The balance between cellular oxidising and reducing forces regulates key transcription factors that influence cell signalling pathways involved in proliferation and differentiation(Reference Schafer and Buettner1, Reference Salas-Vidal, Lomeli and Castro-Obregon2). Oxidising and reducing reactions occur constantly in cells as part of normal aerobic life, resulting in the production of oxygen-derived radicals (reactive oxygen species (ROS)). ROS act as primary or secondary messengers to promote cell growth or death but can activate a variety of signalling pathways that inflict damage on cells if produced at high levels. Antioxidants provide important intracellular defence against damage by ROS. A delicate balance between ROS production and antioxidant capability maintains homeostasis; an imbalance between the two results in oxidative stress.
Throughout development, the embryo requires energy from aerobic respiration; however, the resultant fetal and placental ROS and oxidative stress can greatly affect organogenesis and growth. In addition, there are significant correlations between maternal and fetal oxidative stress biomarkers and antioxidants, suggesting that a maternal oxidant/antioxidant imbalance causes oxidative stress in the fetus. A certain amount of oxidative stress is needed to allow for the normal progression of embryonic and fetal growth, but alterations in oxidative stress and a perturbed antioxidant system can cause pregnancy complications including miscarriage and growth restriction(Reference Myatt and Cui3).
The embryo possesses a set of activities of antioxidant enzymes including Cu- and Zn-containing superoxide dismutase (Cu,Zn-SOD), Mn-containing SOD (Mn-SOD), glutathione peroxidase and thioredoxins (Trx). It is likely that changes in the expressions of antioxidants in the fetus are an adaptive response to counter the effects of changing oxidative stress in the mother(Reference Arikan, Konukoglu and Arikan4), and that the failure of this response has adverse consequences for the fetus. The mechanisms involved in the deterioration of the fetus in response to maternal and placental oxidative stress are multifaceted. Previous studies have shown that oxidative damage to the trophoblast and placental tissues are key factors in early fetal loss(Reference Jauniaux, Greenwold and Hempstock5), that ROS generation induced by high nutrient intake in early pregnancy suppresses placental growth, resulting in reduced placental and fetal size(Reference Sibley, Glazier and D'Souza6), and that placental dysfunction during pregnancy is associated with fetal growth retardation and the metabolic syndrome in later life(Reference Barker7–Reference Robinson, Chidzanja and Kind9). Based on these data, we propose that one process contributing to fetal death during oxidative stress is a compromised placenta and the decreased maternal–fetal transfer of nutrients across an impaired placental–decidual interface.
Maternal obesity and overfeeding alter the oxidant status in the fetus. A maternal diet rich in fat shifts the fetal cellular redox environment to a more oxidised state(Reference Anderson, Lustig and Boyle10), retards embryonic progression, causes embryo toxicity and results in miscarriage(Reference Salas-Vidal, Lomeli and Castro-Obregon2, Reference Hempstock, Jauniaux and Greenwold11). In addition, maternal high-fat diets can alter fetal programming and predispose children to CVD and metabolic disease in adult life(Reference Barker7, Reference Taylor and Poston12). These observations indicate that maternal fat and energy intake can influence the metabolic fate of the fetus. However, the mechanisms by which maternal energy intake affects maternal and fetal oxidant/antioxidant status and the availability of appropriate therapies to obviate these changes are largely unknown.
Previous reports have shown that diets rich in fibre increased antioxidant status and had important implications for health(Reference Molinari, Manzi and Ricci13), and that the consumption of dietary fibre was inversely associated with the risk of CHD(Reference Estruch, Martínez-González and Corella14). This suggests that the addition of dietary fibre to a HF, Western-style diet may be beneficial to health. However, studies investigating the effects of a high-fat diet in combination with maternal fibre intake on maternal and fetal antioxidant capacities and fetal development are limited.
N-acetylcysteine (NAC) is a sulphur-based amino acid and a potent antioxidant that can minimise oxidative stress and its associated downstream negative effects. We investigated whether supplementation of a high-fat diet with fibre and NAC was effective in abrogating dietary fat-induced maternal and placental oxidative stress and promoting fetal development. We compared maternal and fetal oxidative responses to dietary fibre and NAC to study the mechanisms involved in dietary fibre-induced changes in antioxidant status. We investigated the effects of the supplements on the nutrient transfer capacity of the placenta to determine whether maternal and fetal antioxidant capacities affected fetal growth and development through processes that altered placental structure and function.
Materials and methods
Animals and diets
A total of eighty virgin female Sprague–Dawley rats were placed on an ad libitum standard laboratory chow diet (20 % protein) and water until pregnancy was confirmed by the observation of vaginal plugs. Pregnant animals were fed ad libitum on one of the four diets: a normal-protein diet (C group); a high-fat diet (HF group); a high-fat and high-fibre diet (HFF group); a high-fat NAC diet (NAC group, 0·1 g/kg), respectively, from the first day of pregnancy. Dietary fat consisted of soyabean oil, rapeseed oil and pig lard because of their wide consumption in the human diet. Fibre was provided by oat and wheat bran with a ratio of 1:1. Diets were prepared on a weekly basis to reduce the risk of oxidative rancidity, and all diets were stored at 4°C. The compositions of the experimental diets are listed in Table 1. Animals were then housed individually in galvanised-steel cages with free access to food and water until sample collection was performed on day 19·5 of gestation. All aspects of animal handling were approved by the Animal Care and Use Committee of the Sichuan Agricultural University and the National Research Council's Guide for the Care and Use of Laboratory Animals.
Table 1 Compositions of the experimental diets based on the American Institute of Nutrition-93 diet
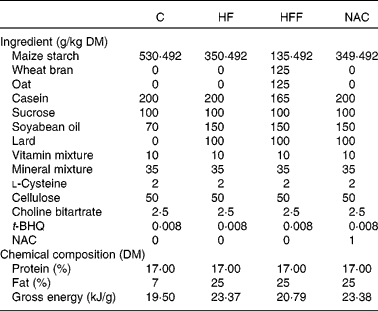
C, control group; HF, high-fat group; HFF, high-fat and high-fibre group; NAC, N-acetylcysteine group; t-BHQ, tert-butyl hydroquinone.
Collection of blood and tissue samples
Rats were anaesthetised in sealed jars containing cotton soaked with 350 μl isoflurane as described previously(Reference Moore and Haig15). Maternal blood was collected and centrifuged at 4000 rpm for 10 min at 4°C; serum was collected and stored at − 20°C until analysis. Immediately after the maternal rats were killed, the fetus and the placenta were removed and weighed. The fetal liver, heart and placenta were frozen in liquid N2, and stored at − 70°C until analysis. Part of the placenta was used for histological examination.
Measurements and analytical methods
Histological examination of placental tissues
Small 0·5 × 0·5 cm segments of placental tissues were cut and rinsed with ice-cold phosphate buffer saline. Samples were fixed in 10 % neutral-buffered formalin, processed routinely for histological examination, cut at 5–6 μm thick, and then stained with haematoxylin and eosin for routine histological examination.
Blood sample biochemical analyses
Maternal serum levels of glucose, TAG, protein and HDL were determined with commercial kits (Leadmanbio, Beijing, China) on an autoanalyser (BMD/Hitachi 705; Hitachi Limited, Tokyo, Japan).
Hydroxyl radical scavenging capacity of maternal serum and the placenta
Hydroxyl radical scavenging capacity was assayed as described previously(Reference Elizabeth and Rao16) with slight modifications. The principle of the assay is the quantification of malondialdehyde (MDA), the degradation product of 2-deoxyribose, by its condensation with tetrabutylammonium. Hydroxyl radical was generated by the Fe3+–ascorbate–EDTA–H2O2 system (Fenton reaction). Absorbance was measured at 532 nm against an appropriate blank solution. All tests were performed five times, and percentage inhibition was evaluated by comparing the test and control groups.
Superoxide anion scavenging capacity of maternal serum and the placenta
Superoxide anion scavenging capacity was measured by a reduction in nitroblue tetrazolium according to a previously reported method(Reference Fontana, Mosca and Rosei17). The xanthine–xanthine oxidase system was used to determine the superoxide anion scavenging activity. All tests were performed five times, and percentage inhibition was evaluated by comparing the test and control groups.
Assay of malondialdehyde and protein carbonyl concentrations in maternal serum and the placenta
MDA concentrations in serum and the placenta were quantified by the tetrabutylammonium method(Reference Buege and Aust18), which is based on the reaction of MDA with thiobarbituric acid to form a pink chromogen. Data were normalised to protein content and are expressed as nmol/mg protein. Protein carbonyl concentrations were determined from reactions with dinitrophenylhydrazine, using a commercially available kit (Oxiselect™ protein carbonyl ELISA; Cell Biolabs, San Diego, CA, USA) according to the manufacturer's instructions. Absorbance was measured at 370 nm, and concentrations were calculated using a molar extinction coefficient of 22 000 m/cm at 370 nm after subtraction of the blank absorbance.
Assay of total superoxide dismutase, copper, zinc-superoxide dismutase and manganese-superoxide dismutase activities in fetal liver
Total SOD (T-SOD) activity was measured using an indirect competition assay between SOD and the indicator compound, nitroblue tetrazolium, for superoxide produced by xanthine–xanthine oxidase according to a previously described method(Reference Spitz and Oberley19). Activity units were determined by defining 1 unit of T-SOD activity as the amount of sample protein capable of inhibiting the reduction in nitroblue tetrazolium by 50 % of maximum inhibition. Data were normalised to protein content and are expressed as U/mg protein. Activities of Cu,Zn-SOD and Mn-SOD were determined by the xanthine–xanthine oxidase method according to the procedures described by Sun et al. (Reference Sun, Oberley and Li20) and Nagai et al. (Reference Nagai, Inoue and Inoue21). Data were normalised to protein content and are expressed as U/mg protein.
RNA preparation and quantitative real-time PCR of the placenta, and fetal liver and heart
Total RNA was isolated from the placenta and fetal liver and heart using a guanidine isothiocyanate-based reagent (TRIzol reagent; TaKaRa, Shiga, Japan) according to the manufacturer's instructions. Quantitative and qualitative analyses of isolated RNA were assessed from the ratio of absorbance at 260 and 280 nm and agarose gel electrophoresis. Total RNA (5 μg) was used as a template to synthesise complementary DNA for quantitative real-time PCR. The genes listed in Table 2 were amplified by DNA-specific primers (Invitrogen, Carlsbad, CA, USA) on a Thermal Cycler (CHRMO4-TM Thermal Cycler; Bio-Rad, Hercules, CA, USA) according to the manufacturer's instructions and the following protocol: 95°C for 10 s, forty cycles at 95°C for 5 s and 60°C for 25 s. Melting curve conditions were from 65 to 95°C, reading every 0·5°C and holding for 5 s every 0·5°C (temperature change velocity 0·5°C). The relative gene expression levels were normalised to those of the eukaryotic house-keeping gene, β-actin.
Table 2 Sequences of primers for PCR amplifications

GPx1, glutathione peroxidase 1; F, forward; R, reverse; Cu,Zn-SOD, Cu- and Zn-containing superoxide dismutase; Mn-SOD, Mn-containing superoxide dismutase; Trx1, thioredoxin-1; Trx2, thioredoxin-2; HIF-1α, hypoxia-inducible factor 1-α; Slc2a1, GLUT1; Slc2a3, GLUT3; Slc7a1, cationic amino acid transporter 1; Slc38a2, system A amino acid transporter gene Slc38a2; Slc38a4, system A amino acid transporter gene Slc38a4.
Statistical analyses
Data are presented as means and standard deviations. All data were analysed using one-way ANOVA. P < 0·05 was considered statistically significant.
Results
Reproductive performance
As shown in Table 3, there were no significant differences in maternal body weight and daily food intake during the 19·5 d experimental period among the four diets. In HFF rats, fetal weight tended to increase (P = 0·052) and placental weight and fetal number were significantly higher (P < 0·05) than in the other groups.
Table 3 Effects of the experimental diets on fetal and placental development and the concentrations of maternal-circulating metabolites
(Mean values and standard deviations)

C, control group; HF, high-fat group; HFF, high-fat and high-fibre group; NAC, N-acetylcysteine group.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Statistical analyses of fetal and placental weights and fetal numbers were based on the average value in each litter.
Maternal blood characteristics
The concentrations of circulating metabolites in maternal serum are shown in Table 3. In HF rats, the maternal serum glucose level was below that in the NAC group (P>0·05) and was significantly lower than that in the C and HFF rats (P < 0·05). In addition, TAG concentration was significantly higher than that in the C and NAC groups (P < 0·05). HDL was lowest in NAC rats (P < 0·05).
Placental histology
Feeding the HF diet had an adverse effect on placental tissue integrity (Fig. 1). In the HF group, placental trophoblasts exhibited evident malformation and were partially necrotic, indicating that consumption of a HF diet had a deleterious effect on placental tissue integrity; however, dietary fibre and NAC supplementation obviously alleviated the phenomenon. In particular, the cellular structure and morphology in the HFF group were better than those in the NAC group.

Fig. 1 Effects of the experimental diets on placental structure. (A) Control group (C), the structure was typical of a normal placenta; (B) high-fat group, the structure showed pronounced placental pathology; (C) high-fat and high-fibre group (HFF), the structure showed some morphological changes compared with the C group, but the pathology was not as pronounced as in the HF group. Placental morphological changes are identified by black arrows (magnification 200 × ). (D) NAC, N-acetylcysteine group.
Superoxide anion and hydroxyl radical scavenging capacities in maternal serum and the placenta
Figs. 2 and 3 show the superoxide anion and hydroxyl radical scavenging capacities in maternal serum and the placenta. Maternal consumption of the HF diet significantly decreased the maternal serum and placental superoxide anion and hydroxyl radical scavenging capacities (P < 0·05). This decrease was not evident in the HFF group (P < 0·05), and the serum and placental scavenging capacities in these rats remained similar to those of the C group. In the NAC group, the maternal serum and placental superoxide anion and hydroxyl radical scavenging capacities were significantly higher than those in the HF group (P < 0·05), and were not significantly different from those in the HFF and C groups (P>0·05).

Fig. 2 Effects of the experimental diets on superoxide anion scavenging capacity in maternal serum and the placenta. The data represent the percentage superoxide anion capacity remaining compared with the control group (□). Values are means, with standard deviations represented by vertical bars (n 6). a,b Mean values with unlike letters were significantly different (P < 0·05). ![]() , High-fat group;
, High-fat group; ![]() , high-fat and high-fibre group; ■, N-acetylcysteine group.
, high-fat and high-fibre group; ■, N-acetylcysteine group.

Fig. 3 Effects of the experimental diets on the hydroxyl radical scavenging capacity in maternal serum and the placenta. The data represent the percentage hydroxyl radical scavenging capacity remaining compared with the control group (□). Values are means, with standard deviations represented by vertical bars (n 6). a,b,c Mean values with unlike letters were significantly different (P < 0·05). ![]() , High-fat group;
, High-fat group; ![]() , high-fat and high-fibre group; ■, N-acetylcysteine group.
, high-fat and high-fibre group; ■, N-acetylcysteine group.
Malondialdehyde and protein carbonyl content concentrations in maternal serum and the placenta
MDA and protein carbonyl concentrations in maternal serum and the placenta were significantly increased in the HF group than in the C group (P < 0·05, Table 4). Placental MDA and protein carbonyl concentrations in serum were similar between the HFF and C treatments, although MDA in serum and protein carbonyl concentrations in the placenta were still higher in the HFF treatment than in the C treatment (P < 0·05). However, all four variables were significantly higher in the NAC treatment than in the C treatment (P < 0·05).
Table 4 Effects of the experimental diets on the content of malondialdehyde and protein carbonyl (nmol/mg protein) in maternal serum and the placenta
(Mean values and standard deviations, n 6)
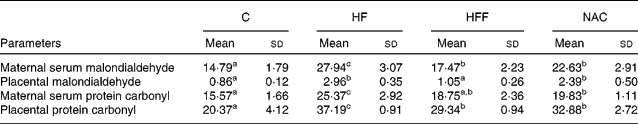
C, control group; HF, high-fat group; HFF, high-fat and high-fibre group; NAC, N-acetylcysteine group.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Antioxidant enzymatic activities in fetal liver
The activities of antioxidant enzymes in fetal liver are shown in Fig. 4. Maternal consumption of the HF diet significantly decreased T-SOD and Cu,Zn-SOD activities in fetal liver compared with the C group (P < 0·05). In the HFF group, T-SOD, Cu,Zn-SOD and Mn-SOD activities were significantly higher than those in the HF group (P < 0·05) but were not different from those in the C group (P>0·05). There were no significant differences between the NAC and HF groups (P>0·05).

Fig. 4 Effects of the experimental diets on enzyme activities in fetal liver. Values are means, with standard deviations represented by vertical bars (n 6). a,b Mean values with unlike letters were significantly different (P < 0·05). T-SOD, total superoxide dismutase; Cu,Zn-SOD, copper- and zinc-containing superoxide dismutase; Mn-SOD, manganese-containing superoxide dismutase. □, Control group; ![]() , high-fat group;
, high-fat group; ![]() , high-fat and high-fibre group; ■, N-acetylcysteine group.
, high-fat and high-fibre group; ■, N-acetylcysteine group.
mRNA expression levels of fetal antioxidant-related genes
The mRNA expression levels of antioxidant genes and hypoxia-inducible factor 1-α (HIF-1α) in fetal liver are shown in Fig. 5. In HF rats, there was a significant suppression of the mRNA expression levels of Trx1, Cu,Zn-SOD and glutathione peroxidase 1 in fetal liver relative to the C group (P < 0·05). In the HFF group, there was a significant up-regulation in the mRNA expression levels of HIF-1α, Trx2 and Mn-SOD in fetal liver relative to the C group (P < 0·05); and the mRNA expression levels of Trx1 and glutathione peroxidase 1 were higher than those in the HF group (P < 0·05).

Fig. 5 Effects of the experimental diets on the mRNA levels of antioxidant-related genes in fetal liver. Values are means, with standard deviations represented by vertical bars (n 6). a,b,c,d Mean values with unlike letters were significantly different (P < 0·05). HIF-1α, hypoxia-inducible factor 1-α; Trx1, thioredoxin-1; Trx2, thioredoxin-2; Cu,Zn-SOD, copper- and zinc-containing superoxide dismutase; Mn-SOD, manganese-containing superoxide dismutase; GPx1, glutathione peroxidase 1. □, Control group; ![]() , high-fat group;
, high-fat group; ![]() , high-fat and high-fibre group; ■, N-acetylcysteine group.
, high-fat and high-fibre group; ■, N-acetylcysteine group.
In maternal rats fed the HF diet, the mRNA expression levels of Trx1, Mn-SOD and HIF-1α in fetal heart were significantly lower than those in the other groups (P < 0·05; Fig. 6). In HFF rats, the mRNA expression level of Mn-SOD in fetal heart was significantly higher than that in the other groups (P < 0·05), but the mRNA expression levels of HIF-1α, Trx1 and Cu,Zn-SOD were not significantly different from those in the C group. In the NAC group, there was no change in the expression levels of antioxidant-related genes in fetal liver relative to the HF group, but increases in the mRNA expression levels of Trx1, Mn-SOD and HIF-1α in fetal heart were observed (P < 0·05).

Fig. 6 Effects of the experimental diets on the mRNA levels of antioxidant-related genes in fetal heart. Values are means, with standard deviations represented by vertical bars (n 6). a,b,c Mean values with unlike letters were significantly different (P < 0·05). HIF-1α, hypoxia-inducible factor 1-α; Trx1, thioredoxin-1; Trx2, thioredoxin-2; Cu,Zn-SOD, copper- and zinc-containing superoxide dismutase; Mn-SOD, manganese-containing superoxide dismutase; GPx1, glutathione peroxidase 1. □, Control group; ![]() , high-fat group;
, high-fat group; ![]() , high-fat and high-fibre group; ■, N-acetylcysteine group.
, high-fat and high-fibre group; ■, N-acetylcysteine group.
mRNA expression of placental nutrient transporter genes
The mRNA expression levels of placental nutrient transporter-related genes are shown in Fig. 7. In maternal rats fed the HF diet, there was a significant up-regulation in the mRNA expression level of the cationic amino acid transporter Slc7a1 (P < 0·05), but there were no significant differences in the mRNA expression levels of the system A amino acid transporter genes Slc38a2 and Slc38a4 (encoding SNAT2 and SNAT4), and the GLUT genes Slc2a1 and Slc2a3 (encoding the GLUT1 and GLUT3), compared with the C group (P>0·05). The HFF group showed a significant up-regulation in the mRNA expression levels of Slc2a1, Slc2a3 and HIF-1α compared with the HF and C groups (P < 0·05). However, no significant differences in the mRNA levels of Slc38a2, Slc38a4 and Slc2a3 were observed between the NAC and C groups. There were no changes in the mRNA expression level of Slc38a4 among all the experimental groups.

Fig. 7 Effects of the experimental diets on the mRNA levels of nutrient transporters and hypoxia-inducible factor 1-α (HIF-1α) in the placenta. Values are means, with standard deviations represented by vertical bars (n 6). a,b,c Mean values with unlike letters were significantly different (P < 0·05). Slc38a2, system A amino acid transporter gene Slc38a2; Slc38a4, system A amino acid transporter gene Slc38a4; Slc7a1, cationic amino acid transporter 1; Slc2a1, GLUT1; Slc2a3, GLUT3. □, Control group; ![]() , high-fat group;
, high-fat group; ![]() , high-fat and high-fibre group; ■, N-acetylcysteine group.
, high-fat and high-fibre group; ■, N-acetylcysteine group.
Discussion
ROS, including the superoxide anion radical and the hydroxyl radical, are formed as a result of the successive one-electron reduction of molecular oxygen. The generation of ROS is an intrinsic characteristic of any living cell and is therefore a consequence of embryo development and metabolism. ROS are beneficial as they serve as key signalling molecules in essential physiological pathways, but they also have a role in pathological processes, including those involving the female reproductive tract(Reference Agarwal, Prabakaran and Said22). The present study showed that maternal consumption of a HF diet during pregnancy decreased fetus number and the scavenging capacities of maternal and placental radicals, and increased the end products of lipid and protein peroxidation. The present results are in accordance with those of other studies, which have shown that failure of implantation and fetal resorption during gestation were increased in female rats fed a HF diet(Reference Niculescu and Lupu23), suggesting that a HF diet is detrimental to embryo development due to high oxidative stress. Taken together, these data indicate that HF diets are associated with maternal and fetal oxidative stress and are detrimental to fetal development. Recent reports have shown that the placenta is both the target of maternal oxidative stress and a modulator of fetal oxidative stress(Reference Liguori, D'Armiento and Palagiano24), and placental oxidative stress contributes to early fetal loss(Reference Jauniaux, Greenwold and Hempstock5, Reference Hempstock, Jauniaux and Greenwold11). Based on these observations, the present results suggest that the maternal redox state transfers to the placenta and the fetus, and affects fetal development and survival.
We found that maternal fibre consumption significantly increased fetal number and placental weight, and decreased placental MDA and protein carbonyl levels. These results are consistent with those of previous studies(Reference Milagro, Campión and Martínez25, Reference Souza, Moreira and Siqueira26), and suggest that supplementation of a HF diet with fibre abrogates high-fat-induced oxidative stress. Fibre interferes with fat digestion and absorption in the intestinal tract, and facilitates the excretion of dietary fat into the faeces(Reference Dégen, Halas and Tossenberger27, Reference Deuchi, Kanauchi and Imasato28). In addition, the fermentation of dietary fibre in the gut produces SCFA, including butyrate and acetate, which prevent H2O2-induced DNA and cell damage(Reference Abrahamse, Pool-Zobel and Rechkemmer29). Butyrate also increases the activity of glutathione S-transferase(Reference Pool-Zobel, Selvaraju and Sauer30), a family of enzymes that utilise glutathione in reactions contributing to the transformation and detoxification of the products of oxidative stress. Based on these data, and the knowledge that NAC elevates intracellular glutathione, we investigated the role of NAC during the dietary fibre-induced abrogation of the effects of a HF diet on maternal and fetal redox status. The present results indicated that NAC increased fetal number and placental weight, and decreased the levels of placental biomarkers of oxidative stress, but that these beneficial effects were significantly lower than those of fibre. Furthermore, as there is no scientific evidence showing that changes in NAC activity are associated with the metabolism of dietary fat, we propose that dietary fibre does not abrogate HF diet-induced oxidative stress by a pathway that directly involves NAC. We suggest that the increases in faecal lipid excretion and SCFA syntheses are of primary importance during the dietary fibre-induced reduction in maternal and fetal oxidative stress and the subsequent increase in the rate of fetal survival.
The oxidative stress programming hypothesis(Reference Luo, Fraser and Julien31) states that oxidative stress is a key link in the fetal or developmental programming of disease. In accordance with this, previous studies have shown that maternal and fetal oxidative status was related(Reference Estruch, Martínez-González and Corella14, Reference Bruin, Petre and Lehman32); that maternal redox status affected transcription factors and altered gene expression in the fetus(Reference Dennery33); that eighteen gene transcripts including Cu,Zn-SOD, Mn-SOD and glutathione peroxidase 1 were down-regulated at least twofold in rats fed a HF diet; and that antioxidant supplementation completely and partially normalised four and twelve of these gene transcripts, respectively(Reference Sreekumar, Unnikrishnan and Fu34). Other studies have suggested that a HF diet could cause oxidative damage to DNA molecules(Reference Slupphaug, Kavli and Krokan35) and lead to lower mRNA expression levels of antioxidant defence genes; and that dietary fibre effectively reduced DNA oxidative damage induced by ROS(Reference Wang, Sun and Cao36) by the up-regulation of antioxidant defence genes. In accordance with these data, we found that a high-fat maternal diet down-regulated the mRNA expression levels of several antioxidant-related genes in the fetal heart and liver, and that these transcripts were up-regulated by maternal supplementation with fibre, and to a lesser extent with NAC. In addition, we observed a marked increase in the activities of SOD in fetal liver with the inclusion of dietary fibre in a high-fat maternal diet. These results suggest that maternal dietary fibre intake during pregnancy reduces oxidative stress in the mother, improves the fetal redox state and, according to the oxidative stress programming hypothesis, may program the susceptibility of the fetus to oxidative stress after birth.
All the metabolic demands of fetal growth are provided by transplacental exchange. The placenta can respond to fetal demand signals through regulation in the expression of specific placental transport systems(Reference Constância, Angiolini and Sandovici37). Slc2a1 and Slc2a3 represent the major GLUT in the human placenta(Reference Shin, Fujikura and Suzuki38); the system A amino acid transporter, Slc38a2 and Slc384, mediates the transport of neutral amino acids; and cationic amino acid transporter 1/Slc7a1 controls the transport of cationic amino acid(Reference Ayuk, Sibley and Donnai39). The present results showed that dietary fibre supplementation of a maternal HF diet significantly up-regulated the mRNA expressions of placental Slc2a1, Slc2a3 and Slc38a2, but the mRNA expression of Slc7a1 was lower than that in the HF group. We propose that the higher fetal number in the HFF group was attributable to up-regulation in the nutrient transfer capacity of the placenta. Marked up-regulation in placental nutrient transport was observed in C57/BL6 mice after maternal exposure to a HF diet before and during pregnancy(Reference Jones, Woollett and Barbour40); but previous reports describing the effects of maternal dietary fibre on the expression of nutrient transfer genes in the placenta during pregnancy are limited. Evidence suggests that maternal low protein intakes down-regulated the activity of transporters for system A and cationic amino acids(Reference Malandro, Beveridge and Kilberg41), and that decreased expressions of Slc2a1 and Slc2a3 in the fetal placenta were related to fetal intra-uterine growth restriction(Reference Hahn, Barth and Graf42). Prolonged maternal malnutrition during late gestation decreased placental GLUT3 expression and maternal plasma glucose content(Reference Lesage, Hahn and Léonhardt43), and intra-uterine growth restriction in human pregnancies was associated with a down-regulation of several important placental amino acid transporters(Reference Dicke and Henderson44). Taken together, these data and the present results suggest that increasing maternal fibre intake during pregnancy improves fetal development by the up-regulation of specific nutrient transporters.
Recently, it has been reported that the placental mRNA expression level of HIF-1α was significantly up-regulated after maternal supplementation with dietary fibre. HIF-1α is a transcription factor directly or indirectly regulated by the cell redox state(Reference Taylor45, Reference Chandel and Schumacker46) and is essential for fetal development(Reference Maltepe, Schmidt and Baunoch47). We found that the expression of HIF-1α in the fetal placenta was associated with the up-regulated expressions of Slc2a1 and Slc2a3. This is in accordance with previous studies that have shown HIF-1α to be involved in the regulation of glycolysis(Reference Lum, Bui and Gruber48) and the expression of Slc2a1(Reference Wenger49). We propose that HIF-1α activates downstream genes, including placental glucose transport 1 and vascular endothelial growth factor, which are advantageous for fetal growth. Interestingly, previous research revealed that a close relationship was observed between ROS and HIF-1α transcription(Reference Pialoux, Hanly and Foster50); that HIF-1 overexpression conferred increased resistance to heat and oxidative stress(Reference Zhang, Shao and Zhai51); and that HIF-mediated hypoxia responses reduced the expressions and functions of system A amino acid transporters in cultured term human trophoblasts(Reference Nelson, Smith and Furesz52). These data indicate that ROS is involved in HIF-1α transcription and support the concept that the redox state in the placenta is closely related to its nutrient transfer capacity, thus affecting fetal development. Future studies are required to validate the concept and explore the mechanisms.
In summary, the present results indicate that maternal dietary fibre consumption during pregnancy has beneficial effects on fetal intrauterine development by alleviating maternal, placental and fetal oxidative stress and by enhancing placental nutrient transfer capacity.
Acknowledgements
The present study was supported by the Educational Commission of Sichuan Province of China (09ZB056) and the National Natural Science Foundation of China (31072044). Y. L. and D. W. designed the study; Y. L., X.-f. H. and Z.-f. F. performed the study; Y. L. and L.-q. C. analysed the data and wrote the manuscript; J. N. and T.-h. Y. assisted in writing the manuscript. The authors declare no conflict of interest.













