Introduction
The Arabian oryx Oryx leucoryx originally occurred in Jordan, Syria, Iraq, Israel, Sinai and the Arabian Peninsula, but was extirpated from the wild in 1972 (Henderson, Reference Henderson1974). It has been on CITES Appendix I (CITES, 2007) since 1975 and is categorized as Endangered on the IUCN Red List (IUCN, 2006). Currently there are five reintroduced populations in large areas of natural habitat, although all have some degree of protection: Oman (Arabian Oryx Sanctuary, 27,500 km2); Saudi Arabia (Mahazat as-Sayd Reserve, 2,244 km2, and Uruq Bani Ma'arid Reserve, 12,500 km2); and Israel (Northern Arava and Negev Desert; Jungius, Reference Jungius1978; Clark, Reference Clark1987; Abu-Jafar & Hays-Shahin, Reference Abu-Jafar, Hays-Shahin, Dixon and Jones1988; Ostrowski et al., Reference Ostrowski, Bedin, Lenain and Abuzinada1998; Bedin & Ostrowski, Reference Bedin and Ostrowski2003).
In Jordan oryx originally occurred throughout the northern steppe grasslands and eastern desert (the badia). They were hunted intensively after 1932, when the construction of the Baghdad–Haifa oil pipeline was accompanied by 'massive mechanized shooting' of oryx (Quemsiyeh et al., Reference Quemsiyeh, Amr and Budieri1996). Some oryx were also killed by pesticides applied across wide areas of the badia to control locusts in the 1950s (Hatough & Al-Eisawi, Reference Hatough and Al-Eisawi1988). The last wild oryx in Jordan was shot in the early 1960s near Qatraneh, c. 75 km south of Amman (Mountfort, Reference Mountfort1965).
In 1978 the Royal Society for the Conservation of Nature in Jordan (RSCN, an NGO) began an oryx reintroduction programme. It obtained 11 oryx from the San Diego Wild Animal Park in the United States (four males and four females) and the Royal herd in Qatar (one male and two females; Table 1). In 1984 the Zurich Zoo in Switzerland provided three males. The founders were from two separate bloodlines: those from San Diego and Zurich were of Yemeni-Saudi stock, whereas those from Qatar were considered a separate stock. They were placed in a captive breeding enclosure consisting of pens and a fenced compound occupying a small portion of the 342 km2 Shaumari Nature Reserve in eastern Jordan (Fig. 1).

Fig. 1 Jordan, with the location of the Shaumari Nature Reserve, the Rum Protected Area, and the proposed nature reserve at Burqu.
Table 1 Provenance or disposition of Arabian oryx imported or exported from the Shaumari Nature Reserve.
In 1983 the RSCN began releasing oryx out of the captive breeding enclosure into the whole Reserve, shared with other large grazers and occasional predators. This was considered a reintroduction into the wild because the reserve outside the captive breeding enclosure is native habitat in a natural state and the herd was managed with minimal human interference (Bauman, Reference Bauman1979; Abu-Jafar & Hays-Shahin, Reference Abu-Jafar, Hays-Shahin, Dixon and Jones1988; Hatough & Al-Eisawi, Reference Hatough and Al-Eisawi1988). Oryx are nomadic, however, ranging over a large area while using a series of separate, suitable areas mostly 100–300 km2 in size for 1–18 months at a time (Price, Reference Price1986), typically with a mean herd size of 5.8, some solitary bulls and a bachelor herd (Spalton, Reference Spalton1993). The situation at Shaumari was therefore transitional to a truly wild, free-ranging population, which the RSCN planned to establish eventually (Mountfort, Reference Mountfort1969; Clarke, Reference Clarke1977, Reference Clarke1979).
Our purpose here is to document the demography of this population from its early, rapid expansion through eventual decline to provide a foundation for further reintroductions within and outside of Jordan.
Study area
The Shaumari Nature Reserve is in arid desert with temperatures frequently above 42°C in summer and down to −10°C in winter. Rain only falls in winter and averaged 62.2 mm per year (range 10.1–149.0) from 1967 to 1997 at nearby Azraq (Government of Jordan, unpubl. data). The habitat is hammada, a flat, treeless terrain covered by flint or limestone pebbles, with many wadis. Wadi vegetation may contain shrubs up to 2 m tall and small trees (e.g. Tamarix spp.), whereas hammada is virtually barren except for sparse forbs and grasses that flourish briefly following rainfall. In 1984, 22 km2 of the Shaumari Nature Reserve was completely fenced to exclude livestock and to contain the oryx and other large wildlife, which include Persian onagers Equus hemionus onager, goitered gazelles Gazella subgutturosa, and blue-necked ostriches Struthio camelus austrellus. By the mid 1980s this protection from grazing had allowed the development of a structurally complex and species-rich plant community that supported a more diverse community of mammals, reptiles and birds than outside the reserve (Al-Eisawi & Hatough, Reference Al-Eisawi and Hatough1987; Hatough-Bouran & Al-Eisawi, Reference Hatough-Bouran and Al-Eisawi1990). Water is provided from a well and mature tamarix and planted eucalyptus trees provide shade.
Methods
Population data
We examined the oryx log (a record of every oryx born at the Reserve) for internal consistency and compared it with independent reports of the population at Shaumari. Finding that a few of the records prior to 1995 were incomplete, we made the following adjustments: (1) For five of 87 (6%) death records prior to 2000, the year of death was missing. To approximate these five death dates we used either the median age of death for that cohort or the date of last observation. (2) Eight of 403 (0.02%) births for which the sex of a calf was not recorded, or was recorded as unknown, were omitted from the population calculations on the assumption that they died before their first year (in all such cases there was no further mention of those individuals).
Another inconsistency in the oryx log was the number of calves born compared to published reports. For example, a visiting ornithologist mentioned that 18 oryx calves had been born at Shaumari by the end of 1981 (Conder, Reference Conder1981) but the oryx log lists only nine up to that date. Budieri (Reference Budieri1995) reported that 328 calves had been born, although the oryx log only listed 272 by the end of 1995. Presumably, some calves were not entered into the log because they died as neonates and the staff at Shaumari felt they had not been recruited to the population and therefore should not be recorded.
Analysis
We reorganized the oryx log data to list the founding population, number of new male and female calves born, number of male and female calf deaths, and number of male and female yearling and adult deaths, by year. Yearling recruitment was calculated as the number of the previous year's female and male calves minus the previous year's male calf and female calf deaths, per 100 adult females. Each year's population of yearlings and adults was therefore the previous year's yearling and adult males and females plus the number of male and female yearlings recruited, plus the new acquisitions, minus the number of male and female yearling and adult deaths, and minus the dispersal of adult oryx to other locations. The total population (N) was these plus the number of calves surviving at the end of the year. We calculated the annual increase in the population, lambda (λ), using the exponential model given by
where N 0 = initial population size, Nt = population at time t, and t = years.
To represent the population increase when density-dependent effects became apparent we used a generalized logistic model (Verhulst, Reference Verhulst1838) in which the rate of increase is a function of the population size relative to the maximum population as given by
where Nt = population at time t, Nt+1 = population size at the end of the next interval (year), R0 = the initial, discrete rate of population growth, and K = maximum population (assumed to be equivalent to carrying capacity).
To express annual population growth in terms of population size, we used the per capita growth rate, or instantaneous rate of increase, r, for continuously increasing populations because of the non-seasonal breeding of oryx
We tested for the Allee effect (Courchamp et al., Reference Courchamp, Clutton-Brock and Grenfell1999) or other hormesis relationships (a U-shaped trend line indicating a reversal of the dependent variable with continued increase of an independent variable) with quadratic models (cf. Treydte et al., Reference Treydte, Williams, Bedin, Ostrowski, Seddon, Marschall, Waite and Ismail2001). All curve estimations and statistical analyses were performed with SPSS (Chicago, USA).
Results
Population growth
The oryx log records 454 births and 166 deaths at Shaumari. During 1984–1989 four females and five males from Shaumari were sent to captive breeding centres in Oman, Saudi Arabia, and Iraq (Table 1). In 1990 there were 69 adult oryx at Shaumari but deteriorating range conditions outside the wildlife refuge prevented further reintroductions. By 1996 the oryx log recorded 273 births at Shaumari and the adult population was 206. In 1997, to reduce overcrowding, the RSCN began dispersing oryx to other countries and to a newly created nature reserve in Jordan, the Wadi Rum Protected Area (Abu-Eid, Reference Abu-Eid2001; Table 1).
By autumn 2003 six adults had survived at Wadi Rum, the other four having died, probably of old age. A female calf born at Wadi Rum also did not survive, and may have succumbed to a snakebite. After initially keeping them in a large, fenced enclosure, they were released into the wild. However, when the entire herd wandered south and were about to cross into Saudi Arabia they were captured and returned to the enclosure. From 2003 to 2005 another five died, leaving one old male at Wadi Rum. In February 2006 the RSCN transferred five more oryx to Wadi Rum. We saw all six there in March 2006.
Growth rates
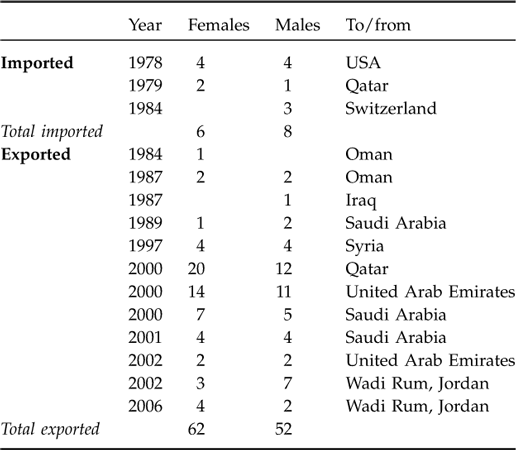
The oryx population grew initially (1979 to 1986) at R 0 = 23% per year (λ = 1.2336) inside the Reserve (Fig. 2). Thereafter, they apparently began to suffer density-dependent effects indicated by increasing aggression among males (Budieri, Reference Budieri1995) and a declining rate of increase commensurate with decreasing productivity and increasing mortality. The per capita growth rate declined linearly with increasing population (r 2 = 0.283, F = 10.3, P = 0.004). When the analysis was limited to 1984–2005 (after most of the herd was released into the reserve from the captive breeding pens) the relationship weakened, but was still statistically significant (r 2 = 0.232, F = 6.1, P = 0.023; Fig. 3). The quadratic model did not show a decrease at low populations, as would have been consistent, for example, with an Allee or other negative founder effect.
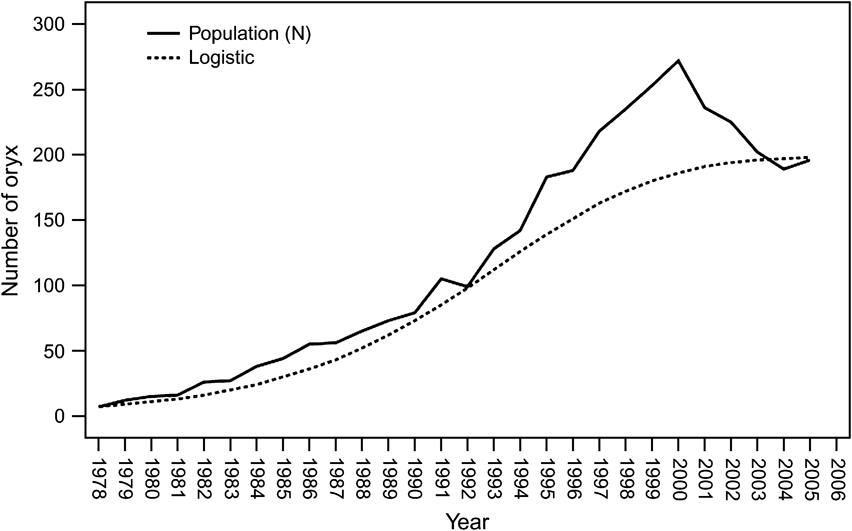
Fig. 2 Oryx population (N) at Shaumari Nature Reserve, Jordan, 1978–2005. The logistic model had an initial growth rate (R 0) of 23% as observed at Shaumari and an assumed maximum population (K) of 200. The sharp decline after 2000 reflects (a) floods that killed 69 oryx in 2000 and 48 in 2002, and (b) a planned dispersal of 119 adult oryx to other Gulf countries and 10 to another nature reserve in Jordan.
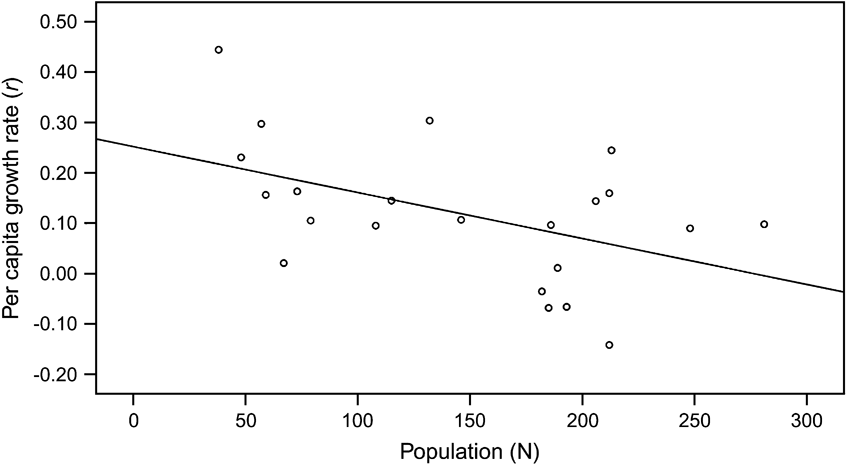
Fig. 3 Annual per capita growth rate (r) in relationship to population for the oryx in Shaumari Nature Reserve, Jordan, 1984–2005, the period after most of the herd was released from the captive breeding pens. The linear trend line is statistically significant at P ≤0.05.
Reproduction and recruitment
Although the overall calf sex ratio was 0.91 male per female, there was no significant difference (paired t test, P >0.05) in the numbers of female and male births per year. They were highly correlated (P <0.001), with both genders increasing as the population rose until 1995, and then declining (Fig. 4). The possibility of some births and neonatal mortality not having been recorded may mean that birth rates were underestimated. The regression for number of calves over time fits a quadratic equation (r 2 = 0.471, F = 10.7, P <0.001), confirming the obvious rise and then fall in the number of births per year. After 1983, when the oryx were released from the captive breeding facility into the Reserve, the birth rate (b, the number of births during the time period, t, divided by the total number of mature females) generally declined as population (N) increased. The trend was close to statistical significance (P = 0.055; Fig. 5).
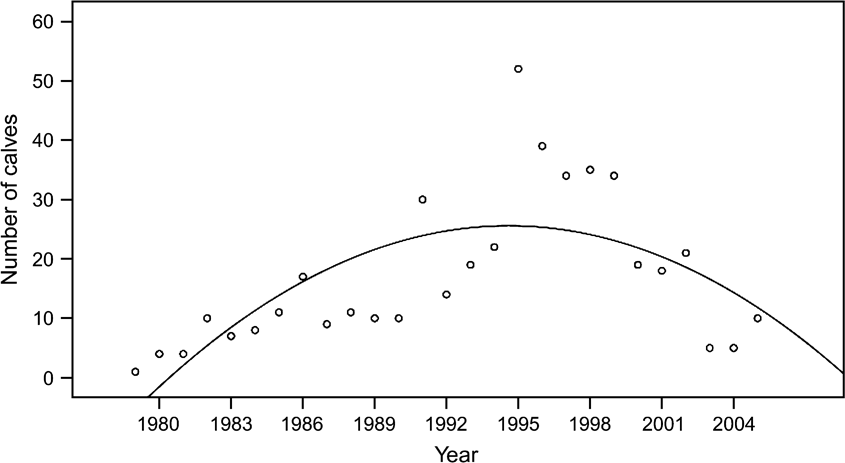
Fig. 4 Annual number of calves born per year at Shaumari Nature Reserve, Jordan, 1979–2005. The fitted line is a statistically significant quadratic model.
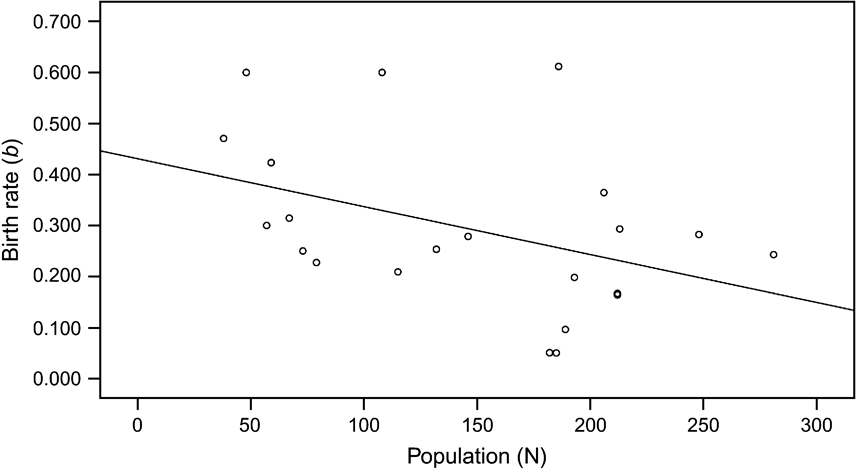
Fig. 5 Annual birth rate (b) in relation to the oryx population at Shaumari Nature Reserve, Jordan, 1984–2005, the period after they were released from the captive breeding facility into the Reserve. The linear regression approaches statistical significance at the 5% level.
Recruitment of yearlings (male and female calves that survived their first year) increased rapidly until 1983, reaching a maximum of 61.5 yearlings per 100 adult females. From 1984 onwards, after most oryx had been released from the captive breeding facility into the Reserve, recruitment declined (quadratic model, r 2 = 0.529, F = 10.7, P = 0.001; Fig. 6). There was no difference in recruitment of males and females per year (paired t test, P >0.05) and the two were highly correlated (P <0.001). Neither the birth rates (b) nor annual recruitment were related to rainfall (P >0.05), a major correlate of forage availability and hence of ungulate productivity in Middle Eastern deserts (Orshan, Reference Orshan, Evenari, Noy-Meir and Goodall1986). Above average rainfall occurred in 1981, 1983, 1988–1991 and 1995; otherwise, rainfall was less than the long term average.
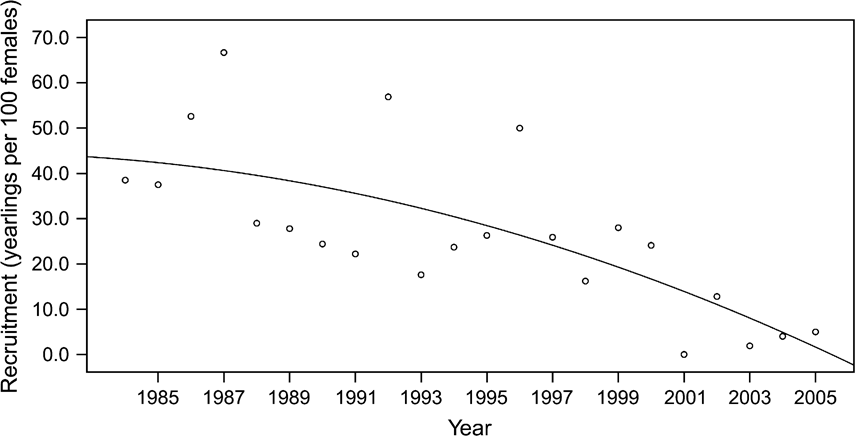
Fig. 6 Recruitment (calves that survived their first year) per 100 adult oryx females for 1984–2005, the period after the oryx were released from the captive breeding facility into the Shaumari Nature Reserve, Jordan. The fitted quadratic line is statistically significant.
Survival and mortality
Calf survival, although often above 90%, occasionally dropped to <80%, in addition to 2 catastrophic years, 2000 and 2002, when floods killed 100 and 92% of calves, respectively (Fig. 7). Male and female calf survival (and, conversely, mortality) were not statistically different (P >0.05). The possibility of some births and neonatal mortality not having been recorded, as noted above, may mean that calf survival was overestimated. Yearling and adult survival was 93–100% for females and 75–100% for males (Fig. 7). Yearling and adult mortality did not change over time (P >0.05) and was not correlated with population size (P >0.05). The numbers of yearling and adult female and male deaths (and, conversely, survival) were not statistically different (P >0.05).
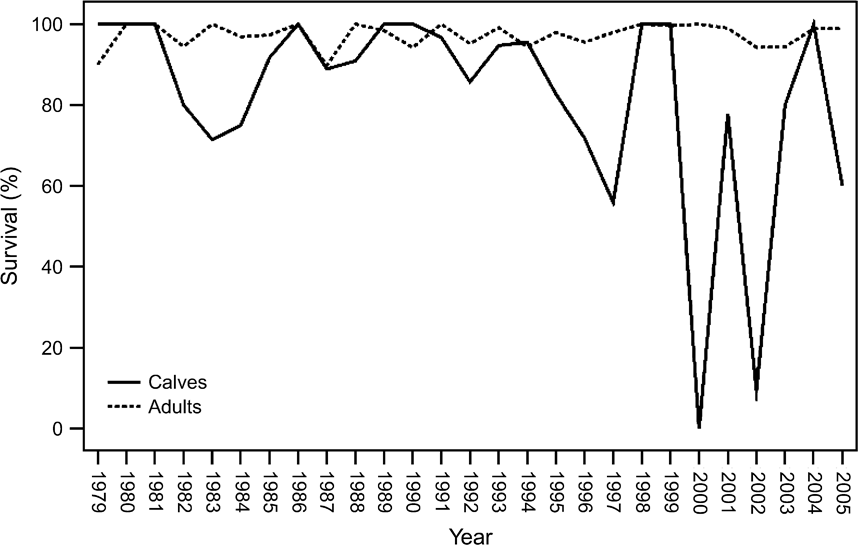
Fig. 7 Annual survival of calves and adults + yearlings at Shaumari Nature Reserve in Jordan, 1979–2005.
The leading mortality factor was predation, accounting for 15% of deaths (Fig. 8). Syrian jackals Canis aureus syriaca, Arabian wolves Canis lupus arabs, red foxes Vulpes vulpes, Ruepelli's sand foxes Vulpes rueppelli, caracals Caracal caracal, sand cats Felis margarita, wild cats Felis sylvestris tristrami, and Syrian striped hyaenas Hyaena hyaena syriaca occur in the area (Bunaian et al., Reference Bunaian, Hatough, Ababaneh, Masaqbeh, Yousef and Amr2001). Caracals, which could easily kill a young oryx, were seen within Shaumari several times during the late 1990s, coincident with a period of high neonatal mortality, and in 2002.
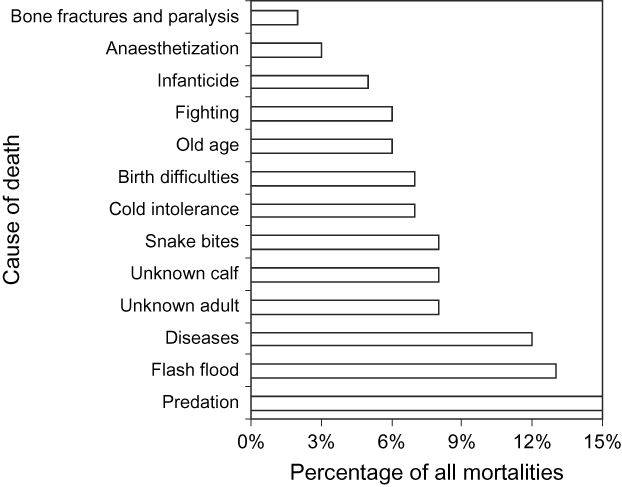
Fig. 8 Frequency of mortality factors for the oryx at Shaumari Nature Reserve, Jordan, 1979–2005.
Floods, although infrequent, were the second highest cause of death in the oryx at Shaumari. Flash floods combined with cold weather were responsible for most of the 19 deaths in 2000 and 30 in 2002. It also is possible that some oryx escaped during floods, as did seven gazelles during a flood in 1994 that damaged the fence. If so, it could account for the five oryx whose death dates were not recorded (i.e. these five oryx either died in the reserve and were not found, or escaped along with the gazelles). Any oryx that escaped would probably not have survived, not only because of the lack of forage, shade and water, but also because of the dispersed population of Bedouins who undoubtedly would have hunted them.
Diseases (including food poisoning, intestinal parasites, and infections) were the third highest mortality factor. Until 1993, veterinarians provided monthly medical care, performed pathological examinations on deceased individuals, and treated accidentally injured oryx. Serological analysis was carried out for possible carriers of antibodies to major zoonotic diseases such as bluetongue, brucellosis, and Pasteurellosis. Faecal samples were taken regularly to check for parasites. After 1993 veterinary care became less regular. Diseases and parasite infestations are likely to have been responsible for some of the deaths from undetermined causes. If this was the case then diseases may have been the highest or second highest source of mortality.
Poisonous snakes killed 2–3 oryx per year (8% of the total mortality) and this factor seemed to the herd's management to be associated with plant cover that provided hiding places for snakes (Budieri, Reference Budieri1995). Hatough et al. (Reference Hatough, Al-Eisawi and Disi1986) also reported that high reptile diversity was associated with increasing plant cover following protection from grazing at Shaumari. Other causes of death included cold intolerance (7%), birth difficulties (7%), intraspecific aggression (males fighting, 6%), old age and weakness (6%), infanticide (males goring neonates with their horns, 5%), anaesthesia during veterinary procedures (3%), and accidents resulting in bone fractures (2%). The herd's management at Shaumari attempted to reduce infanticide by isolating females with calves from the rest of the herd.
Life span
Males and females lived for up to 18 and 16 years, respectively. Of those that survived their first year, mean life span was 6.9 ± SD 4.5 years for males, 8.8 ± SD 4.8 years for females and 7.7 ± SD 4.7 years for both (Fig. 9).
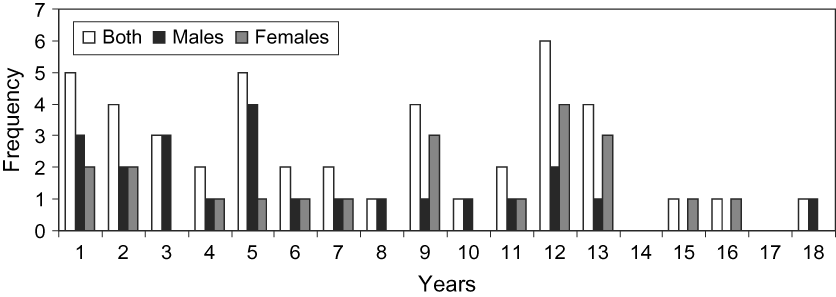
Fig. 9 Frequency distribution of life span of oryx at Shaumari Nature Reserve, Jordan, 1979–2000.
Discussion
This study shows the value of setting up a good record-keeping system at the outset of captive breeding and reintroduction programmes, and then ensuring continuity and standardization to facilitate retrospective analyses. The initial growth rate (R 0) observed at Shaumari, about 23% per year (λ = 1.2336), was similar to those of other reintroduced oryx populations. In 1990, 72 Arabian oryx were reintroduced into a 2,244 km2 wildlife reserve in Saudi Arabia and increased to 350 by 1998 (Seddon et al., Reference Seddon, Ismail, Shobrak, Ostrowski and Magin2003), an annual rate of 22% (λ = 1.22). After 1998, however, the annual growth rate there declined to c. 15% per year (λ = 1.15). In Oman 35 oryx introduced from 1982 to 1989 increased to 100 in 1990 and 450 in 1996 (Spalton et al., Reference Spalton, Lawrence and Brend1999); the 1990–1996 annual growth rate was 28% (λ = 1.28). On the other hand, an oryx herd reintroduced into a small reserve in Israel experienced a rate of increase of only 7.7% from 1978 to 2003 (Saltz, Reference Saltz1998; EPAA, 2003).
These data (the overall population trend, declining per capita birth rate, increasing mortality, declining recruitment at Shaumari) show a classic example of a population approaching and exceeding the carrying capacity of a small nature reserve. It is not certain, however, whether the carrying capacity was a function of the habitat quality or intrinsic density-dependent population controls, such as male aggression to each other and to calves, both of which were sources of mortality. The sharp drop in productivity after 1995 was reportedly in response to deteriorating browse availability within the Reserve (Boef, Reference Boef1996; Budieri, Reference Budieri1995). Although no quantitative measurements were made after 1990 this decline is consistent with the carrying capacity estimate of about 180 oryx (Hatough & Al-Eisawi, Reference Hatough and Al-Eisawi1988) based on the abundant vegetation and forage in 1986 (Al-Eisawi & Hatough, Reference Al-Eisawi and Hatough1987). Probably both extrinsic and intrinsic factors were involved. The inconsistent availability of professional wildlife expertise in several areas was another factor resulting in poor productivity in the early 1990s, not only of oryx, but of other species held at Shaumari (Budieri, Reference Budieri1995).
The gazelle population, for example, began with a founding population of 11 in 1980, increased by 12% per year to a high of 34 in 1990, and then declined to 14 in 1994 (L. Harding, unpubl. data). By 2003, the gazelle population had decreased to two, both males. Because their decline preceded that of the oryx by 5 years, it was probably not caused by a lack of forage that would have affected both species simultaneously. Floristic surveys in 1990 showed the vegetation to be abundant, diverse, and well developed structurally (Hatough-Bouran & Al-Eisawi, Reference Hatough-Bouran and Al-Eisawi1990). However, the herd's manager at the time thought that the gazelles failed to reproduce at higher densities because of improperly designed captive breeding pens, behaviour unsuited to captivity in pens, and high rates of mortality from diseases brought in by domestic livestock (Budieri, Reference Budieri1995). Sheep and goats are not allowed into the Reserve but commonly graze at its edge and occasionally get inside.
A small flock (c. 20 individuals) of ostriches is maintained at Shaumari. Ostriches feed on green annual grasses and forbs when available, otherwise leaves, flowers, and fruits from succulents and woody plants, and can be destructive to rangeland when confined and stocked at high densities (Milton et al., Reference Milton, Dean and Siegfried1994). Similarly, the few (<10) onagers at Shaumari may compete with the oryx for forage. Onagers, predominately grazers, browse a large portion of their diet during the dry season in drier habitats (Feh et al., Reference Feh, Shah, Rowen, Reading, Goyal and Moehlman2002). These other herbivores could have reduced the amount of forage available to the oryx.
There also may have been other causes of declining productivity in oryx, such as genetic inbreeding or outbreeding depression, both of which affected juvenile survival of oryx in Oman (Marshall & Spalton, Reference Marshall and Spalton2000). The genetics of the Shaumari herd have not been investigated but calf survival trends gave no evidence of such effects. The RSCN attempted to reduce genetic effects by obtaining its founders from two separate stocks but in later years was unable to obtain new genetic stock because of political instability in the region.
Although there were problems inside the reserve the most severe difficulty facing the RSCN was overgrazing outside. Jordan's arid rangelands have been overgrazed and subject to declining levels of rangeland productivity since the 1970s (Hatough et al., Reference Hatough, Al-Eisawi and Disi1986). The RSCN and its predecessor, the Royal Jordanian Hunting and Shooting Club, had been seeking to establish new, much larger reserves in the eastern desert since 1965, including one of 950 km2 at Burqu (Mountfort, Reference Mountfort1965; Clarke, Reference Clarke1979), a permanent water body near the Syria and Iraq borders (Fig. 1). During 1990–1991, however, Bedouins fleeing Kuwait and Iraq during the Gulf War brought 690,000 sheep and 580,000 goats into Jordan, approximately doubling the livestock population (Hashemite Kingdom of Jordan, 1991; Dutton, Reference Dutton, Dutton, Clarke and Battikhi1998; CC/EVS/ERM, 2002). After walking up to 1,000 km from Kuwait these animals were starving. They also had higher disease and parasite loads than local animals (Jones, Reference Jones1995; Kamhawi et al., Reference Kamhawi, Hijjawi, Abu-Gazaleh and Abbass1995; Allonby, Reference Allonby1996; Aldomy et al., Reference Aldomy, Wilsmore and Safi1997; Aldomy & Wilsmore, Reference Aldomy and Wilsmore1998) and were the probable source of at least one outbreak of Pasteurellosis among the oryx during 1990–1991. This livestock influx greatly increased the severity of overgrazing throughout the badia but especially around water sources such as Burqu. Burqu was drained dry in 1991 for the first time ever by excessive livestock watering (CC/EVS/ERM, 2002). Therefore, the RSCN was forced to defer the oryx release plans and the new reserve at Burqu was still ‘under establishment’ in 2000 (Budieri, Reference Budieri2000). These events also precluded the expansion of the Shaumari Nature Reserve from its current 22 km2 to the 342 km2 that had originally been allocated.
In 2005 the United Nations Compensation Commission recognized the environmental damages from the 1990 Gulf War and awarded Jordan compensation (UNCC, 2005). Some of these funds are to be directed for rejuvenation of the oryx reintroduction programme. With a renewed reintroduction programme, Jordan may begin releasing oryx into the wild within a few years, completing a project started 28 years ago. Notwithstanding its setbacks, Jordan's captive breeding programme produced enough surplus oryx for dispersal to other countries (Table 1), contributing to the increase in the world oryx population and its genetic heterogeneity. In 2003 there were c. 4,000 oryx outside zoos, a 13.5% increase compared to 2001 and a 38.4% increase compared to 2000 (Ostrowski & Anajariyah, Reference Ostrowski and Anajariyah2003).
Acknowledgments
Field investigations by LEH in 2002, 2003 and 2006 and participation in the United Nations Compensation Commission hearings in 2004 were supported by the Hashemite Kingdom of Jordan. Staff at Shaumari, including Aqel Abu-Hammd and Ahmad El Zoubi, assisted our work at the reserve. Information on current policies and conservation plans of the RSCN in 2002 was provided by Yahya Khaled, the Director of Conservation. We thank Adnan Budieri of Envirotech (Amman, Jordan) for information on the reintroduction programme, Majdi Salameh of the Integrated Management and Information Consultants Company, Amman, for translating portions of the oryx log and other materials from Arabic, and Mohammad Shahbaz of the Badia Research and Development Centre for guiding the Gulf War environmental assessment. We also thank the anonymous referees for their useful reviews.
Biographical sketches
Lee Harding is a wildlife biologist whose research interests include ungulates, large carnivores and fur bearers in tundra, temperate and arid ecosystems. Formerly with the Canadian Wildlife Service, he now owns and manages SciWrite Environmental Sciences Ltd. He led the team that assessed the damage to terrestrial and wetland ecosystems in Jordan caused by the 1990–1991 Gulf War. Omar Abu-Eid has been involved in the captive breeding programmes of the Royal Society for the Conservation of Nature (RSCN) in Amman since 1999. He is currently the Programme Assistant and the Environment Focal Point at the European Union delegation to the Hashemite Kingdom of Jordan. Nashat Hamidan has been an ecologist with RSCN since 2000 and is responsible for providing technical advice on management of the Arabian oryx herd at Shaumari. His research interests include fish, birds and reptiles in desert oasis ecosystems. Ahmad al Sha'lan began working at the Shaumari Nature Reserve in 1984 and has been the Reserve manager since 1998. His interests are in oryx and their management in captivity.












