Introduction
According to the US Census Bureau, around 24 million Asian Americans resided in the USA in 2021 [1]. Of those 24 million, 2.3 million are of Vietnamese descent, making them one of the largest Asian American populations [1]. During the coronavirus disease 2019 (COVID-19) pandemic, finding data regarding the Asian American, Native Hawaiian, and Pacific Islander (AANHPI) populations was challenging, let alone disaggregated data about different ethnic populations [Reference Yan, Hwang, Ng, Chu, Tsoh and Nguyen2]. While some states report disaggregated data on COVID-19 AANHPI healthcare disparities, availability is limited in Texas despite the large AANHPI and Vietnamese population [Reference Tai, Sia, Doubeni and Wieland3].
As researchers raced to develop vaccines and treatments for this new infectious disease, historically marginalized communities were inadequately represented in COVID-19 clinical trials [Reference Craft, Travassos, Palacios and Openshaw4–Reference Murali, Gumber and Jethwa6]. However, increased participation of racial and ethnic minorities is essential for understanding diseases, preventive factors, and treatment effectiveness across populations. Efforts to increase minority groups' enrollment among research participants, such as the Revitalization Act of 1993, require that clinical trials that the National Institutes of Health fund include women and minority participants. Unfortunately, such efforts have failed to gain substantial improvement [Reference Clark, Watkins and Piña7].
Limited research has examined facilitators and barriers to COVID-19 clinical therapeutic trial participation among Vietnamese Americans and associations with levels of trust [Reference Abdelhafiz, Abd ElHafeez and Khalil8,Reference Thompson, Manning and Mitchell9]. Other studies looking at AANHPI participation in clinical trials have found that Asians were less familiar with the term “clinical trials,” and Vietnamese groups may have lower knowledge of clinical trials compared to other AANHPI ethnic groups [Reference Paterniti, Chen and Chiechi10,Reference Ma, Seals, Tan, Wang, Lee and Fang11]. A more recent investigation found that Asian Americans were less willing to participate in health research than other ethnic groups, but they were as likely to trust researchers [Reference Liu, Elliott, Strelnick, Aguilar-Gaxiola and Cottler12].
Under the umbrella of the National Institutes of Health (NIH) Community Engagement Alliance (CEAL) works with historically marginalized communities to conduct community-engaged outreach and research. Through collaborations with the Texas CEAL Consortium and community-based organizations (CBOs), we investigated the association between information source trust levels about COVID-19 and COVID-19 clinical trials and Vietnamese Americans’ willingness to participate in COVID-19 clinical trials.
Methods
NIH CEAL developed the Common Survey 2 instrument, which contains 23 questions about social determinants of health, information, trust, risk perception, testing and disease control, COVID-19 vaccination, and demographics. We examined the questions about trust in sources to provide pertinent information about COVID-19, trust in sources to provide correct information about COVID-19 clinical trials, and if respondents were to get COVID-19, how willing they were to sign up for a hypothetical therapeutic trial. The survey instrument contained questions pertaining to trust in various sources of information about COVID-19 and COVID-19 clinical trials as distinct variables. While various options for COVID-19 information sources, such as news, social media, or governments, exist, fewer and potentially different information sources exist about COVID-19 clinical trials like the NIH, drug companies, and researchers.
To ensure that participants were part of the target population, they self-assessed their race from the following categories: Hispanic or Latino, American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian, Pacific Islander, and/or white. They could then select all ethnicities that applied.
A translator from a partnering CBO translated this survey from English to Vietnamese. Then, the survey was back-translated from Vietnamese to English by a different CBO translator. Native Vietnamese speakers at another CBO reconciled the translations to ensure that they were understandable by the population of interest. The survey and consent form was available online in both English and Vietnamese. The average time to complete the survey was 15 minutes in English and 20 minutes in Vietnamese.
We used a convenience sample of Vietnamese Americans. The inclusion criteria were people at least 18 years old, of Vietnamese heritage, living in Texas, and who could read and write in English or Vietnamese. The survey was open from September 20, 2021, to March 4, 2022. Recruitment occurred virtually in English and Vietnamese through email lists, social media, an online webinar, and an internet ad from January 6 to 23, 2022. Participants were also recruited in person at literacy classes and two health fairs organized by CBOs. Recruitment flyers in English and Vietnamese were also posted in two health clinics serving Vietnamese populations. All participants who completed the survey and provided a valid email address or phone number were entered into a raffle for one of five $50 credit card gift cards. The University of Houston Institutional Review Board approved this research.
We analyzed those willing and unwilling to sign up for a clinical trial for COVID-19 using available case analysis. Those who responded with “prefer not to answer” or “no opinion” were excluded from the analysis. Willingness to participate in COVID-19 trials was originally scored on a 7-point Likert scale with 1 meaning not at all willing and 7 very willing. Willingness was then dichotomized, with unwillingness defined as responses of 1–4 and willingness as responses of 5–7. This threshold (>4) combines all endorsements of clinical trial participation above the neutral option (4). Trust in sources of information about COVID-19 in general or COVID-19 clinical trials was measured as the proportion of participants who responded that they had “a great deal” of trust. Chi-square tests and logistic regression assessed statistical significance and the magnitude of relationships, respectively, between trusted sources of information and willingness. The data analysis was performed using STATA 17.
Results
Of the 224 surveys Vietnamese Texans submitted, the 212 surveys containing complete responses regarding participants’ willingness to sign up for a clinical trial were used. Table 1 provides the demographics of the 212 respondents.
Table 1. Sociodemographic characteristics
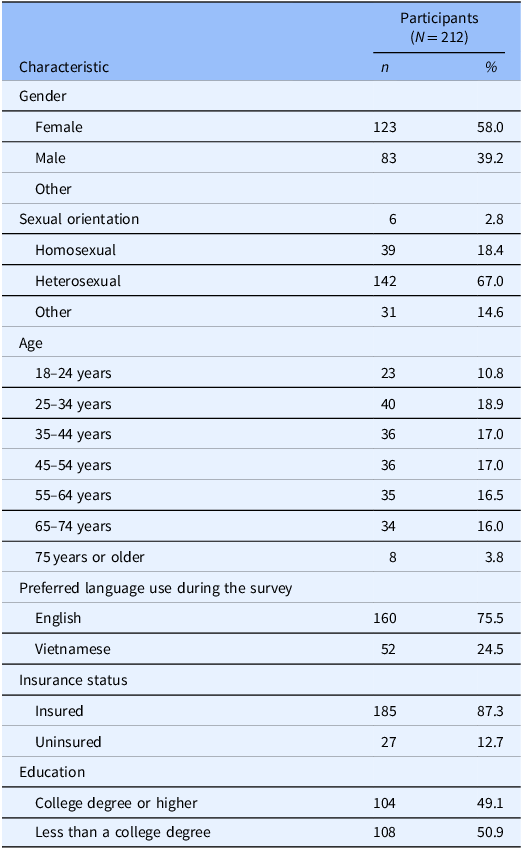
This table highlights the sociodemographic characteristics, including gender, sexual orientation, age, preferred language use during a survey, insurance status, and education from the online survey for Vietnamese adults living in Texas that collected data from September 2021 to March 2022.
Trust in COVID-19 clinical trial sources of information
Willingness to participate in a clinical trial was significantly associated with past participation in a trial (odds ratio (OR) = 4.32; 95% CI 1.64–11.36), trust in information about trials from university hospitals (OR = 4.91; 95% CI 1.35–17.89), and from drug companies (OR = 4.14; 95% CI 1.77–9.67). Conversely, trust in information that local clinics provided was significantly associated with less willingness to participate in a trial (OR = 0.30; 95% CI 0.12–0.73). Table 2 provides the ORs and tests of significance between willingness to participate in COVID-19 clinical trials and previous enrollment in a COVID-19 clinical trial.
Table 2. Association between willingness to participate in COVID-19 clinical trials and trust in information sources regarding COVID-19 clinical trials (odds ratios, 95% confidence intervals, and associated P-values)
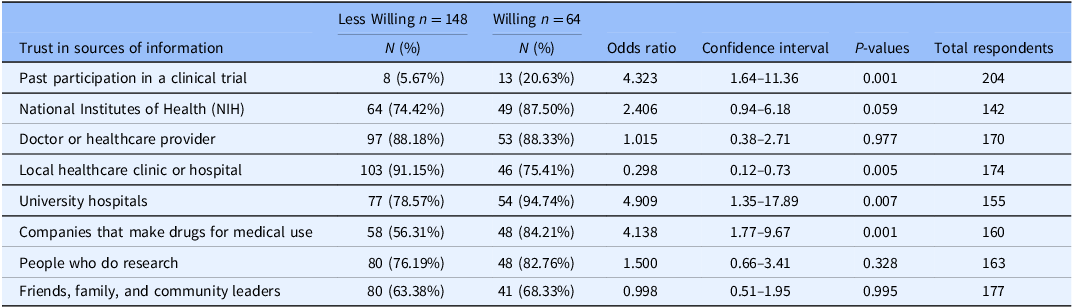
N = 212. This table highlights trust in sources of information regarding COVID-19 clinical trials associated with willingness to participate in COVID-19 clinical trials. Participants who chose “no opinion” or “prefer not to answer” were not included in the analysis.
Trust in COVID-19 sources of information
Participants who trusted information about COVID-19 from the federal government (54.55%) were significantly more willing to participate in COVID-19 clinical trials (OR = 2.27; 95% CI 1.16–4.47). Trust in information about COVID-19 from the local and state government (51.85%) was significantly associated with willingness to participate in COVID-19 clinical trials (OR = 2.30; 95% CI 1.17–4.52). Table 3 provides ORs and tests of significance between willingness to participate in COVID-19 clinical trials and trust in sources regarding COVID-19 information.
Table 3. Odds ratios (OR) from bivariate Chi-square tests of willingness to participate in COVID-19 clinical trials and trust in sources regarding COVID-19

N = 212. This table highlights the trust in sources for COVID-19 information associated with the willingness to participate in COVID-19 clinical trials. Participants who chose “prefer not to answer,” “don't know,” or “does not apply” were not included in the analysis.
Discussion
Our principal finding was that a significant association existed between Vietnamese Americans’ high trust levels in drug companies and university hospitals and their willingness to participate in COVID-19 clinical trials. This finding means that researchers should identify trusted sources of information. After identifying such trusted information sources, researchers can collaborate with them to provide education regarding clinical trials to the Vietnamese American population and increase awareness and recruitment. This increased awareness could help recruit participants for clinical trials from different racial and ethnic subgroups to identify harms and benefits in specific populations.
These findings correlate with other studies that have examined trust and COVID-19 clinical trial participation. For example, Abdelhafiz et al. found that a general lack of trust in pharmaceutical companies, physicians, and hospitals prevented a Middle Eastern population from participating in COVID-19 clinical trials [Reference Abdelhafiz, Abd ElHafeez and Khalil8]. Thompson et al. examined medical mistrust and found that most participants, including Asians, were unwilling to participate in a vaccine trial [Reference Thompson, Manning and Mitchell9]. In general, distrust of pharmaceutical companies discouraged participation in clinical trials involving pregnant people and women [Reference Pahus, Suehs and Halimi13,Reference Palmer, Pudwell, Smith and Reid14]. A common barrier to clinical trial participation among Blacks, Latinos, Asian Americans, and Pacific Islanders is mistrust, which is rooted in fear of being mistreated, taken advantage of by the researchers, and treated as “lab rats” or “guinea pigs” [Reference George, Duran and Norris15].
Another major finding from our analysis was that participants who had greater trust in the federal, state, or local government about general COVID-19 information showed greater willingness to participate in COVID-19 therapeutics trials. Greater willingness is essential because government resources and investment led to the rapid undertaking of the vaccine development for the novel severe acute respiratory coronavirus virus 2 (SARS-CoV-2). A government initiative to collaborate with pharmaceutical companies and university hospitals could raise awareness of COVID-19 clinical trials in Vietnamese Americans and improve representation.
Interestingly, high trust in clinical trial information that local clinics and hospitals provided was linked to a reluctance to join a COVID-19 clinical trial. This linkage might be because community facilities, which focus on healthcare rather than research, might offer less detailed trial information, creating perceptions of inadequate knowledge. This limited exposure could affect participation decisions due to doubts about the clinics' information depth and relevance to the trials.
Although limited data exist regarding the association between AANHPI, trust in government, and participation in COVID-19 clinical trials, research on the key role the government plays in a pandemic and the influence it has on vaccine adherence and clinical trials does. For example, Riersen et al. found that trust in government was associated with fewer COVID-related deaths in a global analysis of the government’s role and COVID-19 outcomes [Reference Reiersen, Romero-Hernández and Adán-González16]. Furthermore, the government has a critical role to play in establishing trust and confidence in the public about clinical trials through monitoring research guidelines and ethics, establishing policies that protect those participating in clinical trials, and communicating about the safety and importance of clinical research [Reference Bhatt17].
Building trust in government is vital to improving the diversity of clinical trial participants. However, it is understandable that historically marginalized populations are hesitant to volunteer. Black bodies have been exploited for centuries by American researchers, including on behalf of the US government [Reference Wailoo18,Reference Scharff, Mathews, Jackson, Hoffsuemmer, Martin and Edwards19]. Native Hawaiians have reported mistrust related to a researcher’s agenda not serving the community [Reference George, Duran and Norris15]. Future research could examine if a broader population of Vietnamese Americans have high trust levels in university hospitals, pharmaceutical companies, and the government and if that trust is associated with participation in clinical trials for other conditions.
This survey study had challenges and limitations. One challenge was that participants were required to complete a captcha verification before responding to the survey questions to prevent the influence of bots and ensure a representative sample. However, one CBO reported that older adults encountered difficulties with this verification process. Thus, future research should explore alternative verification methods and include paper surveys to promote sample representation and address accessibility concerns in this population.
Regarding limitations, the survey instrument was specific to COVID-19 clinical trials and COVID-19 information and may not be generalizable to other conditions. Also, the results of this cross-sectional study do not demonstrate causality. Moreover, the sample was restricted to self-identified Vietnamese Americans in Texas who could complete an online survey. The online survey format introduces biases favoring technologically adept, younger, and more English-acclimated Vietnamese Americans. These format biases skew participation, potentially excluding older or less tech-savvy individuals. Consequently, data might not represent diverse opinions or those lacking digital access. Furthermore, the overrepresentation of highly educated respondents introduces bias, reflecting specific socioeconomic and cultural subsets, possibly misrepresenting those with lower education or diverse backgrounds among Vietnamese Americans.
Additionally, participants who responded as “prefer not to answer” or “no opinion” were removed from the analysis. Excluding participants who chose not to provide an answer or expressed no opinion can lead to a biased sample that does not accurately reflect the diversity of opinions within the population of interest. Therefore, these findings may not be generalizable to those with neutral, ambivalent, or other more nuanced opinions about clinical trial participation.
Nonetheless, this study provides insights into the beliefs and attitudes of Vietnamese Americans to increase the diversity of clinical trials. Vietnamese Americans’ high trust in pharmaceutical companies and university hospitals to provide correct information about COVID-19 clinical trials and governments to provide correct information about COVID-19 is associated with willingness to participate in SARS-CoV-2 therapeutics trials. By striving to identify these trusted sources of information, we can increase the participation of Vietnamese Americans in clinical trials and help to ensure that these treatments are safe and effective for use in this population.
Acknowledgments
The authors would like to thank medical librarian Stefanie Lapka for assisting with the literature search. We also acknowledge Boat People SOS Houston staff and PIVOT members for the assistance with translations and recruitment.
Author contributions
SV: investigation, data curation, formal analysis, writing (original draft), and visualization. SS: investigation, data curation, formal Analysis, writing (original draft), and visualization. CN: investigation, data curation, formal analysis, writing (original draft), and visualization. JD: conceptualization, investigation, resources, and writing – review and editing. LG: conceptualization, methodology, investigation, and writing – review and editing. BK: data curation, formal analysis, and writing – reviewing and editing. BMN: conceptualization, project administration, writing (review and editing), supervision, and funding acquisition.
Funding statement
This research was, in part, funded by the National Institutes of Health (NIH) Agreement OT2HL158287. Disclaimer: The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the NIH.
Competing interests
When this manuscript was initially submitted, Dr Nguyen owned stock in AbbVie, which was not in conflict with this topic. At the time a revised manuscript was submitted, she no longer owned AbbVie equity. The other authors declare no conflicting interests.





