Vitamin E is a lipid-soluble vitamin with antioxidative property. It has been reported to eliminate infertility; however, the main symptom of its deficiency in humans is ataxia, which occurs in people with impaired lipid absorption or genetic disorder caused by defects in the α-tocopherol transfer protein (α-TTP)(Reference Traber1–Reference Euch-Fayache, Bouhlal and Amouri3). Furthermore, it has been reported that low dietary vitamin E intake increases oxidative stress(Reference Traber1). Natural vitamin E has eight forms; namely, α, β, γ, δ-tocopherols and tocotrienols with α-tocopherol being the predominant form in mammals since a specific transporter protein for α-tocopherol, α-TTP exists in the liver and only this vitamin E form is secreted into the circulation and peripheral tissues(Reference Traber and Kayden4–Reference Oram, Vaughan and Stocker6). Most of the other vitamin E forms are metabolised and excreted into the urine(Reference Abe, Uchida and Ohta7). It has been reported that α-TTP knockout mice and patients with α-TTP gene mutations have reduced vitamin E levels in the body and could demonstrate vitamin E deficiency symptoms(Reference Traber and Arai5).
We previously reported an increase in anxiety-like behaviour in rats fed with a vitamin E-free diet for 4 weeks(Reference Okura, Tawara and Kawai8). Further, the anxiogenic effect of vitamin E deficiency has been observed not only in juvenile rats but also in adult rats despite the fact that a vitamin E-free diet was less effective in lowering vitamin E body levels in adult rats than in juvenile rats(Reference Terada, Okura and Kikusui9). We previously demonstrated that behavioural changes in rats fed with a vitamin E-free diet for 4 weeks were not due to ataxia since there was no impairment of motor coordination and locomotor activities(Reference Okura, Tawara and Kawai8). Moreover, increased anxiety-like behaviour has been reported in α-TTP knockout mice, which have low plasma and tissue vitamin E levels(Reference Yokota, Igarashi and Uchihara10). Increased anxiety-like behaviour has been reported in phospholipid transfer protein (PLTP) knockout mice, which have low α-tocopherol levels in the brain but not in plasma, indicating that α-tocopherol in the brain is involved in anxiety(Reference Desrumaux, Risold and Schroeder11). The increased anxiety-like behaviour in PLTP knockout mice was confirmed to be caused by vitamin E deficiency since the anxiety in 6-month-old PLTP knockout mice was prevented by vitamin E supplementation to parents(Reference Desrumaux, Mansuy and Lemaire12). Since vitamin E is among the most important antioxidants in the body, increased oxidative stress could be involved in the anxiety increase. Animals exposed to oxidative stress through buthionine sulfoximine treatment have been reported to show an increase in anxiety-like behaviour in a reactive oxygen species-dependent manner(Reference Masood, Nadeem and Jamal Mustafa13,Reference Salim, Sarraj and Taneja14) . Further, an association between oxidative stress and anxiety-like behaviour has been reported(Reference Hovatta, Juhila and Donner15).
Moreover, anxiety-like behaviour has been reported to be significantly increased by vitamin E deficiency in rats under social isolation stress in our previous study(Reference Okura, Tawara and Kikusui16), indicating that anxiety induced by vitamin E deficiency is possibly related to the stress response mechanism. The hypothalamus–pituitary–adrenal axis is a well-known endocrine system that is strongly associated with stress and anxiety. When animals are exposed to stress, the hypothalamus secretes corticotropin-releasing factor, which triggers the pituitary to secrete adrenocorticotropic hormone(Reference Arborelius, Owens and Plotsky17); consequently, adrenocorticotropic hormone induces corticosterone secretion from the adrenal cortex(Reference Arborelius, Owens and Plotsky17). Corticosterone down-regulates corticotropin-releasing factor and adrenocorticotropic hormone expression and inhibits its own secretion. This negative feedback regulation prevents long-term exposure to high corticosterone levels. Many studies have reported that chronic exposure to high corticosterone levels affects nerve cells and increases anxiety behaviour(Reference Murray, Smith and Hutson18–Reference Cook and Wellman22).
In the present study, we first aimed to investigate the time-dependent effects of vitamin E deficiency on anxiety-like behaviour in individually housed rats. Moreover, we aimed to examine the effect of vitamin E refeeding or excess vitamin E feeding in reducing anxiety. Finally, we aimed to investigate the role of adrenal hormones on increased anxiety-like behaviour due to vitamin E deficiency using adrenalectomised rats.
Methods
Animal experiments
We purchased male SPF Wistar rats (Japan Laboratory Animals Inc.) and housed them in stainless cages at 22–24°C under a 12 h light/dark cycle (06.00–18.00 hours) in a conventional animal room. The rats were fed with a commercial pellet diet (certified diet MF; Oriental Yeast) ad libitum for the first 3–5 d to acclimate to the new environment. After this acclimation period, they were used for each animal experiment as described below. At the end of each experiment, the rats were dissected under anaesthesia with sodium pentobarbital (1 mg/kg, intraperitoneal, Somnopentyl, Kyoritsu Pharmaceutical Co. Ltd). We obtained heparinised plasma and tissues and stored them at −80°C until use. All the experimental procedures followed institutional and national guidelines for the care and use of animals and were approved by the Meiji University Institutional Animal Care and Use Committee (Approval Number: IACUC 15-007).
Experiment with rats fed with a vitamin E-free diet for various periods
We fed 3-week-old rats with a control (CON) or vitamin E-free (−VE) diet (Table 1) throughout the experimental period. We performed separate experiments with different durations (3 d, 1 week and 2 weeks). Rats were randomly divided into the experimental groups (n 6 per group) and housed with wood chip bedding. The rats were individually housed in a stainless cage (12 × 18 × 11 cm) in the 3-d and 1-week experiments. In the 2-week experiment, we housed three rats in a stainless cage (15 × 24 × 11 cm) for the 1st week and individually housed them the following week. This method allowed all the rats to be subjected to social isolation stress for 1 week before behavioural analysis. We performed the elevated plus maze (EPM) test on day 3 (3-d experiment), days 7 and 8 (1-week experiment) and day 14 (2-week experiment) and dissected the rats on days 4, 9 and 17, respectively.
Table 1. Composition of the diets (g/100 g diet)

VE, vitamin E.
* AIN-93 vitamin mixture contains 1500 mg/100 g all-racemic α-tocopheryl acetate.
Experiment with rats fed with a vitamin E-free diet for 4 weeks and refed with a control diet
We fed 3-week-old rats with a CON or −VE diet for 28 d. Next, the rats fed with a −VE diet were divided into three groups and fed with a CON diet for another 1, 3 or 7 d. Rats were randomly divided into the experimental groups (n 6 per group) and housed with wood chip bedding. We housed three rats in a stainless cage (15 × 24 × 11 cm) and then individually housed them in a stainless cage (12 × 18 × 11 cm) during the last week before the EPM test. We performed the EPM test on the last day of the feeding period and dissected the rats after a 12-h fasting period on the next day after the EPM test.
Experiment with rats fed with an excess vitamin E diet or vitamin E-free diet
We fed 3-week-old rats with a CON, −VE or +VE diet (added 500 mg/kg all-racemic α-tocopherol to the −VE diet) (Table 1) for 4 weeks. Rats were randomly divided into the experimental groups (n 6 per group) and housed with wood chip bedding. We housed three rats in a stainless cage (15 × 24 × 11 cm) for 3 weeks and then individually housed them in a stainless cage (12 × 18 × 11 cm) during the last week before the EPM test. The EPM test was performed on day 30, and the rats were dissected on day 34.
Experiment with adrenalectomised rats fed with a vitamin E-free diet
We adrenalectomised or sham-operated 5-week-old rats and fed them with a CON or −VE diet for 4 weeks. Rats were randomly divided into the experimental groups (n 6 per group) and housed with wood chip bedding. All the rats had free access to a 0·88 % NaCl solution after surgery. We housed three rats in a stainless cage (15 × 24 × 11 cm) and then individually housed them in a stainless cage (12 × 18 × 11 cm) during the last week before the EPM test. The EPM test was performed on day 28, and the rats were dissected after a 12-h fasting period on day 29.
Elevated plus maze test
The EPM test was performed as previously described(Reference Oshima, Watanabe and Endo23) with an apparatus composed of two open arms (50 × 10 cm) and two closed arms (50 × 10 × 40 cm) elevated 50 cm from the floor. We video-recorded the rats’ behaviour for 15 min and analysed the activity on the open arms, head dipping, stretch-out posture and locomotion. We used the first three indicators to evaluate anxiety behaviour and used locomotion to evaluate spontaneous motor activity.
α-Tocopherol
We measured tissue or plasma α-tocopherol levels using high-performance liquid chromatography as previously described(Reference Terada, Okura and Kikusui9).
Thiobarbituric acid reactive substances
We measured tissue or plasma thiobarbituric acid reactive substances (TBARS) levels using the fluorescence method as previously described(Reference Terada, Okura and Kikusui9).
Plasma corticosterone
We used heparinised plasma samples that were collected right after the EPM tests. We measured the corticosterone levels using an AssayMax Corticosterone ELISA Kit (Assaypro).
Statistical analysis
We used Student’s t test or Welch’s t test to perform between-group comparisons of values depending on whether they had equal or unequal homogeneity of variances, respectively. We performed the Mann–Whitney U test for non-normal data sets. We used one-way ANOVA and post hoc tests (Tukey–Kramer) to analyse differences among the three experimental groups that received excess vitamin E. We used two-way ANOVA to evaluate the effects of two factors simultaneously in the adrenalectomy experiment. We compared the corresponding CON and −VE groups when we observed a significant interaction be the two evaluated factors. All statistical analyses were performed using Statistics 2008 (Social Survey Research Information Co. Ltd) for Excel and the differences were considered significant at P < 0·05.
Results
Experiment with rats fed with a vitamin E-free diet for various periods
The body weight was not affected by vitamin E deficiency, while there was a significant decrease in plasma, liver and cortex α-tocopherol levels in all the examined durations (Table 2). Vitamin E deficiency induced an increase in plasma and liver levels of TBARS after 1 or 2 weeks of vitamin E deficiency compared with those in the control groups; however, there was no significant increase in the cortex levels. Vitamin E deficiency for 3 d resulted in an increase in TBARS level in plasma, but not in either the liver or the cortex (Table 2). We have demonstrated that 1 week and 2 weeks of vitamin E deficiency induces an increase in plasma corticosterone levels compared with those in the control groups (Table 2). In the EPM test, there was a decrease in open arm activity after 2 weeks of vitamin E deficiency while a change in head dipping and stretch out was observed after 1 week (Fig. 1). There was no effect of vitamin E deficiency on locomotion in all the examined durations (Fig. 1). The EPM test results demonstrated an increase in anxiety-like behaviour after 1 or 2 weeks of vitamin E deficiency depending on the behavioural indicators.
Table 2. Characteristics of the rats fed vitamin E-free (−VE) diet for various periods
(Mean values with their standard errors)

CON, control diet; TBARS, thiobarbituric acid reactive substances; TEP eq., tetraethoxypropane equivalent.
P value P < 0·1; * P < 0·05, ** P < 0·01.
† Food intake; daily food intake during the last 1 week of the individual housing (2 weeks groups).
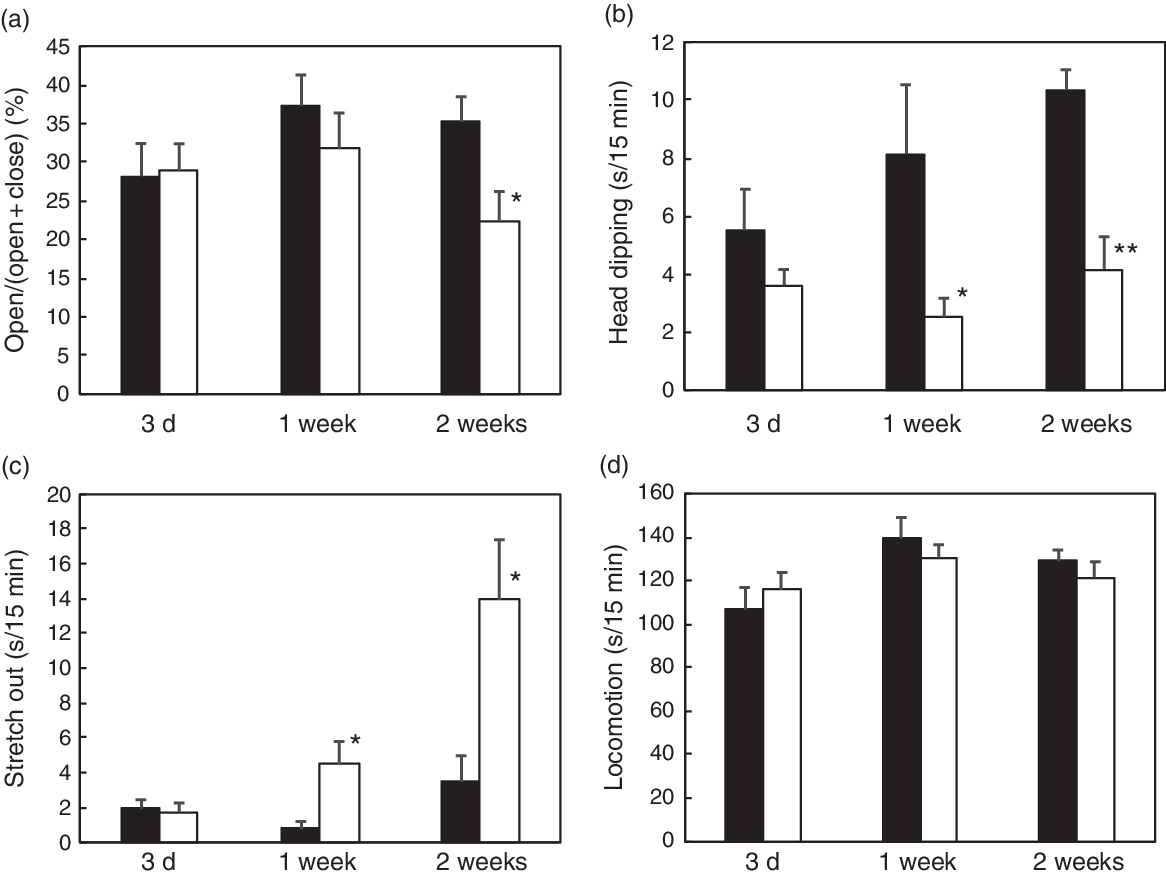
Fig. 1. Effect of vitamin E deficiency for various periods on anxiety-like behaviour in rats. The rats were fed with a control (CON) or vitamin E-free (−VE) diet for 3 d, 1 week or 2 weeks. We analysed open arm activity (a), head dipping (b), stretch out (c) and locomotion (d) in the 15-min elevated plus-maze (EPM) test. Values are means with their standard errors (n 6 per group). Mean value is significantly different from that of the CON group: * P < 0·05, ** P < 0·01. ![]() , CON;
, CON; ![]() , −VE.
, −VE.
Experiment with rats refed with vitamin E after receiving a vitamin E-free diet for 4 weeks
The body weight and food intake were not affected by vitamin E deficiency or refeeding except for a slight increase in food intake in rats refed with vitamin E for 3 d (Table 3). Feeding a vitamin E-free diet decreased the plasma, liver and cortex α-tocopherol levels, which were recovered by vitamin E refeeding (Table 3). Vitamin E deficiency increased the plasma, liver and cortex TBARS levels with the plasma and liver levels being reduced by vitamin E refeeding (Table 3). Vitamin E deficiency tended to increase plasma corticosterone levels, which on subsequent vitamin E refeeding, tended to reduce (Table 3). Vitamin E deficiency increased anxiety-like behaviour in open arm activity, head dipping and stretch out (Fig. 2). Vitamin E refeeding significantly decreased anxiety-like behaviour in stretch out and tended to decrease them in open arm activity and head dipping (Fig. 2). Vitamin E deficiency or refeeding did not affect locomotion (Fig. 2).
Table 3. Characteristics of the rats refed vitamin E after feeding vitamin E-free (−VE) diet for 4 weeks
(Mean values with their standard errors)

CON, control diet; TBARS, thiobarbituric acid reactive substances; TEP eq., tetraethoxypropane equivalent.
P value P < 0·1, * P < 0·05, ** P < 0·01, −VE v. 1, 3, 7 d.
P value P < 0·1, † P < 0·05, †† P < 0·01, CON v. −VE.
‡ Food intake, daily food intake during the last 1 week of the individual housing.
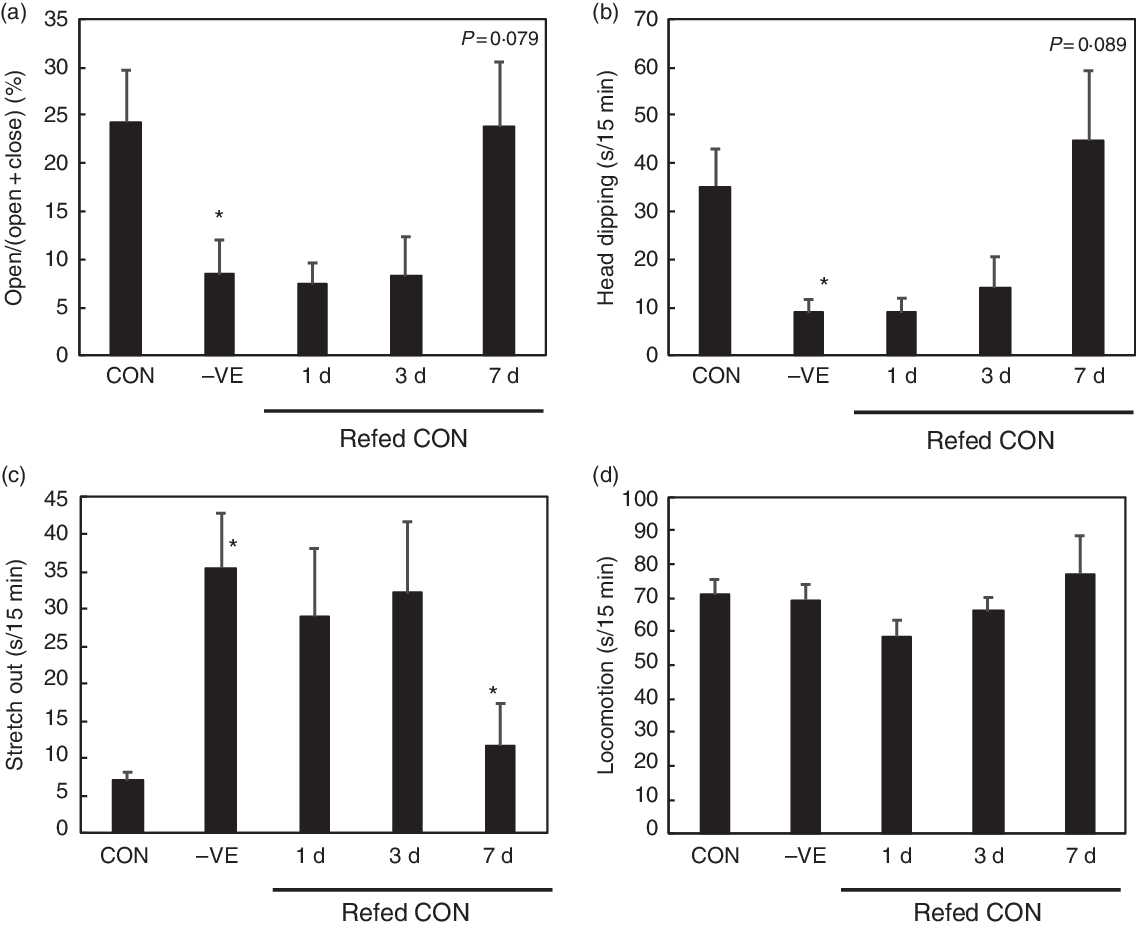
Fig. 2. Effect of vitamin E refeeding on anxiety-like behaviour in vitamin E-deficient rats. The rats were fed with a control (CON) or vitamin E-free diet (−VE) diet for 28 d. Next, the rats fed with a −VE diet were divided into three groups and then refed with a CON diet for another 1, 3 or 7 d. We analysed open arm activity (a), head dipping (b), stretch out (c) and locomotion (d) in the 15-min elevated plus-maze (EPM) test. Values are means with their standard errors (n 6 per group). Differences between CON and −VE are shown as * P < 0·05 on −VE bar chart. Differences between −VE and 1, 3 or 7 d are shown as P value when P < 0·1 or * P < 0·05 on 1, 3 or 7 d bar chart.
Experiment with rats fed with a vitamin E-free or excess vitamin E diet
The body weight and food intake were not affected by feeding with a vitamin E-free or excess vitamin E diet (Table 4). Vitamin E deficiency and excess vitamin E feeding decreased and increased plasma and liver α-tocopherol levels, respectively (Table 4). The amount of dietary vitamin E did not significantly affect cortex α-tocopherol levels (Table 4). Plasma and liver TBARS levels were increased by vitamin E deficiency but were not affected by excess vitamin E intake (Table 4). The amount of dietary vitamin E did not significantly affect cortex TBARS levels (Table 4). Plasma corticosterone levels were increased by vitamin E deficiency but were not affected by excess vitamin E intake (Table 4). Anxiety-like behaviour in the open arm and stretch out activities tended to increase with vitamin E deficiency and subsequently tended to reduce with excess vitamin E intake (Fig. 3). The amount of dietary vitamin E did not affect head dipping and locomotion (Fig. 3).
Table 4. Characteristics of the rats fed vitamin E-free (−VE) diet or diet with excess vitamin E (+VE)
(Mean values with their standard errors)
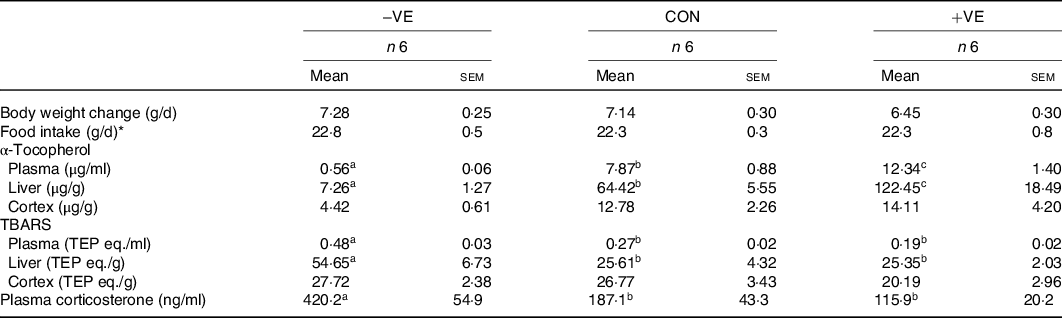
CON, control diet; TBARS, thiobarbituric acid reactive substances; TEP eq., tetraethoxypropane equivalent.
a,b,c Mean values in a row with unlike superscript letters are significantly different (P < 0·05).
* Food intake, daily food intake during the last 1 week of the individual housing.
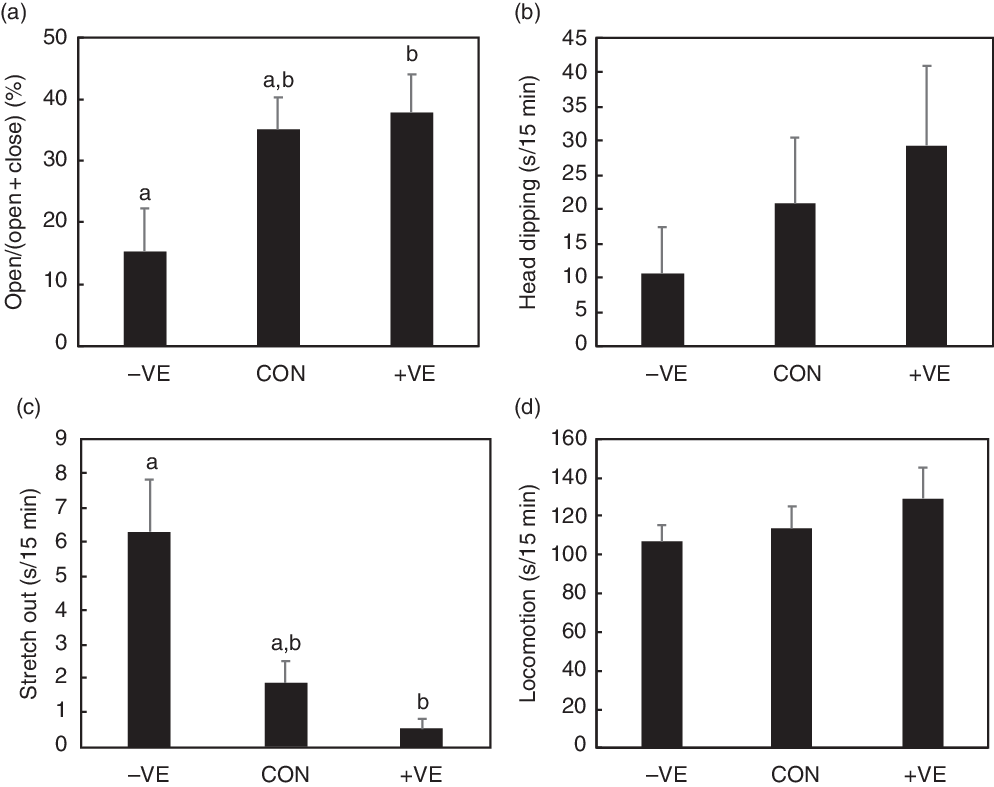
Fig. 3. Effect of vitamin deficiency or excess vitamin E feeding on anxiety-like behaviour in rats. The rats were fed with a control (CON) diet, vitamin E-free (−VE) diet or a diet with 500 mg/kg vitamin E (+VE) for 4 weeks. We analysed open arm activity (a), head dipping (b), stretch out (c) and locomotion (d) in the 15-min elevated plus-maze (EPM) test. Values are means with their standard errors (n 6 per group). a,b Mean values with unlike letters are significantly different according to post hoc analysis (P < 0·05).
Experiment with adrenalectomised rats fed with vitamin E-free diets
Vitamin E deficiency increased food intake but did not affect body weight in either the sham-operated or adrenalectomised rats (Table 5). Vitamin E deficiency decreased plasma, liver and cortex α-tocopherol levels (Table 5). Plasma and liver TBARS levels were affected by vitamin E deficiency, while the cortex levels were affected by adrenalectomy (Table 5). Adrenalectomy decreased plasma corticosterone levels (Table 5). Anxiety-like behaviour was increased by vitamin E deficiency in the sham-operated rats but was not increased in the adrenalectomised rats in open arm activity, head dipping and stretch out (Fig. 4). Locomotion was slightly increased by adrenalectomy (Fig. 4).
Table 5. Characteristics of the rats with or without adrenalctomy and fed control (CON) or vitamin E-free (−VE) diet
(Mean values with their standard errors)

SHAM, sham-operated; ADEX, adrenalectomised; TBARS, thiobarbituric acid reactive substances; TEP eq., tetraethoxypropane equivalent.
* P < 0·05, ** P < 0·01.
† Body weight gain during feeding experimental diet.
‡ Daily food intake during the last 1 week of the individual housing.

Fig. 4. Effect of adrenalectomy on anxiety-like behaviour in rats due to vitamin E deficiency. The rats were sham-operated (SHAM) or adrenalectomised (ADEX) and were fed with a control (CON) or vitamin E-free (−VE) diet for 4 weeks. We analysed open arm activity (a), head dipping (b), stretch out (c) and locomotion (d) in the 15-min elevated plus-maze (EPM) test. Values are means with their standard errors (n 6 per group). Two-way ANOVA results are shown below each graph (NS, * P < 0·05, P value when P < 0·1). Differences between the two dietary groups in the same surgery group were analysed when effect of interaction was observed in two-way ANOVA. ![]() , CON;
, CON; ![]() , −VE.
, −VE.
Discussion
Our findings demonstrate the time-dependent changes of α-tocopherol levels, oxidative stress marker levels and anxiety-like behaviour in rats fed with a vitamin E-free diet. Regarding the oxidative stress marker, there was a slower increase in TBARS levels than decrease in α-tocopherol levels in all the examined tissues. Further, there was a faster increase in anxiety-like behaviour than that of TBARS levels, which could be attributed to the fact that TBARS is generated through a multi-step reaction. Although there was decline in plasma, liver and cortex α-tocopherol levels after 3 d of receiving a vitamin E-free diet, rate of decrease in the cortex was small. This is consistent with the previously reported slower vitamin E decline in the brain than in other tissues in vitamin E-deprived mice(Reference Leonard, Terasawa and Farese24,Reference Cuddihy, Ali and Musiek25) . Regarding brain vitamin E levels, which we hypothesise to be associated with increased anxiety behaviour, the cortex α-tocopherol levels decreased to 75 % of those observed in control rats after 1 week of receiving vitamin E-free diet when the anxiety increase began. These findings, together with the previous findings that brain α-tocopherol levels in PLTP knockout mice were reduced to 70 % of those in wild-type mice(Reference Desrumaux, Risold and Schroeder11), indicate that a decrease in the level of brain α-tocopherol would increase the risk of increased anxiety-like behaviour.
In the EPM test, a 2-week vitamin E-deficient diet significantly increased anxiety-like behaviour in open arm activity, while a 1-week vitamin E-deficient diet increased anxiety-like behaviour in stretch out and head dipping. Similarly, we previously reported that stretch out and head dipping were more sensitive indicators of anxiety in vitamin E-deficient rats(Reference Okura, Tawara and Kikusui16). These findings indicate that the effect of vitamin E deficiency on anxiety-like behaviour is not acute but rather progresses over time. Moreover, 1 week of vitamin E refeeding restored the increased anxiety to normal levels. These results suggest that the increase or decrease in anxiety-like behaviour is caused by a mechanism that gradually changes with an increase or decrease in vitamin E.
However, increased anxiety-like behaviour has been reported to occur much earlier than the physical symptoms of vitamin E deficiency, which were reported to occur after 12 weeks in rats fed with a vitamin E-deficient diet(Reference Machlin, Filipski and Nelson26). This indicates that anxiety-like behaviour is a more sensitive indicator of vitamin E deficiency than physical symptoms. Moreover, we found that rats fed with excess vitamin E had less anxiety-like behaviour and lower TBARS levels than control rats under social isolation. These results suggest that vitamin E supplementation to the control diet might be effective to prevent stress-related anxiety even when the control diet contains enough vitamin E amounts to maintain physical health. Lower vitamin E levels have been reported in patients with major depression(Reference Desrumaux, Mansuy and Lemaire12), as well as a beneficial effect of vitamin E intake on Alzheimer’s disease(Reference Owen, Batterham and Probst27); however, these previous findings remain controversial.
Since plasma corticosterone levels right after the EPM test were higher in the vitamin E-deficient rats than in the control rats, we investigated the involvement of corticosterone in increased anxiety-like behaviour using adrenalectomised rats. We found that adrenal hormones were necessary for the anxiety-like behaviour to appear. Adrenal removal almost completely suppressed the increase in anxiety-like behaviour caused by vitamin E deficiency despite the low α-tocopherol levels. There have been previous reports of an unexplained increase in brain TBARS levels after removal of the adrenal gland(Reference Liu, Yokoi and Doniger28); however, it was not associated with anxiety-like behaviour. Previous studies have reported a close association between corticosterone and anxiety. Chronic corticosterone administration to mice was reported to induce reduced neurogenesis in the hippocampus and increased anxiety-like behaviour(Reference Murray, Smith and Hutson18). Further, glucocorticoid receptor deletion, corticotropin-releasing factor deletion or corticotropin-releasing factor antagonist administration has been reported to decrease anxiety-like behaviour(Reference Smith, Aubry and Dellu19–Reference Heinrichs, Pich and Miczek21,Reference Timpl, Spanagel and Sillaber29) . Additionally, high corticosterone levels under chronic stress were reported to induce morphological spine changes in the prefrontal cortex(Reference Cook and Wellman22). Moreover, high corticosterone levels under stress have been reported to decrease endogenous cannabinoid, which is an important neuroplasticity regulator and increase anxiety-like behaviour(Reference Patel, Roelke and Rademacher30,Reference Qin, Zhou and Pandey31) . These findings demonstrate that chronic exposure to high corticosterone levels affects neural functions and increases anxiety-like behaviour. Previous studies have also reported increased corticosterone levels associated with vitamin E deficiency(Reference South, Smith and Guidry32). Although the mechanism underlying increase corticosterone levels remains to be identified, we speculate that the decreased reactivity to corticosterone in the hypothalamus or hippocampus reduces the negative feedback regulation of the hypothalamus–pituitary–adrenal axis and upregulates plasma corticosterone level during stress. Increased corticosterone levels resulting from this mechanism have been observed in depressed patients and could cause emotional disorders(Reference Kunugi, Ida and Owashi33). Furthermore, vitamin E-deficient rats have been reported to have reduced hippocampal glucocorticoid receptor levels(Reference Kobayashi, Machida and Takahashi34).
Many studies have reported a relationship between vitamin E deficiency and brain functions. Brain monoamine levels, which are important for emotion control, have been reported to be altered by vitamin E deficiency and this alteration might be related to increased anxiety behaviour during vitamin E deficiency(Reference Desrumaux, Mansuy and Lemaire12,Reference Adachi, Izumi and Mitsuma35) . DNA microarray analysis results of α-TTP knockout mice brain indicated reduced expression of genes that determine synaptic plasticity and neuronal development(Reference Gohil, Schock and Chakraborty36). Furthermore, oxidative stress has been reported to cause neuroinflammation(Reference Takahashi, Yanai and Takisawa37) and neurodegeneration and is thought to cause neurodegenerative diseases such as Alzheimer’s disease(Reference Wang, Wang and Li38). Further, PLTP knockout mice have been reported to have enhanced memory impairment caused by amyloid β and that vitamin E administration reduces this disorder(Reference Desrumaux, Pisoni and Meunier39). The increased anxiety-like behaviour observed in present study is considered as one of the early symptoms of emotional disorder occurring from hypothalamus–pituitary–adrenal axis abnormalities that decrease brain function. It has also been reported that oxidative stress may affect human anxiety(Reference Islam, Ahmed and Islam40).
Our findings indicate that increased anxiety-like behaviour is an early symptom of vitamin E deficiency that can be recovered by vitamin E refeeding. Moreover, we confirmed that anxiety-like behaviour under social isolation stress can be reduced by taking vitamin E excess of the required amount. Therefore, taking a higher vitamin E amount than is currently needed might be effective for maintaining a healthy mental state. Further, we found that adrenal hormones are crucial in the increase of anxiety behaviour due to vitamin E deficiency and that there was a close relationship between the anxiogenic effect of vitamin E deficiency and stress. Present study provides novel findings that demonstrate the necessity and importance of vitamin E in mental health.
Acknowledgements
The authors thank the members of the Laboratory of Food Biochemistry at Meiji University for assistance with the animal experiments.
The present study was funded by the Japan Society for the Promotion of Science (JSPS), Grant-in-Aid for Scientific Research (C) 24580202.
The authors’ contributions were as follows: A. T. contributed to the study design; Y. T., H. O., Y. O. and K. T. performed the animal experiments, data collection and data analysis; A. T. contributed to writing the manuscript. All the authors read and approved the final version of the manuscript.
The authors have no financial or personal conflicts of interest to declare.












