumen microbial activities in the rumen, SF animals were in higher plane of nutrition that of GR animals. Moreover the forage to concentrate ratio was also narrower (60:40) in SF than in GR animal diet (77: 23). Required quantities of concentrate intake in SF group would have increased the availability of readily available carbohydrates that are known to improve animal performance and growth (Lee et al., Reference Lee, Jones, Moorby, Humphrey, Thedorou, MacRae and Scollan2001) because of increased efficiency of nitrogen and protein in ruminants (Arroquy et al., Reference Arroquy, Cochran, Villarreal, Wickersham, Llelwellyn, Titgemeyer, Nagaraja, Johnoson and Gnad2004).
Table 5 Wool yields and performance of lambs on complete feed block or grazing with supplement feeding
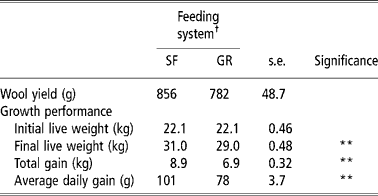
† Feeding system: SF, complete feed block fed; GR, grazing plus supplementation.
Conclusions
The SF animals had higher intake and digestibility, average daily gain and ruminal activity of MCCase and lower ruminal activity of protease than that of GR animals. Therefore the SF feeding system where CFB were offered to animals can be advocated as an alternative to grazing and supplementation feeding strategy for sheep production, especially where the pastures are highly eroded and need resting for regeneration/curing. Feeding of CFB can also be adopted under adverse situations such as drought or famine, which are common phenomena in arid and semi-arid regions.
Acknowledgements
The authors wish to thank the Director for providing the facilities to carry out this experiment. Funding provided by NATP/AED (ARID)/PAL 028/99 is greatly appreciated. The senior author is very grateful to PIU of NATP for providing financial help through a Senior Research Fellowship.







