Hyperemesis gravidarum (HG) is severe nausea and vomiting in pregnancy. HG can be complicated by dehydration, electrolyte disturbances, poor nutritional intake and weight loss(Reference Niebyl1). Vitamin deficiencies, including vitamin B1 deficiency, can further complicate HG, although little is known about the incidence and consequences of such deficiencies(Reference Oudman, Wijnia and Oey2).
The fact that vitamin K deficiency has been frequently described in chronic malnutrition makes it of possible interest in the context of HG(Reference Krzyżanowska, Książyk and Kocielińska-Kłos3,Reference Sherf-Dagan, Goldenshluger and Azran4) . Vitamin K is primarily obtained through dietary intake, but is also synthesised by bacteria in the large intestine(Reference Walther, Karl and Booth5). Although vitamin K is a fat soluble vitamin, the body’s stores of vitamin K are limited, and vitamin K can be depleted after metabolic surgery and in fat malabsorption syndromes(Reference Krzyżanowska, Książyk and Kocielińska-Kłos3,Reference Sherf-Dagan, Goldenshluger and Azran4,Reference Shearer and Newman6) . Vitamin K is important for coagulation, serving as a cofactor in the synthesis of multiple vitamin K-dependent proteins (factors II, VII, IX, X and protein C and S) in the intrinsic pathway(Reference Shearer7). Besides its effects on coagulation, vitamin K deficiency can also lead to abnormal calcium depositions and growth of cartilage(Reference Shearer and Newman6).
Vitamin K deficiency can cause a range of maternal and fetal complications. Maternal and neonatal coagulopathy-related haemorrhage has been described(Reference Menger, Lin and Toriello8,Reference Shearer9) as well as neonatal vitamin K deficiency embryopathy and grey matter heterotopia, most commonly described in the context of maternal vitamin K antagonist medication use(Reference Hall, Pauli and Wilson10,Reference Howe, Webster and Lipson11) . Vitamin K deficiency embryopathy includes Binder phenotype and chondrodysplasia punctata. Binder phenotype is the result of maxillonasal hypoplasia and causes a flat facial profile with a short nose and flat nasal bridge(Reference Binder12). Chondrodysplasia punctata is a skeletal abnormality classified by stippled calcifications of certain bones, most commonly toes, ankles or fingers(Reference Irving, Chitty and Mansour13). Short or misshapen bones can also be present, for example, short distal phalanges, also known as brachytelephalangy(Reference Howe, Webster and Lipson11). Vitamin K deficiency-related chondrodysplasia punctata should not be mistaken for the genetic form of chondrodysplasia punctata, which is caused by mutations in the X-linked arylsulfatase E (ARSE) gene and can be ruled out by genetic testing(Reference Irving, Chitty and Mansour13). Grey matter heterotopia is a neurological disorder classified by common malformations of cortical development, possibly caused by depletions in the vitamin K-dependent growth arrest-specific 6 protein which is widely expressed in the nervous system(Reference Alisi, Cao and De Angelis14–Reference Barkovich and Kuzniecky16).
The fact that HG has a profound impact on nutritional intake, sometimes necessitating enteral or parenteral nutrition, has raised concerns about the possibility that vitamin K deficiency can also occur in pregnancies complicated by HG(Reference Niebyl1,Reference Birkeland, Stokke and Tangvik17,Reference van Stuijvenberg, Schabort and Labadarios18) . Recently, the identification of the immediate and long-term effects of HG for pregnant women and their offspring were selected as urgent research questions by patients and health care professionals, which triggered the current work(Reference Dean, Bierma and Clarke19). In this systematic review, we aimed to summarise the available literature on HG-related maternal and neonatal vitamin K deficiency and determine the relevance of measuring vitamin K-related coagulopathy factors or prothrombin time (PT) in routine work-up for women with HG.
Methods
The study protocol was registered at the website of Prospero, an international prospective register of systematic reviews, on 17 August 2020 (CRD42020199501). This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Search strategy
We performed a search to identify all available studies reporting on vitamin K deficiency in women suffering from HG and their offspring. We searched Medline and Embase from inception to 12 November 2020. Our search included the following terms: ‘hyperemesis gravidarum’, ‘pregnancy sickness’, ‘vitamin K deficiency’, ‘embryopathy’, ‘haemorrhage’ and their synonyms, as shown in online Supplementary Appendix A. De-duplication of database search results was conducted using Endnote software(Reference Bramer, Giustini and de Jonge20). We also searched citation lists of eligible primary studies and reviews.
Study selection
Two reviewers (KN and LM) independently screened titles and abstracts. Conflicts were resolved by discussion until consensus was reached, or by consultation of a third reviewer (RP). All potentially relevant articles were retrieved as full text and assessed on the following inclusion and exclusion criteria. Inclusion criteria were: (1) women diagnosed with or admitted for HG with either (2) Maternal vitamin K deficiency or signs/symptoms of vitamin K deficiency (e.g. prolonged PT or signs of any type of haemorrhage) and/or (3) offspring of women with HG with vitamin K deficiency embryopathy or any type of vitamin K deficiency-related haemorrhage. Exclusion criteria were: (1) non-human subjects and (2) women with vitamin K deficiency due to any other cause than HG. We included observational studies, case reports, case series and research letters. Conference abstracts were included, if they provided sufficient information. We did not apply any language restrictions.
Data extraction
Data extraction was performed independently by two reviewers (KN and LM). We extracted data on study characteristics, demographics, details about pregnancy and specifically about the severity and clinical course of HG (if available), laboratory results (including PT, coagulation factors and vitamin K measurements) and both maternal and neonatal outcomes (vitamin K deficiency-related haemorrhage or embryopathy).
Quality assessment
We assessed the risk of bias of included case reports using the Joanna Briggs Institute checklist for case reports and the Newcastle-Ottawa Scale for included cohort studies(Reference Moola, Munn and Tufanaru21,Reference Wells, Shea and O’Connell22) . The Newcastle-Ottawa Scale assigns up to a total maximum score of 9 based on eight items: a score ≥ 7 was considered as good quality, a score ≥ 5 as fair quality and a score ≤ 4 as poor quality(Reference Wells, Shea and O’Connell22). All included articles were critically appraised and were included, despite of their quality assessment.
Statistical analysis
Data of included case reports were combined by entering available information on baseline characteristics and outcome measures of each reported case of women with HG or their offspring into a SPSS database (SPSS Statistics, version 26.0 for Windows, IBM Corp). If a case report included multiple HG patients or multiple HG-exposed offspring, all of the cases were entered separately. Continuous data were presented as means and standard deviations if they were normally distributed. Not normally distributed continuous data were presented as medians with interquartile ranges. Dichotomous and categorical data were displayed as frequencies with percentages.
Results
Search results
We identified 1741 articles and one additional article through searching citation lists as shown in Fig. 1. After removing duplicates, 1564 articles remained for title and abstract screening, of which thirty-six were deemed possibly eligible. Upon further eligibility screening after full texts for possibly eligible papers had been retrieved, we included fifteen articles reporting on HG and vitamin K deficiency(Reference Alessandri, Ramful and Cuillier23–Reference Toriello, Erick and Alessandri37). Fourteen of the included studies were case reports(Reference Alessandri, Ramful and Cuillier23–Reference Brunetti-Pierri, Hunter and Boerkoel27,Reference Devignes, Grare and Raft29–Reference Toriello, Erick and Alessandri37) and we included one retrospective cohort study(Reference Chraïbi, Ouldamer and Body28). Two of the included studies were conference abstracts(Reference Miller, Mostafavi and Mroczkowski33,Reference Selvarajah, Gupta and Deo35) and two additional included studies were written in French(Reference Chraïbi, Ouldamer and Body28,Reference Devignes, Grare and Raft29) .
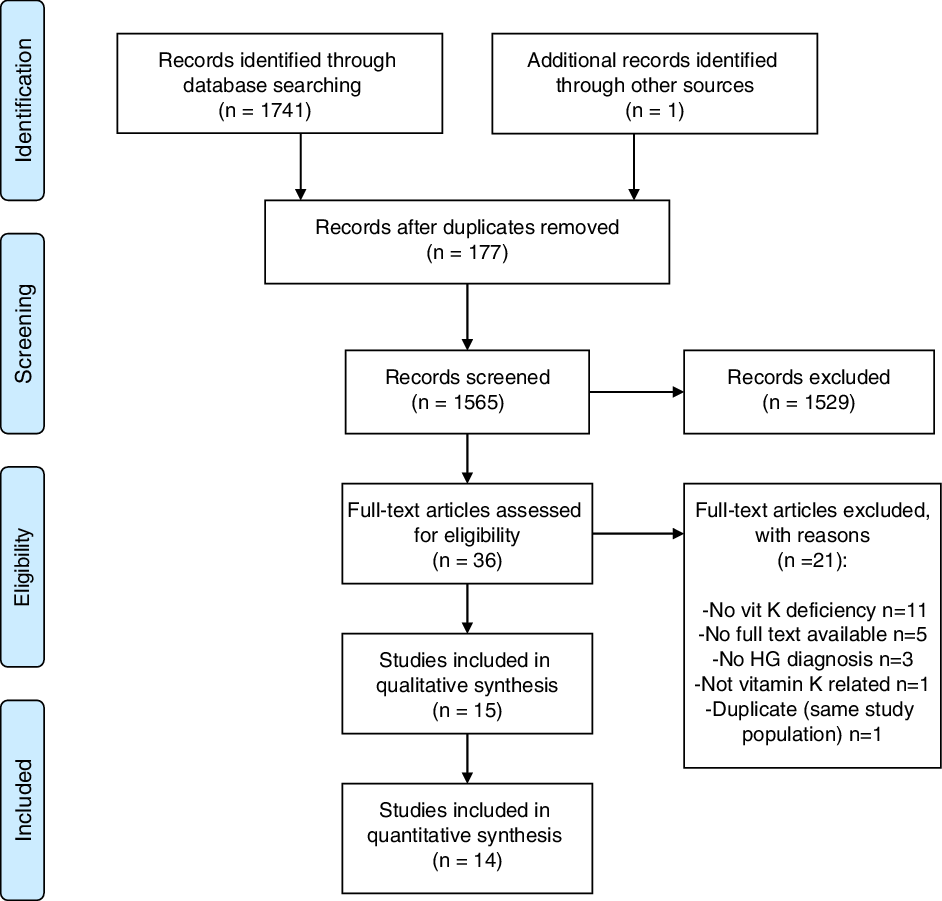
Fig. 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram: selection process of articles.
Two case reports included multiple cases: Miller et al.(Reference Miller, Mostafavi and Mroczkowski33) included three cases and Toriello et al.(Reference Toriello, Erick and Alessandri37) included eight cases. From the eight cases of Toriello et al.(Reference Toriello, Erick and Alessandri37), case 8 was excluded for this review since vitamin K deficiency was caused by Crohn’s disease instead of HG. Case 1 of Toriello et al.(Reference Toriello, Erick and Alessandri37) was identical to the included case report of Robinson et al.(Reference Robinson, Banerjee and Thiet34), but contained follow-up information of the neonate, so we combined data of these two case reports.
Risk of bias assessment
The risk of bias assessment of case reports is shown in Fig. 2. For most domains, case reports were assessed as low risk of bias. However, in half of the studies a patient’s medical history was not or poorly described. In addition, in almost half of the studies which reported a treatment, the treatment was not clearly described in terms of dosage or frequency and therefore was rated as having a high risk of bias. The cohort study was rated to be of fair quality, as shown in Table 1.
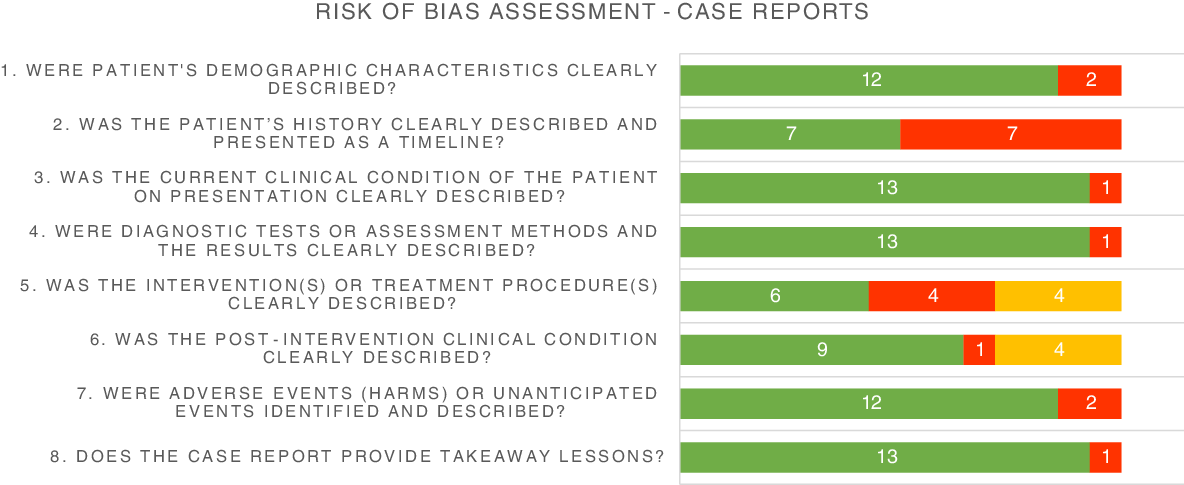
Fig. 2. Risk of bias assessment of included case reports. ![]() , low;
, low; ![]() , high;
, high; ![]() , not applicable.
, not applicable.
Table 1. Risk of bias assessment of the included cohort study using the Newcastle-Ottawa Quality Assessment Scale (NOS)

The NOS risk consisted of eight items with a total maximum score of 9. A score ≥ 7 was considered as good quality, a score ≥ 5 as fair quality and a score ≤ 4 as poor quality.
Baseline characteristics
Baseline characteristics of all included studies are shown in Table 2 and data of the included women from case reports were combined and shown in Table 3. In seventeen women, the gestational age of onset of symptoms was reported; in the vast majority (16/17) symptoms of HG had started in the first trimester (mean 8·47 (sd 3·16) weeks) (Tables 2 and 3). Ten articles (n 14 women) reported whether weight loss during pregnancy due to HG had occurred: thirteen out of fourteen women had some degree of weight loss, ranging from 5 to 28 kg with an average weight loss of 13·64 (sd 8·03) kg compared with 5·6 (sd 3·1) kg weight loss reported in the cohort study(Reference Alessandri, Ramful and Cuillier23,Reference Baba, Morisawa and Saito24,Reference Brunetti-Pierri, Hunter and Boerkoel27–Reference Lane, Stallworth and Eichelberger32,Reference Robinson, Banerjee and Thiet34,Reference Selvarajah, Gupta and Deo35,Reference Toriello, Erick and Alessandri37) . Nine out of twenty-one women of included case reports had more than 10 kg weight loss(Reference Alessandri, Ramful and Cuillier23,Reference Brunetti-Pierri, Hunter and Boerkoel27,Reference Devignes, Grare and Raft29,Reference Lane, Stallworth and Eichelberger32,Reference Robinson, Banerjee and Thiet34,Reference Toriello, Erick and Alessandri37) .
Table 2. Baseline characteristics of included studies
(Means and standard deviations)
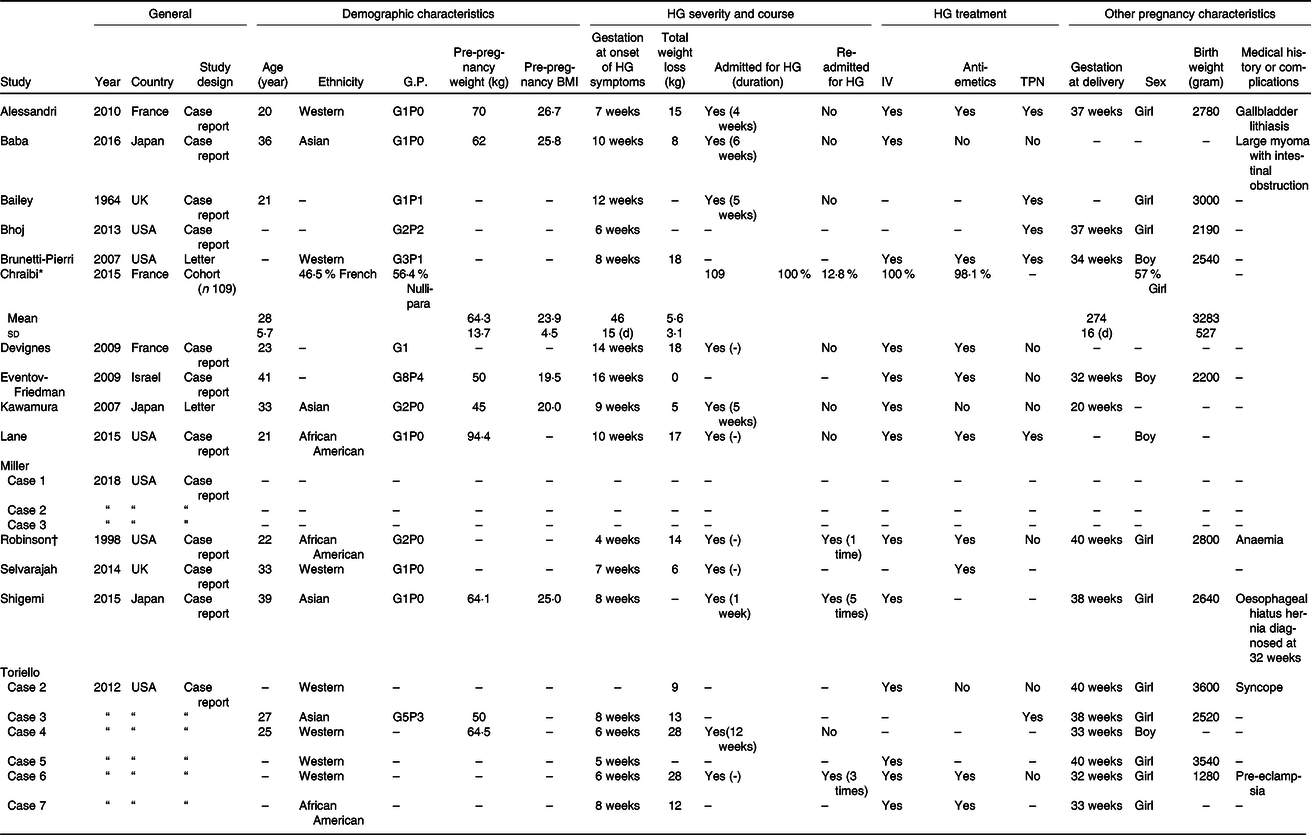
GP, gravidity parity; IV, intravenous; TPN, total parenteral nutrition; UK, United Kingdom; USA, United States of America.
* Cohort study: characteristics presented as means and standard deviations, median (IQR) or frequency (%).
† Case of Robinson et al. is the same case as case 1 of Toriello et al., so available data are combined.
Table 3. Combined baseline characteristics of included case reports in this systematic review
(Numbers and percentages; median and interquartile ranges)
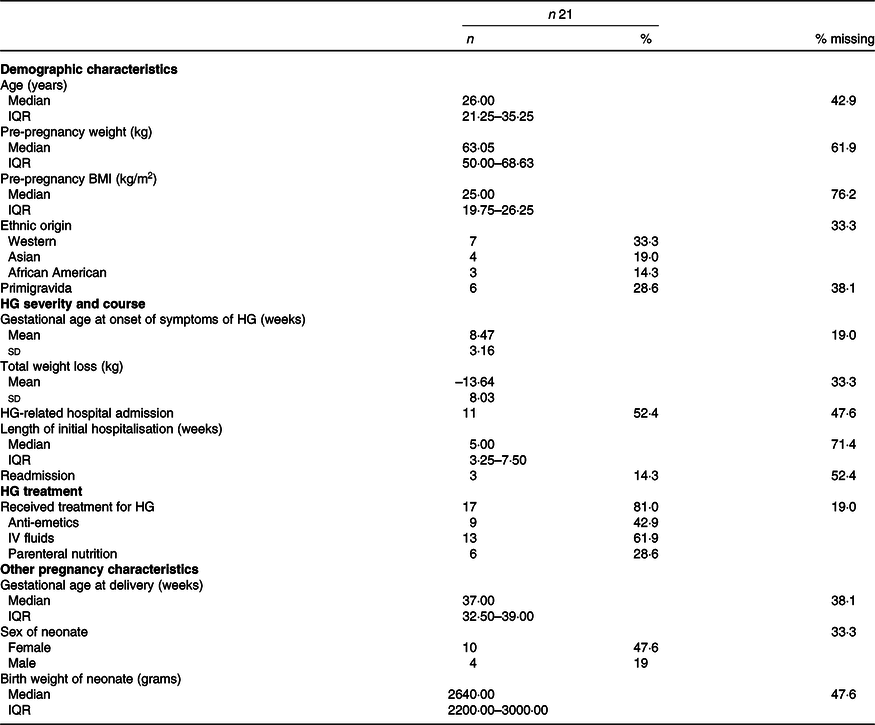
HG, hyperemesis gravidarum; IV, intravenous. Normally distributed continuous variables are presented as means and standard deviations, skewed variables as medians with interquartile ranges (IQR) and dichotomous or categorical variables as frequencies with percentages (%).
In all three cases of Miller et al.(Reference Miller, Mostafavi and Mroczkowski33) and in case 4 of Toriello et al.(Reference Toriello, Erick and Alessandri37) treatment for HG was not described (Table 2). All other seventeen included women of remaining case reports received some form of treatment for HG(Reference Alessandri, Ramful and Cuillier23–Reference Brunetti-Pierri, Hunter and Boerkoel27,Reference Devignes, Grare and Raft29–Reference Lane, Stallworth and Eichelberger32,Reference Robinson, Banerjee and Thiet34–Reference Toriello, Erick and Alessandri37) , varying from receiving anti-emetics (9/17)(Reference Alessandri, Ramful and Cuillier23,Reference Brunetti-Pierri, Hunter and Boerkoel27,Reference Devignes, Grare and Raft29,Reference Eventov-Friedman, Klinger and Shinwell30,Reference Lane, Stallworth and Eichelberger32,Reference Robinson, Banerjee and Thiet34,Reference Selvarajah, Gupta and Deo35,Reference Toriello, Erick and Alessandri37) , intravenous rehydration (13/17)(Reference Alessandri, Ramful and Cuillier23,Reference Baba, Morisawa and Saito24,Reference Brunetti-Pierri, Hunter and Boerkoel27,Reference Devignes, Grare and Raft29–Reference Lane, Stallworth and Eichelberger32,Reference Robinson, Banerjee and Thiet34,Reference Shigemi, Nakanishi and Miyazaki36,Reference Toriello, Erick and Alessandri37) to receiving tube feeding (6/17)(Reference Alessandri, Ramful and Cuillier23,Reference Bailey25–Reference Brunetti-Pierri, Hunter and Boerkoel27,Reference Lane, Stallworth and Eichelberger32,Reference Toriello, Erick and Alessandri37) . Chraïbi et al.(Reference Chraïbi, Ouldamer and Body28) described a cohort of women admitted for HG: all 109 included women (100 %) received intravenous treatment and 106 women (98·1 %) received at least one anti-emetic (Table 2). From the twenty-one women included from case reports, eleven women had been admitted for HG (Table 3)(Reference Alessandri, Ramful and Cuillier23–Reference Bhoj, Dubbs and McDonald-McGinn26,Reference Devignes, Grare and Raft29,Reference Kawamura, Kawamata and Shinya31,Reference Lane, Stallworth and Eichelberger32,Reference Robinson, Banerjee and Thiet34–Reference Toriello, Erick and Alessandri37) .
Vitamin K deficiency diagnosis
In half of the case reports, a vitamin K deficiency diagnosis was made retrospectively based on neonatal clinical signs of embryopathy(Reference Bhoj, Dubbs and McDonald-McGinn26,Reference Brunetti-Pierri, Hunter and Boerkoel27,Reference Lane, Stallworth and Eichelberger32,Reference Miller, Mostafavi and Mroczkowski33,Reference Toriello, Erick and Alessandri37) . The other half performed laboratory measurements to confirm vitamin K deficiency. PT was most commonly used and prolonged PT was reported as prolonged PT in seconds or as decreased prothrombin levels. PT was measured in nine out of twenty-one women included in case reports: 8/21 women (38·1 %) had a prolonged PT (Tables 4 and 5)(Reference Alessandri, Ramful and Cuillier23–Reference Bailey25,Reference Devignes, Grare and Raft29,Reference Kawamura, Kawamata and Shinya31,Reference Robinson, Banerjee and Thiet34,Reference Shigemi, Nakanishi and Miyazaki36,Reference Toriello, Erick and Alessandri37) . In four out of nine women, PT was measured secondary to maternal signs of haemorrhage(Reference Baba, Morisawa and Saito24,Reference Bailey25,Reference Devignes, Grare and Raft29,Reference Robinson, Banerjee and Thiet34) . In the other five cases, PT was included in routine laboratory measurements, without the presence of clinical signs of maternal or fetal haemorrhage or embryopathy(Reference Alessandri, Ramful and Cuillier23,Reference Eventov-Friedman, Klinger and Shinwell30,Reference Kawamura, Kawamata and Shinya31,Reference Shigemi, Nakanishi and Miyazaki36,Reference Toriello, Erick and Alessandri37) .
Table 4. Maternal and neonatal outcomes of included studies
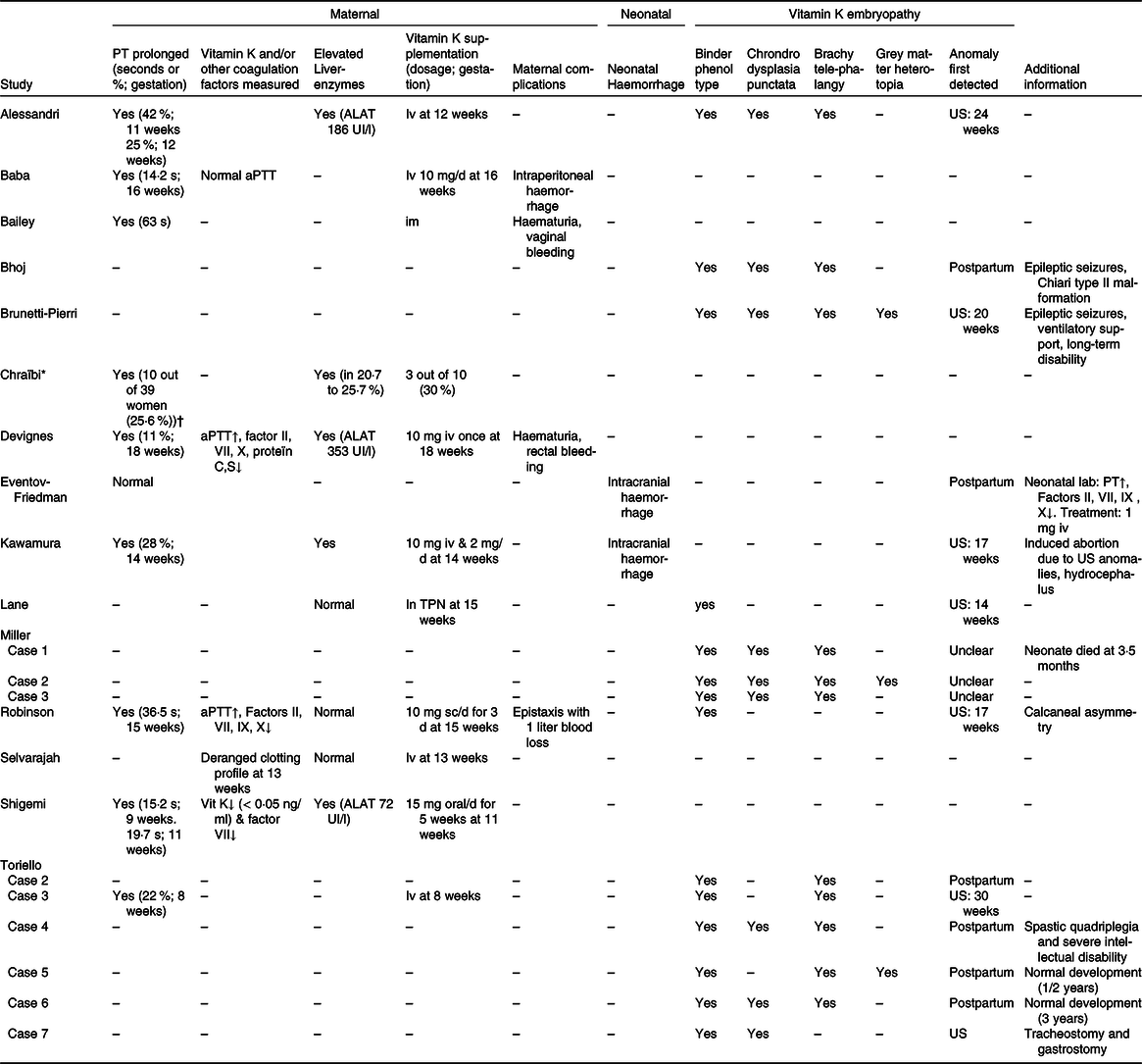
PT, prothrombin time; US, ultrasound (perinatal); aPTT, activated partial thromboplastin time; TPN, total parenteral nutrition.
* Cohort study: data presented as frequencies/percentages.
† PT measured in 39/109 women.
Table 5. Combined outcomes of included case reports in this systematic review
(Numbers and percentages; median and interquartile ranges)
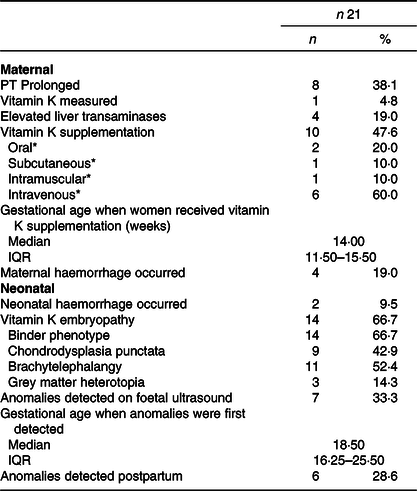
PT, prothrombin time. Skewed variables are presented as medians with interquartile ranges (IQR) and dichotomous or categorical variables as frequencies with percentages (%).
* Percentage shown is percentage of women who received vitamin K supplementation.
Four case reports performed additional coagulopathy laboratory measurements. Three case reports measured activated partial thromboplastin time(Reference Baba, Morisawa and Saito24,Reference Devignes, Grare and Raft29,Reference Robinson, Banerjee and Thiet34) . Two of them found a prolonged activated partial thromboplastin time, but also found a decreased factors II, VII, IX, X and protein C and S, which are vitamin K-dependent coagulation factors(Reference Devignes, Grare and Raft29,Reference Robinson, Banerjee and Thiet34) (Table 4). The fourth study was the only study that measured vitamin K concentrations in addition to PT and that found vitamin K deficiency (below 0·05 ng/ml)(Reference Shigemi, Nakanishi and Miyazaki36). Selvarajah et al.(Reference Selvarajah, Gupta and Deo35) mentioned that the woman included had a deranged clotting profile, but did not further specify which laboratory measurements were performed (Table 4).
In one neonate coagulation, factors were measured postpartum because of low Apgar scores together with signs of haemorrhage: first a haematoma in the hand palm and later intracranial haemorrhage. A prolonged PT together with a decreased factors II, VII, IX and X was found(Reference Eventov-Friedman, Klinger and Shinwell30).
In the cohort study from Chraïbi et al.(Reference Chraïbi, Ouldamer and Body28), PT was measured in thirty-nine out of 109 women (35·8 %) admitted for HG: ten out of these thirty-nine women (25·6 %) had a prolonged PT with a level below 70 % and two out of these ten women (5·1 %) had a PT level below 50 % (Table 5). The cohort study did not describe why PT was initially measured or whether other coagulation factors were measured(Reference Chraïbi, Ouldamer and Body28).
Vitamin K supplementation
Vitamin K was supplemented in all case reports reporting a prolonged PT (n 8 women and n 1 neonate) and in one women described to had a ‘deranged clotting profile’ (Table 4)(Reference Alessandri, Ramful and Cuillier23–Reference Bailey25,Reference Devignes, Grare and Raft29–Reference Kawamura, Kawamata and Shinya31,Reference Robinson, Banerjee and Thiet34,Reference Shigemi, Nakanishi and Miyazaki36,Reference Toriello, Erick and Alessandri37) . One additional woman received vitamin K as part of parenteral nutrition, so in total ten out of twenty-one (47·6 %) women and one neonate received vitamin K supplementation as shown in Table 5 (Reference Alessandri, Ramful and Cuillier23–Reference Bailey25,Reference Devignes, Grare and Raft29–Reference Lane, Stallworth and Eichelberger32,Reference Robinson, Banerjee and Thiet34,Reference Shigemi, Nakanishi and Miyazaki36,Reference Toriello, Erick and Alessandri37) . Vitamin K was administered by different routes, but most women (60·0 %) and the described neonate received intravenous vitamin K supplementation (Table 5)(Reference Alessandri, Ramful and Cuillier23,Reference Baba, Morisawa and Saito24,Reference Devignes, Grare and Raft29–Reference Kawamura, Kawamata and Shinya31,Reference Selvarajah, Gupta and Deo35,Reference Toriello, Erick and Alessandri37) . In all of them, PT normalised after vitamin K supplementation(Reference Alessandri, Ramful and Cuillier23–Reference Bailey25,Reference Devignes, Grare and Raft29–Reference Kawamura, Kawamata and Shinya31,Reference Robinson, Banerjee and Thiet34,Reference Shigemi, Nakanishi and Miyazaki36,Reference Toriello, Erick and Alessandri37) .
In the cohort study of Chraïbi et al.(Reference Chraïbi, Ouldamer and Body28) three out of ten women with a prolonged PT (level below 70 %) received vitamin K, which was not further specified in route of administration, dosage or frequency (Table 4).
Liver function measurements
Liver transaminases tests were performed in seven out of twenty-one women included in case reports of whom four women (19·0 %) had elevated liver transaminases (Tables 4 and 5)(Reference Alessandri, Ramful and Cuillier23,Reference Devignes, Grare and Raft29,Reference Kawamura, Kawamata and Shinya31,Reference Shigemi, Nakanishi and Miyazaki36) . Three out of these four women also had elevated total bilirubin levels and two women had elevated gamma glutamyl transferase levels.
As shown in Table 4, Chraïbi et al.(Reference Chraïbi, Ouldamer and Body28) reported elevated alanine transaminase and aspartate aminotransferase in respectively 20·7 and 25·7 %. PT levels were significantly lower in women with an increased alanine transaminase than in women with normal alanine transaminase levels (68 (sd 14) % v. 78 (sd 9) %).
Maternal complications due to hyperemesis gravidarum-related vitamin K deficiency
We identified four studies, including four women, that reported on maternal complications due to HG-related vitamin K deficiency. All four studies reported coagulopathy-related haemorrhage (Tables 4 and 5). Two women had mild haemorrhage symptoms, not in the context of their delivery, consisting of haematuria, bruising and/or vaginal or rectal bleeding(Reference Bailey25,Reference Devignes, Grare and Raft29) . Two other studies reported more severe cases of haemorrhage. Robinson et al.(Reference Robinson, Banerjee and Thiet34) described a case of severe epistaxis with one litre blood loss, which was initially treated with topical silver nitrate and after the diagnosis of vitamin K deficiency was made vitamin K was supplemented. Baba et al.(Reference Baba, Morisawa and Saito24) described a case of a woman with HG who developed intraperitoneal haemorrhage due to a pedunculated myoma, which was operatively resected at 16 weeks gestation. In total, perioperative blood loss contained 290 ml of which 110 ml intraperitoneal blood loss was noted at the start of the operation. Postoperative laboratory results revealed coagulopathy based on a prolonged PT with a normal activated partial thromboplastin time and international normalised ratio. Coagulopathy was strongly suspected to be secondary to vitamin K deficiency, since PT normalised after intravenous supplementation of vitamin K, and the amount of blood loss was thought to be insufficient to induce secondary coagulopathy.
Neonatal complications due to hyperemesis gravidarum-related vitamin K deficiency
Nine studies reported neonatal complications due to HG-related vitamin K deficiency(Reference Alessandri, Ramful and Cuillier23,Reference Bhoj, Dubbs and McDonald-McGinn26,Reference Brunetti-Pierri, Hunter and Boerkoel27,Reference Eventov-Friedman, Klinger and Shinwell30–Reference Robinson, Banerjee and Thiet34,Reference Toriello, Erick and Alessandri37) . Two case reports, including two neonates, reported neonatal intracranial haemorrhage(Reference Eventov-Friedman, Klinger and Shinwell30,Reference Kawamura, Kawamata and Shinya31) and seven case reports, including fourteen neonates, reported neonatal embryopathy as shown in Tables 4 and 5 (Reference Alessandri, Ramful and Cuillier23,Reference Bhoj, Dubbs and McDonald-McGinn26,Reference Brunetti-Pierri, Hunter and Boerkoel27,Reference Lane, Stallworth and Eichelberger32–Reference Robinson, Banerjee and Thiet34,Reference Toriello, Erick and Alessandri37) .
Neonatal intracranial haemorrhage
Two studies reported neonatal intracranial haemorrhage (Table 5). Kawamura et al.(Reference Kawamura, Kawamata and Shinya31) described a case where fetal intracranial haemorrhage accompanied by hydrocephalus was detected during the mid-trimester ultrasound at 17 weeks gestation. Due to these fetal anomalies, the woman decided to terminate her pregnancy. Autopsy showed a subarachnoid haemorrhage with haemosiderin deposits to the choroid plexus near the foramen of Luschka and on the surface of the brainstem which blocked the pathway of cerebrospinal fluid absorption and subsequently lead to a non-obstructive hydrocephalus. No evidence of chromosomal abnormalities was found, and a diagnosis of a Dandy–Walker syndrome was rejected because of the presence of a non-obstructive hydrocephalus.
Eventov-Friedman et al.(Reference Eventov-Friedman, Klinger and Shinwell30) also reported a case of neonatal intracranial haemorrhage, which was diagnosed postpartum (Table 4). An emergency caesarean was performed at 32 weeks gestation due to suspected fetal distress. The neonate had an Apgar score of 1, 1 and 3, after respectively 1, 5 and 10 min. A cranial ultrasound revealed extensive intracranial haemorrhage and neonatal coagulopathy laboratory results confirmed a vitamin K deficiency. A cranial computed tomography on day two postpartum showed no midline shift and therefore the infant was managed conservatively. The neonate developed recurrent seizures which was treated with phenobarbital. No further neonatal long-term outcomes were described.
Neonatal vitamin K-related embryopathy
From the fourteen neonates diagnosed with vitamin K-related embryopathy in the studies included in our review, all neonates had Binder phenotype, nine neonates also had chondrodysplasia punctata of whom three also suffered from grey matter heterotopia as shown in Table 5 (Reference Alessandri, Ramful and Cuillier23,Reference Bhoj, Dubbs and McDonald-McGinn26,Reference Brunetti-Pierri, Hunter and Boerkoel27,Reference Lane, Stallworth and Eichelberger32–Reference Robinson, Banerjee and Thiet34,Reference Toriello, Erick and Alessandri37) . Also brachytelephalangy was noted in eleven out of fourteen neonates with vitamin K-related embryopathy(Reference Alessandri, Ramful and Cuillier23,Reference Bhoj, Dubbs and McDonald-McGinn26,Reference Brunetti-Pierri, Hunter and Boerkoel27,Reference Miller, Mostafavi and Mroczkowski33,Reference Toriello, Erick and Alessandri37) . Genetic testing was performed in nine out of fourteen neonates, none of which found genetic abnormalities(Reference Alessandri, Ramful and Cuillier23,Reference Bhoj, Dubbs and McDonald-McGinn26,Reference Brunetti-Pierri, Hunter and Boerkoel27,Reference Lane, Stallworth and Eichelberger32,Reference Robinson, Banerjee and Thiet34,Reference Toriello, Erick and Alessandri37) . Three studies specifically described that no mutations in the ARSE gene were found(Reference Bhoj, Dubbs and McDonald-McGinn26,Reference Brunetti-Pierri, Hunter and Boerkoel27,Reference Toriello, Erick and Alessandri37) .
Anomalies detected and timing of vitamin K supplementation
Of the ten women who received vitamin K supplementation, five cases had neonatal complications(Reference Alessandri, Ramful and Cuillier23,Reference Kawamura, Kawamata and Shinya31,Reference Lane, Stallworth and Eichelberger32,Reference Robinson, Banerjee and Thiet34,Reference Toriello, Erick and Alessandri37) . Four cases had neonatal vitamin K deficiency-related embryopathy(Reference Alessandri, Ramful and Cuillier23,Reference Lane, Stallworth and Eichelberger32,Reference Robinson, Banerjee and Thiet34,Reference Toriello, Erick and Alessandri37) and one case had intracranial haemorrhage(Reference Kawamura, Kawamata and Shinya31). As shown in Table 4, in Alessandri et al.(Reference Alessandri, Ramful and Cuillier23), Kawamura et al.(Reference Kawamura, Kawamata and Shinya31), Robinson et al. (Reference Robinson, Banerjee and Thiet34) and case 3 of Toriello et al.(Reference Toriello, Erick and Alessandri37) vitamin K supplementation was started before fetal anomalies were detected on perinatal ultrasound. Here, PT was measured on maternal indication or during routine maternal laboratory measurements and subsequently vitamin K was supplemented at respectively 12, 14, 15 and 8 weeks gestation. In Lane et al.(Reference Lane, Stallworth and Eichelberger32) vitamin K was administered after fetal anomalies were detected on perinatal ultrasound. Vitamin K was included in parenteral nutrition which was started at 15 weeks gestation. The median gestational age when vitamin K supplementation was commenced was 14 weeks (interquartile range 12–16) compared with the median gestational age of 19 weeks (interquartile range 16–26) when fetal anomalies were detected on perinatal ultrasound (Table 5).
Neonatal prognosis
Eleven out of twenty-one neonates had been given a good prognosis by the paediatrician during follow-up visits(Reference Alessandri, Ramful and Cuillier23,Reference Baba, Morisawa and Saito24,Reference Bhoj, Dubbs and McDonald-McGinn26,Reference Devignes, Grare and Raft29,Reference Lane, Stallworth and Eichelberger32,Reference Shigemi, Nakanishi and Miyazaki36,Reference Toriello, Erick and Alessandri37) . One neonate described in Miller et al.(Reference Miller, Mostafavi and Mroczkowski33) died at 3·5 months: she had a severe nasal aperture stenosis, critical cervical spinal stenosis and myelomalacia of the upper cervical cord (Table 4). Two neonates were described as having a poor prognosis(Reference Brunetti-Pierri, Hunter and Boerkoel27,Reference Toriello, Erick and Alessandri37) . One of these neonates suffered from long-term disability due to ventilatory support dependence and severe neurodevelopmental delay(Reference Brunetti-Pierri, Hunter and Boerkoel27). While the other neonate described in case 4 of Toriello et al.(Reference Toriello, Erick and Alessandri37) suffered from severe intellectual disability and spastic quadriplegia following spinal surgery because of severe cervical spinal stenosis (Table 4)(Reference Brunetti-Pierri, Hunter and Boerkoel27). Two neonates described in Bhoj et al.(Reference Bhoj, Dubbs and McDonald-McGinn26) and case 6 of Toriello et al.(Reference Toriello, Erick and Alessandri37) had a mild delay in neurodevelopment.
Discussion
Principal findings
In this systematic review, which identified fifteen articles, we found evidence that vitamin K deficiency secondary to HG can lead to severe adverse maternal and neonatal outcomes. Our review highlights the fact that HG, usually considered a benign and self-limiting condition of early pregnancy, can lead to irreversible morbidity and mortality, and therefore deserves the prompt attention of clinicians to avoid these sequelae. Although selective reporting likely has affected our findings, two-thirds of the neonates included in the case reports suffered from vitamin K embryopathy, making it the most commonly reported vitamin K deficiency-related complication among women with HG, followed by maternal haemorrhage (19 %) and neonatal haemorrhage (10 %). A further 26–38 % of cases showed evidence of disturbed maternal coagulation due to vitamin K deficiency, with 30–48 % receiving vitamin K supplementation.
Strengths and limitations of the study
One of the main strengths of this study is that it presents an overview of a rare complication, and summarises the evidence on vitamin K deficiency in women with HG and their offspring. Besides case reports, research letters and conference abstracts, we were also able to include one cohort study. We did not apply a date or language restriction, which avoided selective inclusion of English language literature. Lastly, all articles included were critically appraised and were rated as low to moderate bias.
Our study also has some limitations. Although we were able to include one cohort study, the remainder of the included studies were case reports. Case reports are subject to publication bias and could result in a bias towards the increased reporting of more unfavourable outcomes. The fact that our review only recovered case reports and one cohort study hampers estimation of the incidence of vitamin K deficiency among women with HG. Furthermore, the case reports suffered from incomplete reporting of data essential to our review, which compromised our ability to link indicators of the severity or course of HG to maternal, fetal and neonatal outcomes in many studies; some articles focused primarily on the course of HG and maternal complications, while other case reports focused more on neonatal complications and did not report extensive details of HG. In addition, direct measures of vitamin K deficiency, for example, PT, were only reported in 43 % of included women, which hampered our ability to determine timing of maternal vitamin K depletion and its relation to fetal and neonatal outcomes in many cases.
Interpretation
Due to the fact that our review included mostly case reports, we are not able to estimate the incidence of vitamin K deficiency among women with HG. In the included cohort study however, ten out of thirty-nine women (26 %) had a prolonged PT, suggesting that the presence of vitamin K deficiency may be more common among women suffering from HG than currently recognised(Reference Chraïbi, Ouldamer and Body28). However, the fact that PT was only measured in thirty-nine out of the 109 women in the cohort raises the possibility of this percentage only being representative of a selected group of more severely affected patients. Unfortunately, we are uninformed about the severity of HG in these specific thirty-nine cases. Unlike the included case reports, the cohort study reported no further vitamin K deficiency complications, suggesting that only a small proportion of cases of vitamin K deficiency lead to complications including haemorrhage and embryopathy. A larger prospective cohort study measuring vitamin K deficiency in women with HG could determine the true incidence of both phenomena. The fact that this systematic review found mostly studies reporting on neonatal complications (nine studies) instead of maternal complications (four studies), could be explained by the given that only very little vitamin K crosses the placenta from mother to fetus, which can decrease even more when there is maternal vitamin K deficiency. This would suggest that the fetus is more at risk to develop a more severe vitamin K deficiency and corresponding complications than mother(Reference Shearer, Rahim and Barkhan38,Reference Greer39) .
It is hypothesised that in women with HG vitamin K deficiency is caused by poor nutritional intake, as is evident from marked weight loss. Most women in the included case reports had severe weight loss, with a mean weight loss of 13·6 kg. In examining the association between the severity of weight loss and presence of vitamin K deficiency-induced complications, we found that in three cases reporting maternal haemorrhage, the maternal weight loss varied from 8 to 18 kg(Reference Baba, Morisawa and Saito24,Reference Devignes, Grare and Raft29,Reference Robinson, Banerjee and Thiet34) . In two included cases where neonates had long-term disabilities, the maternal weight loss due to HG was respectively 18 and 28 kg(Reference Brunetti-Pierri, Hunter and Boerkoel27,Reference Toriello, Erick and Alessandri37) . The woman who lost 28 kg was also admitted to the hospital for 12 weeks in total(Reference Toriello, Erick and Alessandri37). The mean weight loss of 5·6 (sd 3·1) kg in women with HG included in Chraïbi et al.(Reference Chraïbi, Ouldamer and Body28), but also in other HG cohort studies(Reference Grooten, Koot and van der Post40,Reference Stokke, Gjelsvik and Flaatten41) , was considerably lower and they did not report any vitamin K deficiency-related complications This may suggest that a more severe clinical course of HG causes more severe malnutrition which can in line lead to an increased risk of developing vitamin K deficiency and related complications.
Embryopathy is also described in neonates born to women using warfarin, a vitamin K antagonist, during pregnancy, better known as the fetal warfarin syndrome(Reference Hall, Pauli and Wilson10). Studies assessing the fetal warfarin syndrome showed that mainly first trimester deficiency of vitamin K results in embryopathy(Reference Howe and Webster42,Reference Chan, Anand and Ginsberg43) and that warfarin use throughout every trimester of pregnancy can result in neonatal central nervous system abnormalities(Reference Hall, Pauli and Wilson10,Reference Duhl, Paidas and Ural44) . This corresponds to the onset, duration and severity of HG and its relation to neonatal complications reported in included case reports. In all cases reporting embryopathy, the onset of HG lay in the first trimester and the two neonates described to have long-term disabilities were born to mothers with a severe HG with a prolonged disease course(Reference Brunetti-Pierri, Hunter and Boerkoel27,Reference Toriello, Erick and Alessandri37) .
The optimal timing of measuring vitamin K deficiency though is difficult to define. When maternal haemorrhage complications occurred, laboratory tests were performed at the time and vitamin K deficiency was then diagnosed and subsequently supplemented(Reference Baba, Morisawa and Saito24,Reference Bailey25,Reference Devignes, Grare and Raft29,Reference Robinson, Banerjee and Thiet34) . In case reports describing neonatal embryopathy however, in the majority of the cases fetal anomalies were found on antenatal ultrasonography, despite earlier treatment with vitamin K. Since the origin of neonatal embryopathy lays in the first trimester and vitamin K supplementation took place primarily in the second trimester, the most likely explanation for this would be that vitamin K was supplemented too late and that fetal anomalies were already present at the time of vitamin K treatment. Bearing this in mind, a solution would be to prophylactically administer vitamin K in women with HG, which has been proposed in previous studies(Reference Alessandri, Ramful and Cuillier23,Reference Baba, Morisawa and Saito24,Reference Brunetti-Pierri, Hunter and Boerkoel27,Reference Eventov-Friedman, Klinger and Shinwell30,Reference Kawamura, Kawamata and Shinya31,Reference Miller, Mostafavi and Mroczkowski33–Reference Shigemi, Nakanishi and Miyazaki36) . Most of these studies suggested that prophylactic treatment should be given in women with severe HG, undernutrition or severe weight loss but do not further specify this(Reference Alessandri, Ramful and Cuillier23,Reference Baba, Morisawa and Saito24,Reference Selvarajah, Gupta and Deo35) . On the contrary, the Royal College of Obstetricians and Gynaecologists advices that women admitted with HG should be offered thromboprophylaxis because of an increased risk of venous thromboembolism. This might make caregivers reluctant to follow that advice, although it is important to clarify that vitamin K supplementation does not increase the risk of venous thromboembolic complications(45).
HG is known to be associated with raised transaminases and can lead to liver dysfunction. Nonetheless, we think it is unlikely that liver dysfunction due to HG led to increased PT described in a number of articles. This is illustrated by the fact that the four case reports to measure liver transaminases found universally raised PT, which promptly resolved after vitamin K supplementation.
Conclusion
In this systematic review, we have demonstrated that women with HG can develop vitamin K deficiency and the corresponding maternal and neonatal complications. We were not able to derive the incidence among women with HG from the studies we retrieved, but found evidence vitamin K deficiency could affect up to 26 % of HG patients. Which aspects of HG severity or disease course increase the risk of vitamin K deficiency remains unclear; severe weight loss and prolonged disease did appear to be common factors in affected HG patients and may therefore present risk factors. Larger prospective cohort studies of women with HG are needed to assess the incidence of vitamin K deficiency. It remains to be established whether early prophylactic vitamin K supplementation is safe and effective in preventing complications including embryopathy. Meanwhile, in women with HG and severe malnutrition or weight loss, measuring and supplementing vitamin K should be considered to prevent maternal or neonatal complications.
Acknowledgements
None.
Dr. K. Nijsten is funded by the Amsterdam Reproduction & Development (AR&D), project number 23346, and the Dutch Heart Foundation, grant number 2013T085.
K. N. and R. P. conceived the study. K. N. and L. van der M. performed the search, screened for eligible studies and performed data extraction. K. N. and L. van der M. performed all statistical analyses, supervised by R. P.. K. N. and L. van der M. drafted the manuscript. H. W., S. M., M. K., I. G., T. R. and R. P. contributed in interpreting the results and revising the manuscript. All authors approved the final draft of the manuscript.
The authors declare no conflicts of interest relevant to this work.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114521002865









