INTRODUCTION
Data on non-bacterial gastroenteritis outbreaks compiled over several years and from several countries clearly indicate the aetiological role of noroviruses (NoV). In Europe, more than 85% of the non-bacterial gastroenteritis outbreaks between 1995 and 2000 were due to NoV [Reference Koopmans1]. In The Netherlands, 76·4% of viral gastroenteritis outbreaks between 1994 and 2005 were attributed to NoV [Reference Svraka2]. The most commonly identified transmission route was person-to-person contact followed by foodborne and waterborne spread [Reference Svraka2]. The contribution of food or water in NoV outbreaks is generally underestimated. Underreporting, due to a lack of appropriate detection methods for confirmation of NoV as the aetiological agent in food, often hampers identification of the actual number. In 2002, Lopman et al. [Reference Lopman3] reported that only 5/10 European countries had the in-house methodology for detection of viruses in food. Currently more isolation and detection methods for NoV in foods are available but they are not easy to perform in a laboratory setting, and no official method is available [Reference Rutjes4–Reference Baert, Uyttendaele and Debevere6].
The investigation and control of foodborne outbreaks is a multi-disciplinary task requiring information on different areas of clinical medicine, epidemiology, food microbiology, food safety and food control, risk communication and management. The cooperation between microbiologists providing the laboratory findings and epidemiological units is therefore of major importance. In Belgium, different authorities deal with foodborne outbreaks and as a consequence the information is dispersed and difficult to follow up. Moreover, NoV causes a self-limiting gastroenteritis; where not every ill person will visit a physician nor will all physicians request a stool sample, while not every patient will provide a sample if it is requested [Reference MacDougall7]. In Belgium NoV analysis is not reimbursed by the public health security system, which restricts the examination of stool samples for NoV. In September 2006, a NoV extraction and detection protocol was introduced in the Belgian National Reference Laboratory for foodborne outbreaks. In the last 4 months of 2006, three NoV outbreaks were detected that caused gastroenteritis in 79 persons. In 2007, 10 foodborne NoV outbreaks were identified and are discussed in the present study. Furthermore, data collected from foodborne and waterborne outbreaks between 2000 and 2007 reported by Eurosurveillance, Morbidity and Mortality Weekly Reports and internationally available peer-reviewed scientific journals are summarized. In total 40 food- and waterborne outbreak events are described of which the seasonality, the source and the role of the food handler are discussed in the present study with the objective of comparing the Belgian data with those relevant reported outbreaks.
METHODS
Collection of samples
NoV analysis was considered in food and stool samples if (i) the incubation period ranged between 12 and 48 h, (ii) acute non-bloody diarrhoea, vomiting, abdominal cramps, nausea or mild fever were present. NoV analysis was also considered in cases where an outbreak occurred with symptoms that did not correspond to the defined criteria but where shellfish was consumed or the food was prepared by food handlers before serving.
According to the symptoms of patients, the suspected food items and the quantity of food leftovers, a selection of microbiological parameters were established for investigation of the samples at the National Reference Laboratory for foodborne outbreaks in Belgium.
Laboratory investigation
Stool samples were diluted ten times in phosphate-buffered saline (PBS). The supernatant was collected after centrifuging for 15 min at 2000 g [Reference Jothikumar8]. RNA extraction was performed using the RNeasy Mini kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. RNA was eluted from the column by using 30 μl DEPC water. NoV were extracted from food items as described previously [Reference Baert, Uyttendaele and Debevere6]. Briefly, 10 g food product was homogenized with 8 ml TRIzol® reagent (Invitrogen, Paisley, UK) allowing a contact time of 20 min (shaking) at room temperature. After centrifugation, the nucleic acid extract (supernatant) was taken and stored at −20°C. In total, 100 μl of nucleic acid extract was purified with a RNeasy Mini kit and eluted from the column with 50 μl DEPC water. Five microlitres of the purified nucleic acid extract was used for cDNA synthesis in a RT–PCR mixture (20 μl) containing 25 U Multiscribe reverse transcriptase (Applied Biosystems, Foster City, CA, USA), 20 U RNase inhibitor (Applied Biosystems), 2·5 μm random hexamers (Applied Biosystems), 1·5 mm MgCl2 (Applied Biosystems), PCR buffer II (Applied Biosystems) and 1 mm dNTPs (GE Healthcare, Diegem, Belgium). The reverse transcription was carried out in a GeneAmp® PCR System 9700 (Applied Biosystems) with the following cycle profile: 22°C for 10 min, 42°C for 15 min, 99°C for 5 min and 5°C for 5 min. A real-time RT–PCR detection method was applied for the detection of NoV genogroup I (GI) and II (GII) as previously described [Reference Jothikumar8].
Descriptive epidemiological information
Investigations by the Federal Agency for the Safety of the Food Chain (FASFC) are mainly focused on food-related matters whereas the communities (Flemish, French, German and Brussels) deal with people-related matters, e.g. illness. A standardized questionnaire enquiring about the suspected food, the setting where food was prepared and consumed, the processing and storage of the food and other possible contributory factors, was completed by the FASFC inspector (federal) in cooperation with the physician of the specific community and was sent to the National Reference Laboratory for foodborne outbreaks.
Genotyping NoV strains
cDNA prepared from food and stool samples was amplified using the JV12/JV13 primer couple [Reference Vinjé and Koopmans9]. PCR was carried out in a GeneAmp® PCR System 9700 (Applied Biosystems) under the following conditions: 15 min at 95°C and 45 cycles of 30 s at 94°C, 30 s at 50°C, and 1 min at 72°C, followed by 10 min at 72°C [Reference Wollants10]. The PCR products were purified by the High Pure PCR Purification kit (Roche Diagnostics, Mannheim, Germany) and sequenced with the JV12 primer using the ABI BigDye 3.1 sequencing kit (Applied Biosystems) and an automated DNA sequencer (ABI3130XL, Applied Biosystems).
Collection of internationally reported food- and waterborne outbreaks due to NoV
Data available in Eurosurveillance, Morbidity and Mortality Weekly Reports and other international peer-reviewed journals concerning food- and waterborne outbreak events that had occurred between 2000 and 2007 were included. An outbreak event was defined as an outbreak with a common contamination source. Peer-reviewed reports were selected by the keywords: ‘outbreaks’ and ‘viral’; ‘outbreaks’ and ‘norovirus’; ‘outbreaks’ and ‘Norwalk’; ‘norovirus’; ‘Norwalk virus’ between 2000 and 2007. An attempt was undertaken to list all food- and waterborne NoV outbreaks from these sources. The authors are aware that presumably more food- and waterborne outbreaks have been reported. However, the fact that particular outbreaks were overlooked, was probably because data on these outbreaks were only published in national databases or only available in a non-English language and therefore did not reach a wide international public. The outbreak events reported in the present study were considered because of their detailed epidemiological information, of which the majority was well funded with laboratory investigations towards NoV detection. Consequently, the ongoing tendency of food- and waterborne NoV outbreaks is reflected by these reported outbreak events.
RESULTS
Ten foodborne NoV outbreaks in Belgium during 2007
In 2007, 48 food samples from 11 suspected foodborne outbreaks were analysed for NoV. In six of these outbreaks NoV was detected in the food sample analysed. In the other outbreaks no agent was detected in the food and they were therefore classified as unknown. Additionally, another four outbreaks were also classified as NoV foodborne outbreaks because of the detection of NoV in the faecal specimen and the collected epidemiological information.
In total 392 persons became ill after a NoV outbreak. Symptoms began in most cases between 12 and 24 h after food consumption with mainly vomiting, diarrhoea and slight fever reported. Hospitalization was not necessary. The majority of outbreaks occurred at work (30%), the second most important settings were at camp (20%) and in nursing homes (20%) while one outbreak each took place in a restaurant (10%), a recreation centre (10%) and at home (10%). The implicated source (food/water) of the outbreak and the number of reported cases with gastroenteritis are shown in Table 1. NoV analysis was performed in outbreaks 1, 2, 7 and 8 because specific symptoms of a NoV infection were observed. Although in six outbreaks (outbreaks 3, 4, 5, 6, 9, 10) samples (food, faecal) were examined for NoV because a food handler was involved. The role of the food handler was suspected in eight outbreaks. In one outbreak (outbreak 1) NoV was detected both in food (3/3 samples contained NoV) and in human faecal samples (1/1). Chicken with rice and soup were served to children making a daytrip to a recreation centre in January and NoV was detected in the leftovers. The unopened bags of the same production date tested negative for the presence of NoV. It is likely that an infected food handler serving the children's meal at the recreation centre was responsible for the spread of NoV through the food. Once the children returned home another 34 persons became ill with the same symptoms because of satellite outbreaks in the families (representing the second attack rate). In outbreaks 2, 4 and 7, respectively, mashed potatoes (1/1), meat stew (1/1) and a composite meal (1/2) were found positive but no stool samples were available for testing. Stool samples were not taken (outbreaks 4 and 7) or not tested for NoV presence but did test negative for bacterial pathogens (outbreak 2). Outbreak 2 occurred in an old peoples' home in March and outbreak 7 took place in a home for disabled persons in June. Outbreak 4 was located at the workplace in April. The implicated food items of outbreaks 2, 4 and 7 were handled and served by kitchen personnel before consumption and, according to the collected epidemiological information, food handlers were the suspected cause of these outbreaks.
Table 1. Foodborne outbreaks due to NoV reported in Belgium during 2007
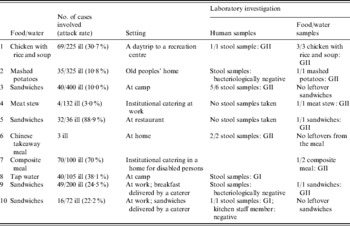
In 4/10 (40%) outbreaks sandwiches were the most likely source of the NoV outbreak. In two of those cases (outbreaks 3, 5), history of gastroenteritis was reported by persons preparing the food. In May a member of the restaurant staff (outbreak 5) suffered from gastroenteritis in the week before the outbreak and sandwiches (1/1), prepared by the staff including this particular food handler, tested NoV positive. Outbreak 3 took place at camp in March where a sick child assisted in the preparation of sandwiches but no leftovers from the sandwiches were available because of the late reporting (>1 week), although stool samples were NoV positive (5/6). In outbreak 9, NoV was detected in sandwiches (1/1) that were delivered by a caterer to the workplace in July. Stool samples were bacteriologically negative but not tested for the presence of NoV. Contrary to outbreak 9, NoV was detected in stool samples (1/1) collected in outbreak 10. Because of the lack of leftovers from the sandwiches implicated in outbreak 10, NoV analysis could not be performed. One outbreak (outbreak 6) occurred at home in May. After eating a Chinese takeaway meal, three persons showed symptoms of gastroenteritis. Stool samples indicated the presence of NoV (3/3). Outbreak 8 was the only suspected waterborne outbreak at a camping site in July. Epidemiological information indicated tap water as the most likely source of the outbreak. However, due to the lack of an appropriate concentration/extraction method of NoV for water, negative results were obtained.
NoV genogroup I (GI) was associated with two outbreaks (outbreaks 8 and 10) while the majority of the outbreaks were due to NoV GII (8/10). NoV detected in three stool samples originating from outbreak 3 were sequenced and revealed NoV/GII.4/Terneuzen70/2006 with 99% similarity. One stool sample was sequenced in outbreak 6 and showed a similarity of 97% with No/GII.2/Kuenzelsau/3870/05. No other faecal material was available for sequencing. It was not possible to obtain sequence data from food samples.
Internationally reported data of food- and waterborne NoV outbreaks from 2000 until 2007
International food- and waterborne outbreak events that occurred between 2000 and 2007 were compared with the Belgian results obtained in 2007 [Reference Boxman11–Reference Gallimore48]. In total 40 events of food- and waterborne outbreaks were included of which 29 outbreaks (72·5%) took place in Europe and 11 outside Europe (27·5%). Four reported events were attributed to contaminated raspberries (10·0%). One of these four events included four outbreaks that were linked in Sweden by a common origin of contaminated raspberries [Reference Hjertqvist47]. In another event, six outbreaks were clustered in Denmark [Reference Falkenhorst45]. Bivalve shellfish was defined as the source in 7/40 listed outbreak events (17·5%). Although three out of those seven events included several clusters of outbreaks whereby each cluster within an event had a common contamination source. Eleven reports were water associated (27·5%). Five waterborne outbreaks indicated environmental water as the origin of the outbreak. Six other waterborne outbreaks took place through consumption of contaminated drinking water.
For 17 outbreak events (42·5%), the role of a food handler was implicated as the origin of the outbreak. One outbreak event in the United States indicated three clustered outbreaks due to a caterer, where one of the food handlers with history of gastroenteritis was involved in three separate catered meals at work [Reference Payne21].
Figure 1 depicts the number of outbreaks in the year and shows that outbreaks took place throughout the year. Outbreaks were additionally categorized according to the place and the implicated food item (Fig. 2). Sandwiches and salads were the major implicated food items at work. Shellfish was responsible for the majority of outbreaks in restaurants and households. Outbreaks that occurred in the community and in holiday resorts were caused by water. Regarding parties and meetings, food prepared by caterers was the most important source of NoV outbreaks.

Fig. 1. The number of international food- and waterborne outbreak events according to the source and month. Histograms show outbreaks due to raspberries (□), bivalve shellfish (![]() ), water (
), water (![]() ) or handling by a food handler (
) or handling by a food handler (![]() ).
).
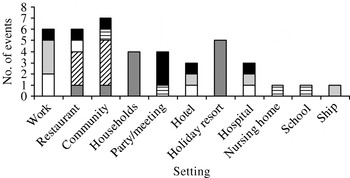
Fig. 2. The number of international food- and waterborne outbreak events according to the place and suspected food item. The implicated food items are classified in categories: raspberries (![]() ), sandwiches (□), salads (
), sandwiches (□), salads (![]() ), water (
), water (![]() ), shellfish (
), shellfish (![]() ), other food items (
), other food items (![]() ).
).
Table 2 shows the implicated food/water item, attack rate and concise laboratory results of 40 international outbreak events including 50 detailed outbreaks. From these outbreaks 16 did not determine sequences, of which 15 did not even report the specific genogroup. Food handler-associated outbreaks where the NoV genogroup was known, revealed the presence of GI in four outbreaks and GII in seven outbreaks. None of the outbreaks revealed both genogroups. Conversely, both GI and GII were frequently detected in shellfish, water and raspberry outbreak events.
Table 2. Internationally reported foodborne and waterborne outbreaks from 2000 to 2007
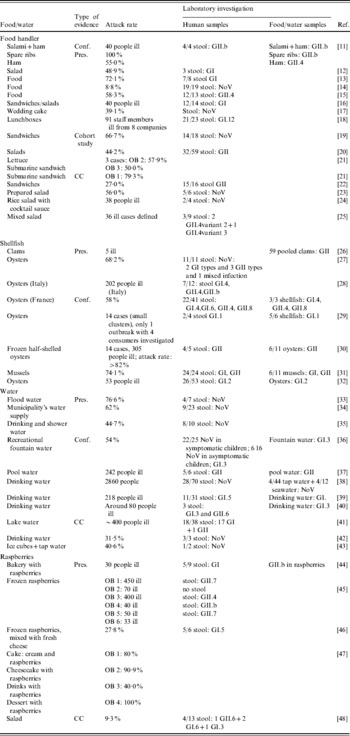
Conf., Confirmed; detection of causative agent in food/water and stool; CC, outbreaks where a case-control study revealed the most probable causative agent; Pres., outbreaks where the causative agent is ‘presumably’ indicated but not funded by a case control study, cohort study or was not confirmed; OB, outbreak.
Attack rates mentioned in Table 2 varied between 8·8% and 100% for food handler-associated outbreaks. Attack rates of 58–74·1%, 31·5–76·6% and 27·8–100% were involved in shellfish, water and raspberry outbreaks, respectively.
DISCUSSION
Ten out of the 75 reported foodborne outbreaks in Belgium during 2007 were due to NoV and these exceeded the number of Salmonella outbreaks (eight reports). Reporting of foodborne outbreaks to the EFSA is mandatory for European member states. In 2006, 53·9% of the reported foodborne outbreaks by 25 European countries were due to Salmonella. Campylobacter (6·9%) was the second most commonly reported causative agent followed by caliciviruses (6·2%) [49]. The introduction of clinical profiles specific for NoV in foodborne outbreaks of unknown aetiology reported to the Centers for Disease Control and Prevention (CDC) between 1982 and 1997, showed an increase from 1% to 38% of NoV outbreaks, even exceeding outbreaks with Salmonella as the causative agent [Reference Hedberg50]. Consequently, these numbers indicate that NoV is an important causative agent of foodborne outbreaks, not only in Belgium, but also in Europe and the United States.
In 2007 all inspectors of the Belgian FASFC, which has responsibility for control of foodstuffs and their raw materials at all stages of the food chain as well as investigation of foods implicated in foodborne outbreaks, followed a mandatory specific training on NoV. Furthermore, since 2006 the medical health inspectors of the Belgian regional communities have had the opportunity to send faecal samples to the National Reference Laboratory for foodborne outbreaks.
Despite the measures taken to improve the investigation regarding NoV outbreaks in Belgium, there was only one outbreak where NoV was detected in both food and stool samples. Underreporting, late reporting, the lack of clinical and environmental samples as well as the lack of laboratories to test water and food for NoV hamper the recognition of the role of this virus in outbreaks [Reference Rizzo51]. Moreover, the FBVE network has encountered similar problems [Reference Lopman3]. The percentage of outbreaks with complete data including epidemiological data and laboratory findings are increasing each year, indicating better cooperation between laboratories and epidemiological units [Reference Kroneman52].
In 6/10 foodborne outbreaks due to NoV in Belgium, the causative agent, i.e. NoV, was detected in food samples which is unique. Internationally reported NoV outbreaks mostly include just epidemiological information and/or stool sample analysis. Rarely is NoV detection described in food other than shellfish or water. The foods implicated in the Belgian outbreaks (sandwiches, soup, meat stew, mashed potatoes, a composite meal) were probably contaminated with low levels of NoV on the surface of the food item. Contrary to these composite and complex food matrices, shellfish bioaccumulate and concentrate NoV, facilitating the detection of the virus in this food category [Reference Schwab53]. Moreover, the food items cover diverse types of foods and were all tested with the same NoV extraction and detection method. This extraction protocol enabled the isolation of NoV in ham, salami and spare ribs in The Netherlands [Reference Boxman11]. Consequently, this method has proved useful in outbreak investigations as suggested by Boxman et al. [Reference Boxman11].
The Belgian data were compared with 40 international outbreak events between 2000 and 2007. It should be noted that the number of international outbreaks considered are extremely low and probably biased because peer-reviewed publications of food- and waterborne outbreaks report merely large, well documented, unusual or novel events [Reference Kroneman52, Reference O'Brien54].
In the Belgian NoV outbreaks reported, NoV GII was detected in 80% of the NoV outbreaks and NoV GI in the remaining 20%. GII.4 and GII.2 were confirmed in two outbreaks. An attempt was undertaken to sequence other samples as well but was unsuccessful. The time between detection and sequencing (up to more than 1 year) and the low levels of NoV on food are factors that resulted in the failure of obtaining sequencing data from food samples.
The Belgian NoV outbreaks were characterized by an attack rate ranging between 3·0% and 100%, similar to the international data. This broad range of observed attack rates is not surprising because outbreaks are caused by accidental point contamination. Less fluctuating attack rates were noted for shellfish. Raspberries are likely to be contaminated by water in the field, affecting large batches. One batch of raspberries can be used for several desserts on different locations and can therefore cause outbreaks at several settings such as in households, at meetings and parties, nursing homes and schools with a considerable high attack rate. Raspberry outbreaks were recorded in particular in summer and in Nordic countries where desserts based on raspberries are very popular.
The role of the food handler was epidemiologically suggested in 80% of the NoV outbreaks reported in Belgium. This high percentage was found because NoV analysis was considered in outbreaks where foods were prepared by food handlers irrespective of the food type. In 17/40 studied internationally reported outbreak events, the role of the food handler was indicated. In eight of these 17 outbreaks, a sick food handler or food handler with a recent history of gastroenteritis was observed [Reference Boxman11, Reference Lederer14, Reference Sakon18–Reference Schmid23]. Moreover, the majority of these outbreaks were linked to sandwiches as the contaminated source of the outbreak. Similarly, in two Belgian outbreaks, history of gastroenteritis was reported by persons handling sandwiches. In January and May, a high incidence of food handler-associated outbreaks occurred. This trend was not observed in Belgium because of the limited number of outbreaks.
NoV GII.4 outbreaks and sporadic cases involving food handlers with gastroenteritis in Japan revealed that many asymptomatic food handlers also tested positive for NoV GII.4 strain. Moreover, the number of virus shed by symptomatic and asymptomatic food handlers was similar, indicating the potential hazard of these highly contagious viruses [Reference Ozawa55]. Food handler-associated outbreaks tend to affect more people due to large catering establishments compared to oyster-related outbreaks [Reference Noda, Fukuda and Nishio56]. Poor personal hygiene was also identified as a contributory factor in outbreaks where NoV was assigned as the causative agent [Reference Rizzo51]. Therefore, it is of major importance to inform food handlers of their responsibilities for the prevention of large-scale NoV outbreaks. Food handlers must have knowledge of good hygienic practices. If family members of food handlers or food handlers themselves show symptoms of gastroenteritis, the employer should be alerted in order that the appropriate preventive measures can be taken.
The present study shows that the introduction of a detection method increased the number of reported NoV outbreaks in Belgium from none in 2005 to becoming the leading cause of foodborne outbreaks in 2007. Food handlers appeared to be the most probable source of foodborne NoV outbreaks and these outbreaks were frequently associated with sandwiches.
ACKNOWLEDGEMENTS
We thank Elke Wattijn (Scientific Institute of Public Health) for technical assistance with the Norovirus analysis.
DECLARATION OF INTEREST
None.






