During immune system activation in animals, nutrients are redistributed from anabolic and maintenance processes towards processes involved in immunity and disease resistance( Reference Klasing and Johnstone 1 , Reference Spurlock 2 ). A cascade of cytokine-induced metabolic alterations occur, including anorexia, increased breakdown and decreased synthesis of skeletal muscle protein, increased hepatic acute-phase protein (APP) synthesis, and increased deamination of gluconeogenic amino acids (AA)( Reference Klasing and Johnstone 1 , Reference Lochmiller and Deerenberg 3 , Reference Le Floc'h, Melchior and Obled 4 ). The acute-phase response is the early innate immune response to injury, trauma or infection, and increases serum concentrations of positive APP while decreasing concentrations of negative APP( Reference Baumann and Gauldie 5 ). Synthesis of positive APP during an acute-phase response is considered to be nutritionally more costly than the adaptive response to inflammation, i.e. leucocyte proliferation and antibody production( Reference Iseri and Klasing 6 ). Reeds et al. ( Reference Reeds, Fjeld and Jahoor 7 ) calculated that an APP response increases the demand for aromatic AA in particular. For the synthesis of APP, AA are provided either from dietary protein or from the breakdown of skeletal muscle protein. The AA composition of APP differs, however, largely from that of muscle protein( Reference Reeds, Fjeld and Jahoor 7 ), and from that of commercial diets, which are formulated mainly to enhance muscle protein deposition. It is hypothesised that, as a consequence, there can be an imbalance in available AA for body protein deposition, leading to increased oxidation of AA and N loss, which is close to the quantitative N loss observed in uncomplicated trauma( Reference Reeds, Fjeld and Jahoor 7 ). Moreover, the cytokine-induced metabolic change after immune system activation generally results in increased breakdown and decreased synthesis of skeletal muscle protein( Reference Breuille, Voisin and Contrepois 8 , Reference Zamir, Hasselgren and Higashiguchi 9 ).
In pigs, immune system activation by continuous exposure to major vectors of antigen transmission( Reference Williams, Stahly and Zimmerman 10 ), or by intramuscular (i.m.) lipopolysaccharide (LPS) injection( Reference Daiwen, Keying and Chunyan 11 ) reduces feed intake, body-weight (BW) gain and N retention. Recent studies in pigs have revealed that immune system activation by i.m. LPS administration increases the optimal dietary Met:Met+cysteine (Cys) ratio( Reference Litvak, Rakhshandeh and Htoo 12 ), and reduces the efficiency of Trp utilisation for body protein deposition( Reference De Ridder, Levesque and Htoo 13 ). These findings indicate that the utilisation for AA in growing pigs may change due to variation in health status. However, quantitative information about the effect of immune system activation on the utilisation for AA is lacking, and measurements on changes in responses of multiple AA simultaneously to immune system activation are largely absent. Alterations in AA metabolism, e.g. an increased protein synthesis rate, can occur without concomitant changes in plasma AA concentrations or pool size, as plasma AA concentrations can be maintained when AA fluxes alter by changes in dietary protein intake, breakdown and synthesis of body protein, and oxidation of AA( Reference Waterlow 14 ). The irreversible loss rate (ILR) of AA, reflects the amount of free AA that disappears per unit of time from the plasma pool for protein synthesis and oxidation. The combination of ILR measurements with N balance and pool size measurements can provide insight into the metabolic changes in multiple AA simultaneously. It is hypothesised that an increase in blood APP concentrations during immune system activation affects the utilisation of AA, associated with an increased incorporation of, in particular aromatic, AA into APP.
In the present study, intravenous (i.v.) administration of complete Freund's adjuvant (CFA), which has been previously shown to induce chronic lung inflammation in pigs( Reference Melchior, Sève and Le Floc'h 15 , Reference Le Floc'h, Melchior and Sève 16 ), was used to activate the immune system. It is hypothesised that the effect of a CFA challenge on protein metabolism is more pronounced under conditions of a marginal dietary protein supply, which would increase the competition for indispensable AA used for immune system functioning and for protein deposition in muscle as a main determinant of the animal's growth. In addition, there is increasing evidence that the dietary protein or AA supply can affect the inflammatory response during immune system activation( Reference Le Floc'h, Melchior and Sève 16 – Reference Li, Changting and Shiyan 23 ). A Trp-deficient diet, for instance, has been suggested to deteriorate the immune response to a CFA challenge, as indicated by increased indoleamine 2,3 dioxygenase (IDO) activity in the lungs and heart, and increased lung weight( Reference Le Floc'h, Melchior and Sève 16 ) in contrast to a Trp-supplemented diet. As IDO is induced by cytokines( Reference Moffett and Namboodiri 24 ), its activity is associated with the degree of immune system activation. In another study, the addition of Cys to a protein-deficient diet increased liver weight and hepatic glutathione concentrations, following an intraperitoneal injection of TNF-α in rats( Reference Grimble, Persaud and Wride 17 ). The authors of the latter study have suggested that Cys supplementation improved the immune response following TNF-α administration, enabling a full metabolic response to cytokines that improves the ability to maintain antioxidant defences. Although it is debatable whether a change in immunological response is beneficial or not, these findings show that dietary protein or AA supply can affect the inflammatory response during immune system activation. The aim of the present study was to quantify the effect of immune system activation on N retention and AA metabolism in growing pigs, depending on dietary protein supply.
Materials and methods
Animals and treatments
The experiment was approved by the Animal Experimental Committee of Wageningen UR Livestock Research. A total of sixteen barrows (Dutch Landrace × York) with an initial BW of 28·5 (sem 0·5) kg were individually housed in metabolism cages (1·3 × 1·3 m) at a room temperature ranging between 18 and 22°C. Based on litter weight and BW, pigs were allocated to one of two treatment groups receiving either an adequate (Ad) or restricted (Res, 70 % of Ad) dietary protein supply at a similar daily supply of other nutrients. To this end, a basal mixture was designed without protein sources. The Ad diet included the basal mixture with the additional protein sources casein, wheat gluten meal, soya protein isolate and potato protein, and met the requirements for essential AA for growing pigs in the range of 35–45 kg BW( 25 ) (Table 1). The Res diet included the basal mixture to which 70 % of the quantities of additional protein sources (casein, wheat gluten meal, soya protein isolate and potato protein) were added compared with the quantities included in the Ad diet. In order to supply all pigs with the same amount of the basal mixture, relative to their metabolic BW, the feed allowance of pigs assigned to the Res diet was 94·3 % of that of pigs receiving the Ad diet. The experimental diets were provided in mash form and mixed with water using a feed:water ratio of 1:3. The pigs were fed at 07.00 and 15.30 hours in equal amounts at 2·7 times the energy requirements for maintenance (458 kJ metabolisable energy/kg BW0·75 per d( 26 )). Feed refusals were collected 30 min after feeding.
Table 1 Composition of the experimental diets (as-fed basis)

NE, net energy; AID, apparent ileal digestible.
* Two levels of dietary protein supply (adequate or restricted (70 % of adequate)) were used in the study, at a similar daily supply of other nutrients. In the restricted dietary protein supply, the proportion of protein-containing ingredients in the diet was reduced by 30 % relative to the proportion in the adequate-protein diet. In order to supply all pigs with the same amount of the basal mixture, relative to their metabolic body weight, the feed allowance of pigs fed the restricted-protein diet was 94·3 % of those fed the adequate-protein diet.
† Vitamin and mineral premix provided per kg of adequate or restricted diet, respectively: 2·4 or 2·5 mg vitamin A; 50 or 52·5 μg cholecalciferol; 14·7 or 15·7 mg vitamin E; 1·5 or 1·6 mg vitamin K; 1·0 or 1·1 mg thiamin; 4·0 or 4·2 mg riboflavin; 12·0 or 12·6 mg pantothenic acid; 20·0 or 21·0 mg niacin; 20·0 or 21·0 μg vitamin B12; 0·20 or 0·21 mg folate; 1·0 or 1·1 mg vitamin B6; 100 or 105 mg choline chloride; 100 or 105 mg Fe as FeSO4; 10·0 or 10·5 mg Cu as CuSO4.5H2O; 65·0 or 68·3 mg Zn as ZnO; 30·0 or 31·5 mg Mn as MnO; 0·15 or 0·16 mg Co as CoSO4; 0·75 or 0·79 mg K as KI; 0·30 or 0·31 mg Se as sodium selenite.
‡ Protastar®, AVEBE Feed.
§ Unless indicated otherwise.
∥ NE was calculated based on Centraal Veevoederbureau( 59 ).
At day − 16 or − 14 before the start of immune system activation, the pigs were surgically fitted with a jugular vein and a carotid artery catheter for blood collection and injection of a mixture of U-13C-labelled AA, respectively. Neopen (Neomycin 5 mg/kg BW and Procaïne benzylpenicillin 10 000 IU/kg BW; Intervet) was given intramuscularly 1 d before surgery, at surgery and 1 d after surgery. Flunixin (Finadyne 2·2 mg/kg BW; Schering-Plough) was given intramuscularly at surgery and for 2 d after surgery. A timeline of the experiment is shown in Fig. 1, with day 0 being the start of immune system activation by the i.v. administration of CFA (F5881; Sigma-Aldrich). CFA is a mineral oil containing 1 mg of dead Mycobacterium tuberculosis cells per ml. The dose of CFA administered per pig was 0·2 ml/kg BW, diluted with saline in a ratio of 1:2. The dose was spread over four equal sub-doses, of which two were infused at day 0 and two at day 1, in the morning between 09.15 and 10.30 hours, and in the afternoon between 15.15 and 16.30 hours. Of the pigs, eight did not receive the fourth sub-dose of CFA, as clinical observations after the first infusions on eight pigs showed a more severe response, i.e. greater and persistent feed refusals, and greater increase in respiratory rhythm, than expected based on a preliminary study (E Kampman-van de Hoek, unpublished results).
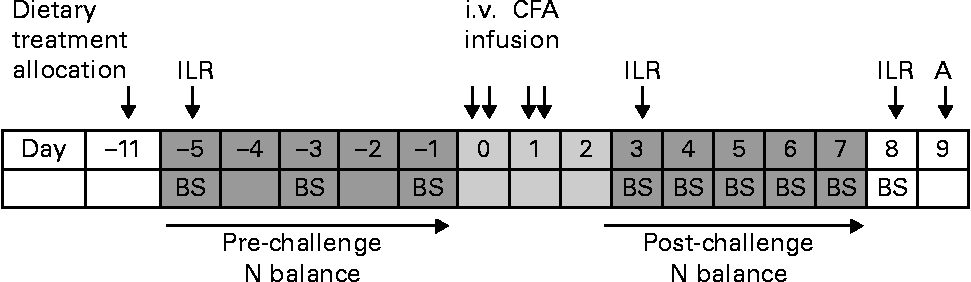
Fig. 1 Timeline of the experimental period. A, autopsy; BS, blood sampling; ILR, irreversible loss rate measurement by the injection of the U-13C-labelled amino acid mixture; i.v., intravenous; CFA, complete Freund's adjuvant.
Immunological response parameters
At days − 5, − 3, − 1, 3, 4, 5, 6, 7 and 8, blood samples were collected into serum tubes (Vacuette; Greiner Bio-One) and allowed to clot for 1 h at room temperature. Serum was collected after centrifugation for 10 min at 1800 g and was stored at − 20°C until analyses of albumin (Randox Bromocresol Green assay, catalogue no. AB 362), C-reactive protein (CRP, ELISA; Reactivlab Limited), haptoglobin (Tridelta Phase Haptoglobin Assay, catalogue no. TP-801), pig major APP (pigMAP, ELISA; Reactivlab Limited), and total protein (biuret reaction( Reference Doumas, Bayse and Carter 27 )). At days − 5, 0, 1, 3, 5 and 8, blood samples were collected into EDTA tubes (Vacuette; Greiner Bio-One) and analysed for the number of total leucocytes. At day 9, all pigs were euthanised and autopsy was performed by an experienced pathologist. Autopsy observations included assessment of body condition, visual inspection for abnormalities of the lungs, spleen, stomach, small intestine, large intestine, kidneys, liver, heart, and mesenteric lymph nodes, determination of lung weight, and histological evaluation of the lungs, liver, tracheobronchial lymph nodes and kidneys.
Nitrogen balance
Pigs were equipped with a Velcro support system to allow separate collection of the faeces( Reference Van Kleef, Deuring and van Leeuwen 28 ) and urine. Faeces and urine were collected quantitatively from each pig during two periods of five subsequent days each, i.e. in the pre- and post-challenge periods (Fig. 1). Faeces were stored at − 20°C until analysis. Urine was collected via funnels, which were sprayed with an acetic acid buffer (sodium acetate 0·08 m, formic acid 0·025 m and acetic acid 0·013 m) to prevent evaporation of NH3, into buckets containing sulphuric acid (4·5 m), to maintain a pH < 3 for conservation. Urine was collected daily from the buckets, weighed, sampled and stored at − 20°C until analysis. N content in the urine and fresh faeces was analysed using the Kjeldahl method( 29 ). DM content of the faeces was determined by drying at 103°C( 30 ).
Amino acid metabolism
At pre-challenge (day − 5), early post-challenge (day 3) and late post-challenge (day 8), the fluxes of plasma lysine, tryptophan, methionine, isoleucine, leucine, valine, phenylalanine and tyrosine (Lys, Trp, Met, Ile, Leu, Val, Phe and Tyr, respectively) were studied by measuring the change in plasma isotopic enrichment of individual AA in time after an intravenously administered bolus of these U-13C-labelled AA. To create a steady state in dietary AA supply at the day of injection of the U-13C-labelled AA mixture and frequent blood sampling, daily feed allowance was spread over ten equal meals. Of these, two meals were fed at 07.30 hours, followed by hourly meals from 09.10 hours until 16.10 hours. At 12.30 hours, a mixture of eleven U-13C-labelled AA (97–99 atom percent 13C; Cambridge Isotope Laboratories) was injected. The composition of the mixture (mg/g saline) was as follows: l-Lys, 0·17; l-Thr, 0·18; l-Trp, 0·07; l-Met, 0·06; l-Cys, 0·04; l-Ile, 0·16; l-Leu, 0·16; l-Val, 0·19; l-Phe, 0·13; l-Tyr, 0·16; l-histidine, 0·08. The mixture was injected as a bolus (0·50 g/kg BW; 0·25 ml/s) in the carotid artery. If the carotid artery catheter was blocked, the mixture was injected in the jugular vein. Blood samples (4 ml each) were collected from the jugular vein and transferred into tubes containing lithium heparin (Vacuette; Greiner Bio-One) at 10 min before the injection of the U-13C-labelled AA mixture and at 3, 5, 7, 9, 11, 15, 25, 45, 80 and 120 min after injection. The tubes were immediately placed on ice and centrifuged for 10 min at 2000 g at 4°C, after which plasma was collected and stored at − 20°C until analysis. In each blood sample, 13C enrichment was measured in plasma Lys, Trp, Met, Ile, Leu, Val, Phe and Tyr as ethyl chloroformate ester (ECF; Merck Schuchardt OHG) derivatives by GC–combustion–isotope ratio MS (Isotope Ratio MS, Delta V Advantage, Thermo Scientific; GC Trace Ultra, Thermo Scientific (column no. CP8982, VF-17ms 30 m × 0·25 mm, film 0·25 μm; Agilent Technologies); and combustion, Combustion III, Thermo Scientific), as adapted from Huang et al. ( Reference Huang, Hogewind-Schoonenboom and de Groof 31 ). Briefly, 20 μl hydrogen chloride (1 m) and 200 μl Dowex ion exchange resin (AG 50W-X8 H+ form, 200–400 mesh; Dow Chemical Company) were added to 180 μl plasma and eluted with 0·7 ml ammonium hydroxide (6 m) to isolate free plasma AA. The supernatant was evaporated with a centrifugal concentrator (Jouan RC 1022; Thermo Scientific) under vacuum at room temperature. Derivatisation was performed by adding 140 μl ethanol–pyridine (4:1, v/v) and 20 μl ECF to the dry supernatant. Derivates were extracted by adding 4 × 200 μl hexane–dichloromethane–ECF (50:50:1, by vol.), and the supernatant was dried in a vial under N2 gas at room temperature. After dissolving in 50 μl ethyl acetate, the sample was injected in triplicate into the GC. The 13C enrichment of histidine could not be successfully analysed due to high losses of histidine during the derivatisation step. The 13C enrichment of Thr and Cys could not be determined with the current procedure, and additional derivatisation steps would be required for their measurement.
Calculations
Dietary N intake, faecal and urinary N excretion and whole-body N retention were expressed relative to metabolic BW (kg BW0·75). Relative lung weight was calculated as a percentage of BW. 13C enrichment in plasma AA was expressed as the tracer:tracee ratio (TTR). To calculate a change in the TTR in time, for each AA, the background enrichment (obtained from plasma samples taken before the injection of the U-13C-labelled AA mixture) was subtracted from 13C enrichment in samples after injection.
The ILR of AA from plasma was calculated from the change in the 13C enrichment of plasma AA after the intravenously administered bolus of U-13C-labelled AA using the model and calculations as described by Holtrop et al. ( Reference Holtrop, Lapierre and Lobley 32 ). The following assumptions were made: there is a physiological steady state during the measurement, i.e. a constant size of the plasma AA pool, so that the inflow of AA into the plasma pool equals the outflow from the plasma pool( Reference Waterlow 14 ); the tracer transfers along with the tracee between compartments with a constant fractional rate( Reference Waterlow 14 ); the ILR for an AA occurs as an output from the plasma pool, and only by the incorporation of the AA into synthesised protein or by the loss of the AA via oxidation( Reference Reeds, Cadenhead and Fuller 33 ). Finally, once the tracer has entered the body protein pool, there is no recycling of the tracer into the plasma pool, as the whole-body protein pool is a large pool with a low turnover rate compared with the plasma pool( Reference Waterlow 14 ). A double exponential model was fitted to the 13C enrichment of individual plasma AA after the administration of the bolus injection:
where E(t) is the predicted 13C enrichment in plasma AA (TTR) at time t (min); and a 1, b 1, a 2 and b 2 are parameter estimates from which the ILR (μmol/kg BW per h) was calculated:
where d is the dose of the administered U-13C-labelled AA (μmol/kg BW).
For each AA and pig, the pool size, i.e. the amount of AA in the pool (μmol/kg BW), was calculated as:
An ILR index was calculated for each pig and time point as the ILR at day − 5, 3 or 8 divided by the mean ILR at days − 5, 3 and 8 of that particular pig, multiplied by 100. This index indicates the change in the ILR within animals as affected by the challenge.
AA released from protein breakdown was calculated as the difference between the ILR and dietary intake, using the steady-state model of Waterlow( Reference Waterlow 14 ), i.e. ILR = protein breakdown+dietary intake = protein synthesis+AA oxidation. Intake was estimated by multiplying the feed intake by the dietary apparent ileal digestible content of each AA and divided by 24 h and the molar mass.
The following two indices were calculated from serum concentrations of APP: a nutritional acute-phase index (NAPI), and a health status acute-phase index (HAPI). The NAPI was considered to be associated with the nutritional costs of APP synthesis, implying that the half-lives of the APP should be taken into account. The half-life of a positive APP was considered to be inversely related to the requirements for AA for APP synthesis. To amplify the nutritional costs of an APP response, all the measured positive APP are divided by the half-life (CRP 19 h( Reference Vigushin, Pepys and Hawkins 34 ), haptoglobin 132 h( Reference Dobryszycka, Osada and Woźniak 35 ) and pigMAP 132 h, with the latter value being assumed to be similar to that of haptoglobin, based on the response pattern( Reference Petersen, Nielsen and Heegaard 36 )):
The HAPI was calculated as the sum of positive APP indices divided by the index for albumin as a negative APP, in which each index reflects the change in APP within a pig relative to the mean APP at days − 5, 3 and 8 of that pig. The HAPI was considered as a general indicator of health status. By including the indices for positive and negative APP in the HAPI, the range in values is amplified( Reference Toussaint, Ederen and Gruys 37 , Reference Gruys, Toussaint and Niewold 38 ):
where the APP index (e.g. CRP index) was calculated for each pig and time point as the APP concentration at days − 5, 3 or 8 divided by the mean APP concentration at days − 5, 3 and 8 of that particular pig, multiplied by 100.
Statistical analysis
Pig was considered as the experimental unit. The effects of dietary treatment and collection day or period on leucocytes, N balance measures, ILR, AA release in plasma from protein breakdown, and AA plasma pool size were analysed with a mixed model, with collection day, or period, taken as the repeated measure. Fixed effects also included the interaction between dietary treatment and collection day, or period. The effects were analysed by pairwise comparisons using Tukey–Kramer adjustment. A covariance structure was chosen based on the lowest value for the Akaike and Bayesian information criteria. The effects of dietary treatment, collection day and the interaction between the two on APP and total protein serum concentrations were analysed separately per period (pre- and post-challenge) with a mixed model, with collection day taken as the repeated measure. For the post-challenge APP serum concentrations, the mean of pre-challenge serum APP concentrations (days − 5, − 3 and − 1) was used as a covariate. The effect of dietary treatment on relative lung weight was analysed by ANOVA. To associate a change in AA utilisation with a change in APP concentrations or N balance, the correlation between the ILR index for AA and the NAPI or HAPI, and the correlation between N retention and the ILR or ILR index were determined using a Pearson correlation analysis. To distinguish between the 2 d post-challenge (days 3 and 8), the correlation analyses were performed separately in two parts, i.e. including data of pre-challenge and day 3 post-challenge, and including data of pre-challenge and day 8 post-challenge.
The normality of the distribution of studentised residuals was assessed. Data on pigMAP and CRP were log transformed to obtain the normal distribution of model residuals. All statistical procedures were conducted in SAS (SAS Institute, Inc.). Data are presented as means with their pooled standard errors, and the effects were considered significant at P≤ 0·05.
The goodness-of-fit of the double exponential model used to fit the 13C enrichment of individual plasma AA was assessed by the mean square prediction error (MSPE). The root MSPE was scaled to the observed mean (mean prediction error), and the correlation between the predicted and observed values was calculated. Errors due to overall bias, due to the deviation of the regression slope from unity, and due to random variation were calculated( Reference Bibby and Toutenburg 39 ). Enrichment data of some AA were excluded due to unrealistic parameter estimation and concomitant unrealistic ILR.
Results
During the post-challenge measurement period, four pigs had feed refusals that exceeded 10 % of their daily allowance. Data from these pigs were excluded from the experiment. Subsequently, one pig was excluded from the experiment due to illness occurring before the start of the N balance measurements.
Immunological response
At pre-challenge, serum CRP concentrations were lower (P= 0·02) in Res-pigs than in Ad-pigs. In the pre-challenge period, dietary protein supply did not affect serum concentrations of haptoglobin, pigMAP and total protein. In the pre- and post-challenge periods, Res-pigs tended to have lower (P= 0·09) serum albumin concentrations than Ad-pigs. In the post-challenge period, dietary protein supply did not affect serum concentrations of CRP, haptoglobin, pigMAP and total protein.
In the pre-challenge period, serum albumin concentrations were higher (P= 0·007) at day − 5 than at days − 3 and − 1 (Fig. 2). In the post-challenge period, collection day affected serum concentrations of all APP and total protein. Serum concentrations of CRP peaked at day 5 post-challenge (P< 0·001) and declined thereafter. Serum concentrations of haptoglobin peaked at day 3 (P< 0·001) and declined thereafter. Serum concentrations of pigMAP peaked at day 3 (P< 0·001) and declined thereafter (Fig. 2). Serum concentrations of albumin (P= 0·001) and total protein (P= 0·002) showed a drop at day 4.

Fig. 2 Effect of complete Freund's adjuvant (CFA) challenge and dietary protein supply (adequate (□, ■) or restricted (◇, ♦)) on serum acute-phase proteins (APP: albumin (A); C-reactive protein (CRP) (B); pig major APP (pigMAP) (C); haptoglobin (D)), serum total protein concentrations (E) and leucocyte count (F) in growing pigs (n 11). Open and closed symbols indicate pre- and post-challenge measures, respectively. Values are means, with their standard errors represented by vertical bars. a,b,c,dWithin the pre- or post-challenge period, mean values with unlike letters were significantly different (P< 0·05; effect of day). Covariate, in the analysis of post-challenge APP serum concentrations, the mean of pre-challenge APP concentrations (days − 5, − 3 and − 1) was used as a covariate.
Leucocyte counts were unaffected by dietary protein supply. The leucocyte count was lower at day 1 post-challenge (P= 0·03) than at day − 5 pre-challenge and day 8 post-challenge (Fig. 2). The leucocyte count was lower at day 0 pre-challenge (P= 0·03) than at day 8 post-challenge (Fig. 2).
Autopsy results revealed that intravenously administered CFA induced a moderate to severe granulomatous interstitial pneumonia. The relative lung weight was 1·52 (sem 0·10) % of BW at day 9 post-challenge, and was unaffected by dietary protein supply. Overall, six pigs showed signs of lymphohistiocytic focal hepatitis, and one pig showed signs of lymphocytosis. In one pig, sinus histiocytosis was observed in the tracheobronchial lymph nodes. No abnormalities were found in the other organs.
Performance and nitrogen retention
Dietary protein supply did not affect faecal N excretion. Total-tract N digestibility was greater in Ad-pigs than in Res-pigs (P= 0·001). Urinary N excretion was greater in Ad-pigs than in Res-pigs (P< 0·001), and N retention was 20 % lower in Res-pigs than in Ad-pigs (P< 0·001). N utilisation for retention was greater in Res-pigs (P= 0·02) than in Ad-pigs (Table 2). The CFA challenge did not affect N intake, faecal N excretion and apparent total-tract N digestibility (Table 2). Urinary N excretion was greater at post-challenge than at pre-challenge (P= 0·04). N retention (P= 0·07) and N utilisation for retention (P= 0·07) tended to be greater in the pre- than in the post-challenge period.
Table 2 Effect of complete Freund's adjuvant challenge and dietary protein supply (adequate or restricted) on growth performance and nitrogen balance in growing pigs (Mean values with their pooled standard errors)
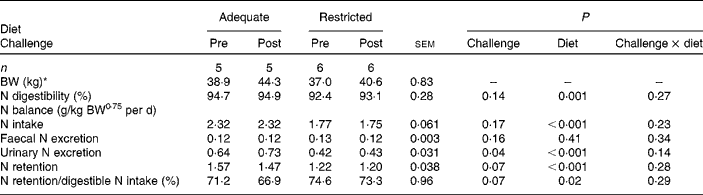
BW, body weight.
* Average BW based on measurements at 1 d before the start of each N balance period and at the last day of the N balance period.
Amino acid metabolism
The double exponential model accurately described the decrease in the 13C enrichment of individual plasma AA after the injection of the bolus with 13C-labelled AA. The average root MSPE of the studied AA ranged between 4·8 and 6·9 %, with >95 % of the prediction error attributable to random variation.
Res-pigs had a lower ILR for Lys (P= 0·02), Met (P= 0·03), Ile (P= 0·05), Val (P< 0·01) and Tyr (P< 0·01) than Ad-pigs, and tended to have a lower ILR for Phe (P= 0·09). The ILR for Leu and Trp was not affected by dietary protein supply. Res-pigs had a lower Lys (P= 0·03), Val (P= 0·02) and Tyr (P= 0·03) release from protein breakdown than Ad-pigs, and the Met release from protein breakdown tended to be lower in Res-pigs (P= 0·06). The Trp, Ile, Leu and Phe release from protein breakdown was not affected by dietary protein supply. Lys pool size tended (P= 0·08) to be lower in Res-pigs than in Ad-pigs (Table 3).
Table 3 Effect of complete Freund's adjuvant challenge and dietary protein supply (adequate or restricted) on the irreversible loss rate (ILR, μmol/kg body weight (BW) per h), release from protein breakdown (μmol/kg BW per h) and pool size (μmol/kg BW) of plasma amino acids (AA) in growing pigs (Mean values with their pooled standard errors)

a,bMean values with unlike superscript letters were significantly different (P< 0·05).
* AA released from protein breakdown was calculated as the difference between the ILR and intake, using the steady-state model of Waterlow( Reference Litvak, Rakhshandeh and Htoo 12 ), i.e. ILR = protein breakdown+dietary intake = protein synthesis+AA oxidation. Intake was estimated by multiplying the feed intake by the dietary apparent ileal digestible content of each AA and dividing by 24 h and the molar mass.
† There was a significant interaction.
The ILR for Val was affected by day of collection (P= 0·04); it remained constant until 3 d post-challenge, but was 7 % lower (P= 0·05) at day 8 post-challenge (Table 3). The ILR for Tyr tended to be affected by day of collection (P= 0·06), with a 9 % lower (P= 0·06) ILR at day 8 post-challenge than at day − 5 pre-challenge. Val release in plasma from protein breakdown tended to be affected by day of collection (P= 0·10), with a 6 % lower (P= 0·09) breakdown rate at day 8 post-challenge than at day 3 post-challenge. Tyr release from protein breakdown tended to be affected by day of collection (P= 0·09), with a 10 % lower (P= 0·09) breakdown rate at day 8 post-challenge than at pre-challenge. The pool size of Lys, Trp, Ile, Leu, Val, Phe and Tyr was not affected by day of collection. The Met pool size was about 230 % greater at day 8 than at day 3 post-challenge in Ad-pigs, but not in Res-pigs (P= 0·03; Table 3).
Correlations between irreversible loss rate v. nutritional acute-phase index, health status acute-phase index and nitrogen retention
The results of the correlation analyses between the ILR or the ILR index of AA and the NAPI, HAPI or N retention are presented in Table 4. The ILR index was not affected by dietary protein supply. Positive correlations were observed between the ILR and N retention for all AA, except for Trp. The ILR of the sum of all measured AA was positively correlated with N retention. The ILR index did not correlate with N retention for any of the AA measured.
Table 4 Correlation coefficients for the relationships between the irreversible loss rate (ILR) index and the nutritional acute-phase index (NAPI), for the ILR index and the health status acute-phase index (HAPI), and for the ILR and nitrogen retention*
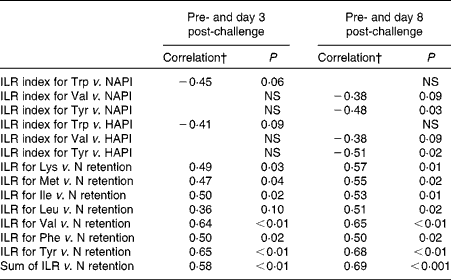
* Correlations were calculated for data obtained at day 3 post-challenge and pre-challenge, and for data obtained at day 8 post-challenge and pre-challenge in growing pigs.
† Pearson's correlation coefficients are presented with their P value. Each correlation analysis included data of eleven pigs.
Discussion
The aim of the present study was to quantify the effect of immune system activation on N retention and AA metabolism in growing pigs, depending on dietary protein supply.
The i.v. administration of CFA has been previously used in pigs as a model for immune system activation( Reference Melchior, Sève and Le Floc'h 15 , Reference Melchior, Mézière and Sève 40 , Reference Edwards and Slauson 41 ). In the present study, i.v. CFA administration activated the innate immune system, as indicated by a 2- to 4-fold increase in serum concentrations of CRP, haptoglobin and pigMAP. The observed increase in haptoglobin concentration is in accordance with a study of Melchior et al. ( Reference Melchior, Mézière and Sève 40 ) in CFA-challenged pigs. Similarly, haptoglobin and pig-MAP concentrations increase up to 6-fold and CRP concentrations up to 4-fold in response to bacterial and parasitic infections, or inflammation induced by subcutaneous turpentine challenge in pigs( Reference Heegaard, Stockmarr and Pineiro 42 ). The observed reduction in leucocytes at day 1 post-challenge might be due to leucocyte migration into the lungs, as infiltration of neutrophils and eosinophils has been reported in lung tissue after i.v. CFA administration in pigs( Reference Edwards and Slauson 41 ). Autopsy results in the present study also indicate increased infiltration of lymphocytes and macrophages in the lungs and liver following the CFA challenge. The drop in leucocytes at day 0 pre-challenge was, however, unexpected.
In the present study, dietary protein supply did not affect the relative lung weight of the pigs following CFA administration. In contrast, Le Floc'h et al. ( Reference Le Floc'h, Melchior and Sève 16 ) observed greater lung weight in CFA-challenged pigs fed a Trp-deficient diet than in pigs fed an adequate-Trp diet. In the present study, the restricted dietary protein supply reduced pre-challenge serum CRP concentrations and tended to reduce serum albumin concentrations pre- and post-challenge. In line with the present results, plasma albumin concentrations, and albumin fractional and absolute synthesis rates decreased in pigs( Reference Jahoor, Wykes and Del Rosario 18 ) fed low-protein diets and after subcutaneous turpentine challenge. In addition, a lower plasma albumin concentration and albumin fractional synthesis rate was observed in intramuscularly LPS-challenged pigs fed a low-Met+Cys diet than in pigs fed a diet with an adequate Met+Cys content( Reference Litvak, Htoo and de Lange 43 ). These findings may suggest that dietary AA supply can be insufficient for albumin synthesis, independent of immune system activation. Albumin serves as a nutrient carrier and depot by binding to nutrients( Reference Cray 44 , Reference Fanali, di Masi and Trezza 45 ). This carrying capacity is possibly reduced when dietary protein supply is restricted. Houdijk et al. ( Reference Houdijk, Campbell and Fortomaris 46 ) found a reduction in plasma CRP concentrations when the dietary protein content decreased in pigs with subclinical colibacillosis. The present results indicate that serum CRP concentrations are sensitive to dietary protein supply in the absence of immune system activation. Upon immune system activation, however, serum CRP concentrations were unaffected by dietary protein supply. This is in line with the concept that immune functions are prioritised over other body functions during immune system activation( Reference Klasing and Johnstone 1 , Reference Lochmiller and Deerenberg 3 ). The tendency for lower serum albumin concentrations during the pre- and post-challenge periods in Res-pigs than in Ad-pigs, however, indicates that a restriction in dietary protein supply can reduce albumin concentrations independent of immune system activation in growing pigs. Furthermore, this suggest that, in contrast to prioritising for immune functions, in this case APP synthesis, there is a competition for AA between immune functions and other body functions such as body protein deposition in growing pigs.
The ILR of an AA reflects the amount of free AA that disappears per unit of time from the blood plasma pool. The ILR includes the use of AA for protein synthesis and oxidation, and does not distinguish between the two fluxes. The ILR of AA in plasma in combination with the pool size or concentration of AA is, however, more useful for quantifying changes in AA metabolism than merely plasma AA concentrations or pool sizes. Changes in AA metabolism, e.g. an increased protein synthesis rate, can occur without concomitant changes in plasma AA concentrations or pool size, as AA concentrations can be maintained when fluxes from protein intake, breakdown and synthesis of body protein, and oxidation of AA are changing( Reference Waterlow 14 ). Yet, changes in plasma AA concentrations have been used previously as a measure to assess the effects of immune system activation on AA metabolism( Reference Melchior, Sève and Le Floc'h 15 , Reference Melchior, Mézière and Sève 40 , Reference Le Floc'h, Jondreville and Matte 47 , Reference Maes, Meltzer and Scharpè 48 ). In the present study, the lower ILR for Val at day 8 post-challenge than at day 3 post-challenge was not associated with a change in pool size. Therefore, the use of pool size or AA plasma concentrations as a single measure to quantify the effects of immune system activation on AA metabolism can be misleading. Furthermore, N retention reflects the total whole-body protein deposition, and does not distinguish between N retained in muscle protein or APP, neither does the ILR.
The restricted dietary protein supply reduced apparent faecal N digestibility compared with the adequate dietary protein supply. This is probably attributed to a proportionally greater excretion of basal endogenous N in Res-pigs, as the relative contribution of endogenous N to total faecal N excretion decreases with increasing dietary protein supply( Reference Fan, Sauer and Hardin 49 ) or when AA are administered intravenously( Reference de Lange, Sauer and Souffrant 50 ). N retention was 20 % lower in Res-pigs than in Ad-pigs, and corresponded with the observed reduction in the ILR for the presented AA, except for Trp. The ILR for Lys was 19 % lower in Res-pigs, followed by Met ( − 18 %), Leu ( − 16 %), Phe ( − 15 %), Val ( − 14 %) and Ile ( − 13 %). In addition, positive correlations were observed between N retention and the ILR for Lys, Met, Ile, Leu, Val, Phe and Tyr, but not for Trp. These positive correlations were mostly attributed to the differences in dietary protein supply, as the ILR indices for all AA, reflecting the changes in the ILR within the animals due to the challenge, did not correlate with N retention. As expected, restricted dietary protein supply resulted in lower urinary N excretion (absolute in g/kg BW0·75 per d as well as relative to the percentage of N intake), indicating that oxidation of AA was reduced in Res-pigs compared with Ad-pigs.
In the present study, immune system activation induced by CFA altered N retention and AA metabolism, independent of dietary protein supply. It was hypothesised that the effect of the CFA challenge on protein metabolism would be more pronounced under conditions of a marginal dietary protein supply. This would increase the competition for indispensable AA used for immune system functioning and for body protein deposition in muscle as a main determinant of the animal's growth. The effects of the CFA challenge on variables related to protein metabolism, however, were less pronounced in Res-pigs than in Ad-pigs. In Res-pigs there was no drop in N retention post-challenge compared with pre-challenge, suggesting that there is a high priority for the allocation of AA for body protein deposition in Res-pigs. N utilisation for retention, i.e. N retention:digestible N intake ratio, was greater in Res-pigs than in Ad-pigs as expected due to the difference in dietary protein supply. As shown by Fuller et al. ( Reference Fuller, Cadenhead and Mollison 51 ), the increase in N retention associated with an increase in dietary protein supply is proportionally smaller than the increase in N digestibility( Reference Fuller, Cadenhead and Mollison 51 ). In Ad-pigs, N retention numerically decreased by 6 % post-challenge, and the post-challenge drop in the ILR for Val and Tyr is, therefore, most probably attributed to a reduction in protein synthesis. In contrast, N retention in Res-pigs was unaffected by the CFA challenge. Therefore, the post-challenge drop in the ILR for Val and Tyr in Res-pigs can probably be attributed to a reduction in oxidation rather than to a reduction in body protein synthesis, as also indicated by the concomitant numerical decrease in Val pool size. This indicates that immune system activation reduced Val and Tyr oxidation in Res-pigs, but not in Ad-pigs. In humans, Leu oxidation decreased substantially more than Leu utilisation for protein synthesis, with 77 and 30 %, respectively, when a low-protein diet compared with a high-protein diet was provided( Reference Hoerr, Matthews and Bier 52 ). A decrease in AA oxidation is possibly a compensatory mechanism in Res-pigs to spare AA from catabolism, when AA for protein synthesis are scarce. The CFA challenge increased urinary N excretion, and tended to reduce N retention and N utilisation for retention. In line with this finding, greater urinary N excretion and lower N retention have been observed in pigs with an activated immune system by continuous exposure to major vectors of antigen transmission( Reference Williams, Stahly and Zimmerman 10 ) or by repeated i.m. LPS injections( Reference De Ridder, Levesque and Htoo 13 ). The observed greater urinary N loss in the post-challenge period might be caused by increased AA oxidation of unbalanced AA, as suggested by Reeds et al. ( Reference Reeds, Fjeld and Jahoor 7 ). An increase in the synthesis of APP has been suggested to increase the demands for AA, especially Phe, Trp and Tyr, which can be released by the breakdown of muscle protein( Reference Reeds, Fjeld and Jahoor 7 ). As the AA composition of muscle protein differs from that of APP, an imbalance in AA available for body protein synthesis can occur, leading to greater urinary N losses( Reference Reeds, Fjeld and Jahoor 7 ). It was hypothesised that an increase in serum APP during immune system activation affects the ILR of AA, with a concomitant reduction in pool size, due to increased incorporation of (in particular aromatic) AA into APP, and increased pool size and oxidation of non-limiting AA resulting from the related AA imbalance. The 2- to 4-fold increase in APP concentrations following immune system activation was, however, not associated with an increase in the ILR of any of the AA. In contrast, the ILR for Val was lower at day 8 post-challenge than at day 3 post-challenge, and the CFA challenge tended to reduce the ILR for Tyr at day 8 post-challenge compared with day − 5 pre-challenge. In addition, negative correlations were observed between the NAPI or HAPI and the ILR index for Tyr, and tendencies were observed for negative correlations with the ILR index for Trp and Val. These findings could, on the one hand, suggest that the changes in AA utilisation for growth due to the incorporation into APP are quantitatively less important than expected based on the findings in other studies( Reference Iseri and Klasing 6 , Reference Reeds, Fjeld and Jahoor 7 ). On the other hand, and more likely, a decrease in muscle protein synthesis during immune system activation( Reference Breuille, Rose and Arnal 53 ) might have balanced the increase in AA utilisation after immune system activation due to increased incorporation of AA into APP. In the present study, however, no distinction could be made between AA utilisation for APP synthesis or for muscle protein synthesis related to growth.
Another explanation for the lower ILR for Tyr at day 8 post-challenge may be a reduced formation of Tyr from Phe. Phenylalanine-hydroxylase catalyses the formation of Tyr from Phe. Pro-inflammatory cytokines (e.g. interferon-γ) induce the expression of the guanosine–triphosphate–cyclohydrolase-1 enzyme pathway and concomitantly release reactive oxygen species, which, in turn, inhibit phenylalanine-hydroxylase activity( Reference Capuron, Schroecksnadel and Féart 54 ). Thus, the formation of Tyr could be reduced after the CFA challenge.
The Met pool size was about 230 % greater at day 8 post-challenge than at day 3 post-challenge in Ad-pigs, but not in Res-pigs (P= 0·03). The greater pool size at day 8 in Ad-pigs might be attributed to the greater release of Met from protein breakdown, i.e. Met released from breakdown increased by 11 % compared with pre-challenge, and by 13 % compared with day 3 post-challenge. An increase in plasma pool size at a similar ILR may indicate Met oxidation (transsulfuration) rather than utilisation for protein synthesis. Hence, Met may have been released in excess of its requirement, which corresponds with the relatively high Met content in muscle protein( Reference Conde-Aguilera, Barea and Le Floc'h 55 ) compared with average APP( Reference Reeds, Fjeld and Jahoor 7 ). It can be expected that Met is increasingly used for conversion into Cys in order to produce glutathione, which plays an important role in maintaining antioxidant defences( Reference Grimble and Grimble 56 , Reference Malmezat, Breuillé and Capitan 57 ), and supports proliferation of T lymphocytes( Reference Grimble 58 ). As shown by Litvak et al. ( Reference Litvak, Rakhshandeh and Htoo 12 ), immune system activation by i.m. LPS administration increased the optimal dietary Met:Met+Cys ratio for whole-body protein deposition.
In conclusion, the effect of the CFA challenge on N retention and AA metabolism was largely independent of the dietary protein supply. A deficient dietary protein supply decreased blood serum concentrations of CRP and, to a lesser extent, albumin, stressing the importance of an adequate dietary AA supply for the production of APP in growing pigs. Immune system activation via i.v. CFA administration increased urinary N excretion in growing pigs, and tended to reduce N retention and N utilisation of digestible N for retention. Immune system activation reduced the ILR for Val and Tyr, but did not lead to a significant change in the pool size of the measured AA, except for Met. The ILR of all AA measured, except for Trp, were strongly affected by dietary protein supply and were positively correlated with N retention. Correlations between the ILR and APP indices were absent or negative, indicating that changes in AA utilisation for APP synthesis are quantitatively unimportant in growing pigs, or, more likely, outweighed by a decrease in muscle protein synthesis during immune system activation.
Acknowledgements
The authors appreciate H. van Beers-Schreurs, G. J. Deetman, D. Wamsteeker and S. Thus for their assistance during the experiments.
The present study was financially supported by the Product Board Animal Feed (PDV) in The Netherlands and the Product Board for Livestock and Meat (PVV) in The Netherlands. The Product Board Animal Feed and the Product Board for Livestock and Meat had no role in the design and analysis of the study or in the writing of this article.
The authors' contributions are as follows: E. K.-v. d. H. and P. S. conducted the experiment; J. J. G. C. v. d. B. and E. K.-v. d. H. analysed the data. All authors were involved in the design of the experiments and in the writing of the paper, and read and approved the final manuscript.
The authors had no conflicts of interest.









