The intake of long-chain n-3 PUFA (LC n-3 PUFA) in Western diets is low. For example, while the UK minimum daily recommended intake is 450 mg/d(1), the mean population intake is approximately 244 mg/d(Reference Gibbs, Rymer and Givens2), and for many individuals, intakes will be lower still given that 73 % of the population consume no oily fish(Reference Gibbs, Rymer and Givens2). Enriching chicken meat with LC n-3 PUFA can make a nutritionally significant contribution to population LC n-3 PUFA intakes, given the high consumption of poultry products by a wide sector of the population(Reference Gibbs, Rymer and Givens2) and the extent to which poultry meat can be enriched with LC n-3 PUFA. To achieve this enrichment with LC n-3 PUFA, fish oil is usually included in the broiler diet(Reference Hulan, Ackman and Ratnayake3–Reference Mirghelenj, Golian and Taghizadeh8), although algal biomass can also be used(Reference Mooney, Hirschler and Kennedy9, Reference Rymer and Givens10). The long-term sustainability of using fish oil in this way has been questioned(11), while the high price of algal biomass prevents its wide-scale inclusion in poultry diets.
Vegetable sources of n-3 PUFA have been investigated, but these generally provide only C18 : 3n-3 (α-linolenic acid (LNA)). The low conversion efficiency of LNA to LC n-3 PUFA and the relatively low concentration of LNA in most vegetable oils result in very low accumulations of LC n-3 PUFA in the edible tissues of birds even when fed relatively LNA-rich oils such as linseed(Reference Rymer and Givens7, Reference López-Ferrer, Baucells and Barroeta12). Stearidonic acid (SDA; C18 : 4n-3) is further along the pathway of conversion of LNA to LC n-3 PUFA, and is the product of desaturation by Δ-6 desaturase. It is therefore possible that feeding birds an oil rich in SDA might result in a greater accumulation of LC n-3 PUFA in their edible tissues compared with feeding LNA. However, there are few naturally occurring vegetable sources of SDA. Echium (Echium plantagineum) is one of the richest sources, but this only contains approximately 13 g SDA/100 g total fatty acids(Reference Mir13), and feeding birds with echium oil produced breast meat of which a 100 g serving would provide just 2 % of the recommended daily intake of 450 mg of LC n-3 PUFA(Reference Kitessa and Young14). Plants developed using biotechnology that produce more SDA would increase the amount of this acid that could be fed to birds. This may then increase the degree to which meat might be enriched with LC n-3 PUFA. The objective of the present study was to compare the effect of feeding conventional soya oil, oil from soyabeans genetically engineered to produce relatively high concentrations of SDA (240 mg/g oil), or fish oil to broilers on the fatty acid composition of their edible tissues and the sensory characteristics of their meat.
Materials and methods
Oils
Soyabean oils were prepared by POS (Saskatoon, SK, Canada) from soyabeans provided by Monsanto (St Louis, MO, USA). The oil was produced using extraction procedures representative of standard industry practices. Two soyabean oils were produced; the first was derived from soyabeans genetically engineered to produce relatively high concentrations of SDA (SDASOY). The other was derived from a near-isogenic conventional soyabean variety that did not contain SDA (denoted CON). The refined, bleached and deodorised soya oils (SDASOY and CON) were stabilised with 120 mg/kg tert-butylhydroquinone and 60 mg/kg citric acid.
Feed-grade blended fish oil was also used (denoted FISH). This was purchased from United Fish Industries (UK) Limited (Grimsby, Lincolnshire, UK). It was not chemically stabilised and so was kept frozen ( − 20°C) until use. A sample (approximately 25 g) of each oil was taken, stored at − 20°C and analysed for fatty acid composition (Table 1).
Table 1 Fatty acid composition (mg/g oil) of the oils used in the study
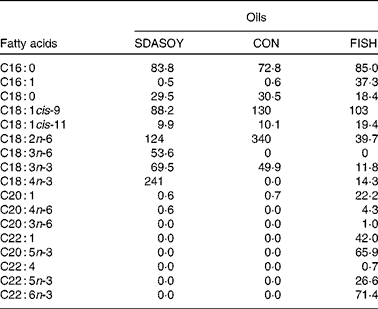
SDASOY, oil derived from soyabeans genetically engineered to produce relatively high concentrations of stearidonic acid (SDA); CON, oil derived from a near-isogenic conventional soyabean variety that did not contain SDA; FISH, feed-grade blended fish oil.
Birds
Ross 308 broiler chicks (n 150) were purchased (PD Hook Hatchery, Bampton, Oxfordshire, UK). The birds used in this experiment were maintained according to the UK Department of the Environment, Food and Rural Affairs Welfare Code for Poultry. On arrival, the birds were placed in a single pen surrounded with a cardboard ring (to minimise draughts) and kept on wood shavings at a depth of approximately 4 cm. They were fed a common starter diet for 14 d. On day 15, the birds were weighed. A total of 120 birds were now required; thirty additional birds had been purchased at the start of the experiment to allow for mortality in the first 14 d, and to enable the heaviest and lightest birds to be excluded from the study. Those selected were then assigned to pens randomly within eight blocks (location within the house) of three pens each (total of twenty-four floor pens). The pens had a solid floor with a bedding of wood shavings. There were three treatment diets, and treatments were randomly allocated among each block of three pens. There were a total of eight blocks, and five birds were placed in each pen. The three treatments were diets incorporating either SDASOY, CON or FISH. Water was provided ad libitum via automatic drinkers, and feed was provided ad libitum via hoppers (Super Feeder Hopper 3 kg, 07 400; Stockshop, Exeter, Devon, UK). The birds were fed a grower diet from days 15 to 28 and a finisher diet from days 29 to 50. Feed added and removed from pens was weighed and recorded. Diet changes were conducted at the same time for all pens.
Birds were weighed again at 41 d, and the amount of feed consumed per pen from 15 to 41 d was recorded. Performance data were calculated and summarised by average weight gain per bird between 15 and 41 d of age. The mean adjusted feed:gain ratio was calculated per pen by dividing total feed consumption by the weight gain of surviving birds plus the weight gain of birds that died. Birds were slaughtered in blocks of two on days 41, 43, 48 and 50 and left to hang (after being plucked and eviscerated) in a cold store overnight. The following day, the total amount of breast skin, leg skin, breast meat and boned leg meat was recorded, and samples of breast skin, breast meat and leg meat were taken, composited by pen and stored frozen ( − 20°C) for the pending analysis of fatty acid composition. Breast skin was analysed rather than leg skin, as it was considered that breast skin would be less prone to damage from bruising. Samples of skinless breast and leg meat, composited by treatment, were also taken for sensory analysis, and stored frozen ( − 20°C).
Diets
Seven diets (Table 2) were manufactured (SDS, Witham, Essex, UK). The common starter diet contained CON. The grower and finisher diets incorporated one of the three oils (CON, SDASOY or FISH). The respective oil was added as the final ingredient in the manufacture of each diet, and mixed for between 5 and 15 min in a horizontal ribbon mixer. The starter diet was fed as a mash, but the other diets were pelleted (Kubex Pellet Mill, Bühler, Uzwil, Switzerland) using a 4 mm die. The pelleting temperature was kept between 50 and 60°C. Knives were set to make the pellet size as small as possible for the grower diets, but up to 7 mm length for the finisher diets. The pellets were screened and placed on trays before being placed in a drier at approximately 30°C overnight (Laboratory Thermal Equipment, Greenfield, Oldham, UK). Diets were stored in sealed plastic buckets. The grower and finisher diets were stored frozen until approximately 3 d before being fed; they were then transferred to a cold room (approximately 4°C) until they were fed to the birds. Within each phase, diets were formulated to have equal concentrations of apparent metabolisable energy, lipid, available lysine, methionine and other essential amino acids. The composition of the diets is detailed in Table 2, and the composition of the vitamin/mineral premixes that were added to the diets is detailed in Table 3. The vitamin E content of the finisher diets was dl-α-tocopherol (50 mg/kg). A running sample was taken of each diet by taking approximately 50 g of feed each day and adding it to a bulked sample that was kept at − 20°C. At the end of the feeding trial, this bulked sample was thoroughly mixed and then stored at − 20°C before being analysed for fatty acids.
Table 2 Formulation of diets used
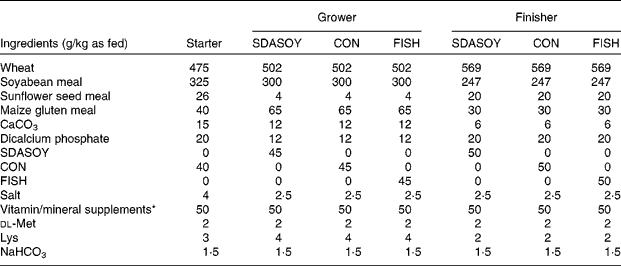
SDASOY, oil derived from soyabeans genetically engineered to produce relatively high concentrations of stearidonic acid (SDA); CON, oil derived from a near-isogenic conventional soyabean variety that did not contain SDA; FISH, feed-grade blended fish oil.
* For composition of the supplements, see Table 3.
Table 3 Composition of the vitamin/mineral supplements that were used
Fatty acid analysis
Samples of milled (1 mm screen) feed or homogenised tissue were defrosted and analysed for fatty acids. Samples of feed (10 g), meat (approximately 20 g) or skin (approximately 10 g) were homogenised with 40 ml methanol and 20 ml chloroform for 2 min. A further 20 ml chloroform was added, and the mixture was homogenised for 30 s. The chloroform contained butylated hydroxytoluene (0·01 g/100 ml). Distilled water (20 ml) was then added, and the mixture was homogenised for a further 30 s. The mixture was then filtered, and the filtrate was poured into a separating funnel. The chloroform extract was then poured into a round-bottomed flask and evaporated on a rotary evaporator. A mixture of chloroform–methanol (2:1, v/v) was then added (25 ml for feed, 20 ml for meat and 100 ml for skin). A sample (1 ml) of this extract was then pipetted into a 10 ml tube and dried under a stream of N2. The residue was dissolved in dried toluene (1 ml) and sodium methoxide (0·5 m in methanol, 2 ml) to methylate the fatty acids. The tube was capped and then heated on a heating block set at 60°C for 30 min. Glacial acetic acid (0·1 ml) and hexane (2 ml, containing an internal standard, docosane) were then added and mixed, followed by water (5 ml). The presence of docosane in these particular samples was not separately analysed, but previous (unpublished results) determinations at this laboratory have detected only negligible amounts of this compound in poultry meat. The organic layer (3 ml) containing the fatty acid methyl esters was then separated off and analysed using a GC–MS (GCD Series 2; Hewlett Packard, Palo Alto, CA, USA). Samples were injected (split injection) onto a capillary column (60 m and 0·32 mm in diameter) with He (flow rate 1 ml/min) used as the carrier gas. The column was held at 70°C for 2 min before being raised to 165°C (5°C/min) for 5 min. It was then raised to 180°C (6°C/min) for 10 min and then 230°C (3°C/min) for 3 min. The inlet temperature was 260°C, and the detector temperature was 250°C. Fatty acid methyl esters were identified by comparing retention times with those of standards, and quantification was done by calculating the areas under the curve and comparing these with standards. Concentrations were calculated as μg/ml solution injected on the column. Fatty acid concentrations were then calculated in terms of mg fatty acid/100 g tissue (fresh weight).
Sensory analysis
Samples of breast and leg meat were subjected to sensory analysis (Sensory Dimensions Limited, Reading, UK). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the University of Reading School of Agriculture, Policy and Development ethics committee. Written informed consent was obtained from all subjects. Breast meat was assessed when freshly cooked, while leg meat was assessed when freshly cooked and when it had been cooked, refrigerated and then reheated. This was because the higher lipid content of the leg meat was likely to make it more prone to oxidative deterioration, and reheating the meat would provide a greater oxidative stress so as to increase the likelihood of detecting any differences in the oxidative stability of the lipid component of the meat(Reference O'Keefe, Proudfoot and Ackman15).
Boneless, skinless fillets of the meat were supplied vacuum packed and frozen. They were then transferred to 20°C and defrosted completely 12 h before cooking. Samples were wrapped in aluminium foil and cooked in a preheated oven at 180°C for 30 min/500 g. The meat was then cooled to room temperature (20°C) before serving. The reheated samples were prepared by cooling cooked samples and then refrigerating them (5°C) overnight. The following day, samples were reheated at 180°C for 30 min and then allowed to cool to room temperature (20°C) before serving. Sensory analysis of the meats was done (breast meat and leg meat was done separately, but freshly cooked and reheated leg meat was analysed together) by ten trained assessors using quantitative descriptive analysis(Reference Stone, Sidel and Oliver16), with each panelist assessing three replicates of each sample over a period of 6 d. Plain crackers and mineral water were used as palate cleansers between samples. Samples were tasted and chewed, and then spat out rather than being swallowed. The aftertaste of samples was determined 1 min after the samples had been removed from the mouth.
Data analysis
The effect of the broiler diet on bird performance and tissue fatty acid composition was determined by ANOVA, taking block into account. Tukey's multiple comparison test was used to identify which treatments were significantly different from each other when the significance was P < 0·05. Skinless breast meat, skinless leg meat and skin were analysed separately. The calculated fatty acid composition of breast meat and leg meat (with the skin on) was then determined, taking into account the relative proportions of skinless meat and skin in the breast and leg. This estimate of proportion of skinless meat to skin was done on a pen basis. The effect of tissue type and broiler diet on the sensory attributes of the meat was analysed by ANOVA and Fisher's least significant difference multiple comparison test.
Results
Bird performance
Between 15 and 41 d, a total of four birds died; there was, however, no evidence to suggest that the diet fed to the birds had any effect on bird health. The effects of the diet on mean feed intake from 15 to 41 d, weight gain, adjusted feed:gain ratio and yields of meat are presented in Table 4. There was no significant difference between treatments in DM intake, weight gain or feed conversion efficiency. The slaughter, dressed and cold carcass weights, and the weight of the breast meat and the whole leg of birds fed FISH were lower than those of birds fed CON. Yields of the total and breast meat (% of slaughter weight) were also lower for birds fed FISH than for birds fed SDASOY. The proportion of skin in the breast and leg was not affected by the diet.
Table 4 Effect of the diets on bird performance
(Mean values with their standard errors)
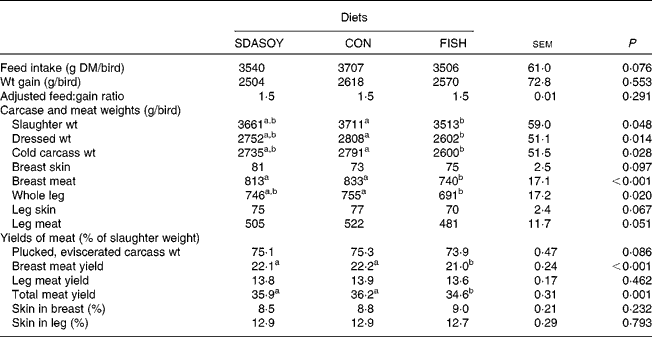
SDASOY, oil derived from soyabeans genetically engineered to produce relatively high concentrations of stearidonic acid (SDA); CON, oil derived from a near-isogenic conventional soyabean variety that did not contain SDA; FISH, feed-grade blended fish oil.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Fatty acid composition
The fatty acid composition of the diets is summarised in Table 5. Diets containing fish oil (FISH) had much higher concentrations of C16 : 1, C18 : 1cis-11, C20 : 1, C22 : 1, EPA, docosapentaenoic acid (DPA) and DHA compared with the other diets. The CON diets had a higher concentration of C18 : 2n-6. The SDASOY diets had higher concentrations of C18 : 3n-6 and SDA compared with the other diets.
Table 5 Fatty acid composition (mg/100 g feed, fresh weight) of the diets used in the present study
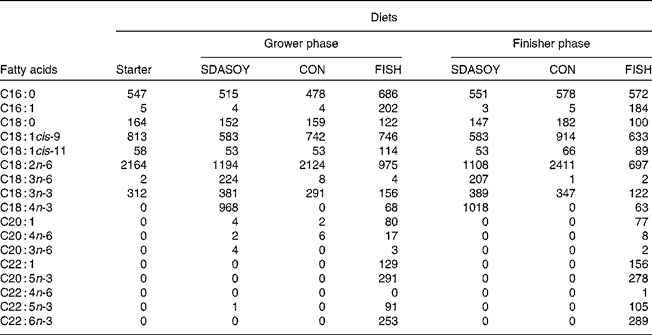
SDASOY, oil derived from soyabeans genetically engineered to produce relatively high concentrations of stearidonic acid (SDA); CON, oil derived from a near-isogenic conventional soyabean variety that did not contain SDA; FISH, feed-grade blended fish oil.
The effect of the diet on the fatty acid composition of skinless breast meat, skinless leg meat and skin is presented in Tables 6–8. The calculated fatty acid composition of breast and leg meat (with the skin on) is also presented (Tables 9 and 10), taking account of the relative proportions of skin and meat recorded in the breast and leg. There was no effect of the diet on the concentrations (mg/100 g fresh tissue) of C16 : 0, C16 : 1, C18 : 0 and C18 : 1 in the skinless breast meat and skinless leg meat. However, the concentration of C16 : 0 was greater in the skin, and in the meat with skin, when birds were fed CON rather than FISH. The concentration of C16 : 1 and C18 : 1cis-11 was lower in these tissues when birds were fed SDASOY compared with the other diets, while the concentration of C18 : 1cis-9 was higher when birds were fed CON. The concentration of C18 : 0 was lower in the skin and breast meat with skin when birds were fed FISH.
Table 6 Effect of the diets on the fat content and fatty acid composition (mg/100 g fresh tissue) of skinless breast meat
(Mean values with their standard errors)
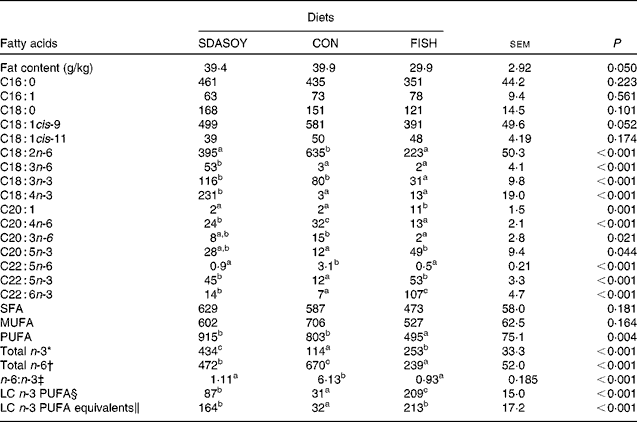
SDASOY, oil derived from soyabeans genetically engineered to produce relatively high concentrations of stearidonic acid (SDA); CON, oil derived from a near-isogenic conventional soyabean variety that did not contain SDA; FISH, feed-grade blended fish oil; LC n-3 PUFA, long-chain n-3 PUFA.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Total n-3 fatty acids.
† Total n-6 fatty acids.
‡ Ratio of n-6:n-3 fatty acids.
§ Total LC n-3 PUFA.
∥ Calculated as (C18 : 4n-3 × 0·33) plus total LC n-3 PUFA.
Table 7 Effect of the diets on the fat content and fatty acid composition (mg/100 g fresh tissue) of skinless leg meat
(Mean values with their standard errors)
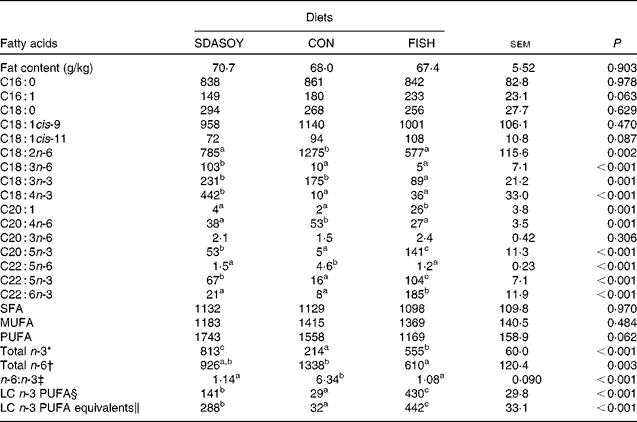
SDASOY, oil derived from soyabeans genetically engineered to produce relatively high concentrations of stearidonic acid (SDA); CON, oil derived from a near-isogenic conventional soyabean variety that did not contain SDA; FISH, feed-grade blended fish oil; LC n-3 PUFA, long-chain n-3 PUFA.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Total n-3 fatty acids.
† Total n-6 fatty acids.
‡ Ratio of n-6:n-3 fatty acids.
§ Total LC n-3 PUFA.
∥ Calculated as (C18 : 4n-3 × 0·33) plus total LC n-3 PUFA.
Table 8 Effect of the diets on the fat content and fatty acid composition (mg/100 g fresh tissue) of skin
(Mean values with their standard errors)
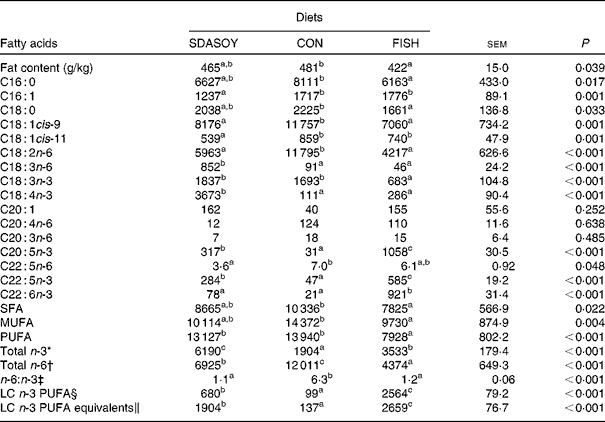
SDASOY, oil derived from soyabeans genetically engineered to produce relatively high concentrations of stearidonic acid (SDA); CON, oil derived from a near-isogenic conventional soyabean variety that did not contain SDA; FISH, feed-grade blended fish oil; LC n-3 PUFA, long-chain n-3 PUFA.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Total n-3 fatty acids.
† Total n-6 fatty acids.
‡ Ratio of n-6:n-3 fatty acids.
§ Total LC n-3 PUFA.
∥ Calculated as (C18 : 4n-3 × 0·33) plus total LC n-3 PUFA.
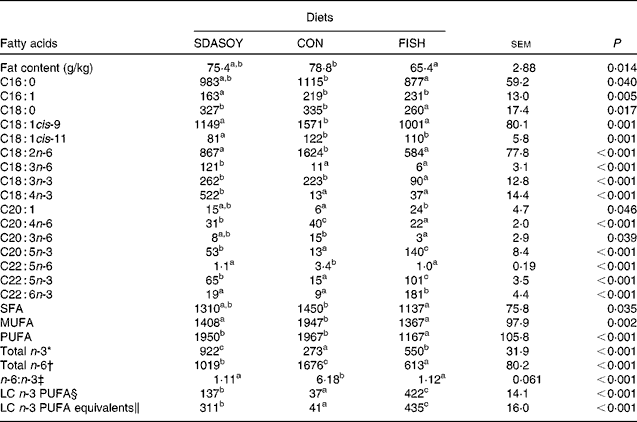
Table 9 Effect of diets on the fat content and fatty acid composition (mg/100 g fresh tissue) of breast meat with skin
(Mean values with their standard errors)
SDASOY, oil derived from soyabeans genetically engineered to produce relatively high concentrations of stearidonic acid (SDA); CON, oil derived from a near-isogenic conventional soyabean variety that did not contain SDA; FISH, feed-grade blended fish oil; LC n-3 PUFA, long-chain n-3 PUFA.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Total n-3 fatty acids.
† Total n-6 fatty acids.
‡ Ratio of n-6:n-3 fatty acids.
§ Total LC n-3 PUFA.
∥ Calculated as (C18 : 4n-3 × 0·33) plus total LC n-3 PUFA.
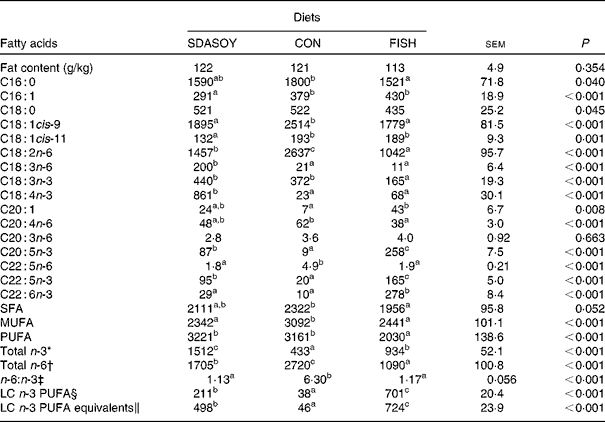
Table 10 Effect of diets on the fat content and fatty acid composition (mg/100 g fresh tissue) of leg meat with skin
(Mean values with their standard errors)
SDASOY, oil derived from soyabeans genetically engineered to produce relatively high concentrations of stearidonic acid (SDA); CON, oil derived from a near-isogenic conventional soyabean variety that did not contain SDA; FISH, feed-grade blended fish oil; LC n-3 PUFA, long-chain n-3 PUFA.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Total n-3 fatty acids.
† Total n-6 fatty acids.
‡ Ratio of n-6:n-3 fatty acids.
§ Total LC n-3 PUFA.
∥ Calculated as (C18 : 4n-3 × 0·33) plus total LC n-3 PUFA.
The concentrations of C18 : 2n-6, C20 : 4n-6 and C22 : 5n-6 were greater in all tissues (except skin for C20 : 4) when birds were fed CON rather than SDASOY or FISH. However, the concentration of C18 : 3n-6 was low or negligible in all tissues apart from those that contained skin or from birds that had been fed SDASOY. In the n-3 series, the concentration of LNA was greater when birds were fed soya oil (CON or SDASOY) rather than FISH, but the concentration of SDA was much higher in all tissues when birds were fed SDASOY. The concentration of EPA, DPA and DHA was highest in all tissues when birds were fed FISH. However, there were significantly higher concentrations of both EPA and DPA when birds were fed SDASOY rather than CON. The exception to this was in the skinless breast meat where the difference in EPA content in SDASOY and CON was not significant, but in the skinless breast meat, the DPA content was significantly greater in the SDASOY meat compared with the CON meat and, indeed, there was no significant difference in DPA content between birds fed either FISH or SDASOY. The concentration of DHA was greatest in all tissues when birds were fed FISH, and apart from the skinless breast meat, there was no significant difference in the DHA content of tissues from birds fed either SDASOY or CON.
The concentrations of total SFA and MUFA were not affected by the diet in the skinless leg or breast meat, but in tissues with skin, SFA were higher in CON compared with FISH, while MUFA were higher in CON compared with both FISH and SDASOY. Total PUFA content was higher in all tissues (except in the skinless leg meat) when birds were fed soya oil (CON or SDASOY) rather than FISH. Total n-3 PUFA content was greatest in tissues when birds were fed SDASOY, but the content was also significantly greater in birds fed FISH compared with those fed CON. However, LC n-3 PUFA content was greatest in all tissues when birds were fed FISH, although birds fed SDASOY still had significantly higher LC n-3 PUFA contents in their tissues compared with birds fed CON. A 100 g serving of skinless breast meat or leg meat with skin would provide 46 and 156 %, respectively, of the minimum recommended LC n-3 PUFA intake of 450 mg/d(1) if birds were fed FISH. The equivalent figures for meat from birds fed SDASOY were 19 and 47 %, while for the CON meat, they were just 7 and 8 %. The concentration of LC n-3 PUFA equivalents was also calculated as the sum of LC n-3 PUFA and 0·33 of the SDA content. This assumes that human subjects consuming these poultry tissues would convert 33 % of the SDA to LC n-3 PUFA(Reference James, Ursin and Cleland18). Some caution should be applied to these figures as it seems likely that this is an overestimate of the conversion efficiency as the birds in this experiment appeared to be much less efficient at converting SDA to LC n-3 PUFA (see below), and it seems unlikely that birds would be so much less efficient than human subjects in this respect. The concentration of LC n-3 PUFA equivalents was still greatest when birds were fed FISH (although this was not significant in the skinless breast meat), but the difference between SDASOY and CON tissues was more marked. If the estimate of 33 % for the conversion efficiency of SDA to LC n-3 PUFA in human subjects is correct, then a 100 g serving of breast and leg meat with skin from birds fed SDASOY would provide 69 and 111 % of LC n-3 PUFA equivalents. Total n-6 PUFA content was greatest in birds fed CON, although birds fed SDASOY had higher concentrations of total n-6 PUFA compared with those fed FISH (except in the skinless leg meat). The ratio of n-6:n-3 fatty acids was greatest in tissues from birds fed CON.
Pool sizes of fatty acids
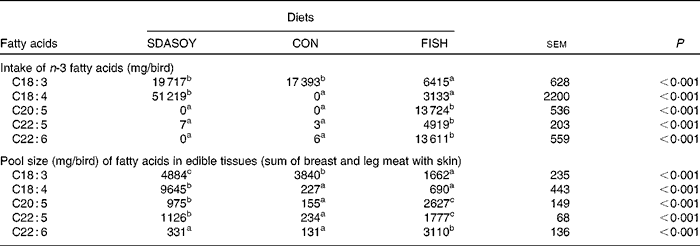
The intake (over the grower and finisher period) and edible tissue pool size (sum of breast meat, leg meat, breast skin and leg skin) of fatty acids for the different diets are summarised in Table 11. Birds fed CON accumulated some SDA and LC n-3 PUFA, despite having no detectable amounts of these fatty acids in their diet.
Table 11 Effect of the diets on the intake and pool sizes of n-3 fatty acids
(Mean values with their standard errors)
SDASOY, oil derived from soyabeans genetically engineered to produce relatively high concentrations of stearidonic acid (SDA); CON, oil derived from a near-isogenic conventional soyabean variety that did not contain SDA; FISH, feed-grade blended fish oil.
a,b,c Mean values within a row with unlike superscript letters were significantly different within a dataset (P < 0·05).
Sensory analysis
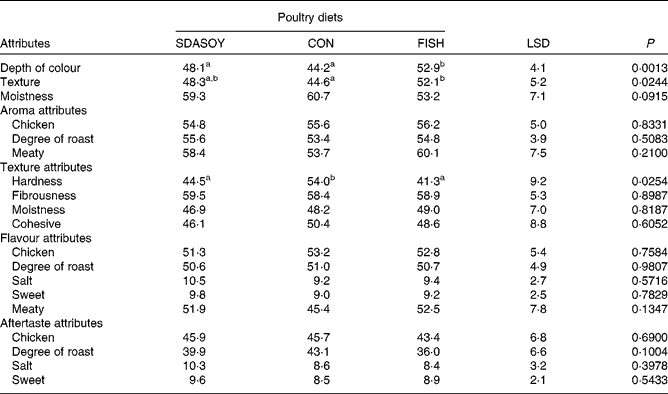
The results of the sensory analysis are summarised in Tables 12 and 13. Differences that were perceived in the breast meats were associated with the texture and appearance of the meat, but not its flavour, aroma or aftertaste. There were no significant differences between the leg meats in terms of their appearance and texture. However, significant differences were perceived between the leg meats in terms of their aroma, flavour and aftertaste, especially among those that had been reheated. Reheating the meats reduced their meaty aroma. Reheated meat from birds fed FISH had significantly less chicken flavour than the other meats, and significantly less degree of roast aroma than the other meats apart from the reheated SDASOY meat. The fish aroma and flavour were stronger in the meat from birds fed FISH (both when the meat was freshly cooked and when it was reheated). Reheated meat from birds fed SDASOY also had a stronger fishy aroma and flavour than meat from birds fed CON, although these fishy notes were not as marked as in the meat from birds fed FISH. The oily aroma of meat from birds fed SDASOY and FISH was also stronger than that from birds fed CON (when the meat was both freshly cooked and reheated). Although there was no difference in the freshly cooked meats, the chicken flavour and the degree of roast aftertaste were significantly lower in the reheated SDASOY and FISH meats compared with the CON meats. Reheated meat from birds fed FISH had a significantly higher oily aftertaste than the other meats. Reheated meat from birds fed SDASOY and FISH had a significantly higher oily mouthfeel than reheated meat from birds fed CON. Birds fed FISH also had a significantly higher fishy aftertaste than birds fed CON and SDASOY, when the meat was both freshly cooked and reheated. The reheated SDASOY meat also had a significantly higher fishy aftertaste than the reheated CON meat, but it was much less marked than the fishy aftertaste of the FISH meat.
Table 12 Effect of the diets on the sensory attributes (scores from 0 to 100) of freshly cooked breast meat
SDASOY, oil derived from soyabeans genetically engineered to produce relatively high concentrations of stearidonic acid (SDA); CON, oil derived from a near-isogenic conventional soyabean variety that did not contain SDA; FISH, feed-grade blended fish oil; LSD, least significant difference.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Table 13 Effect of the diets on the sensory attributes (scores from 0 to 100) of freshly cooked and reheated leg meat
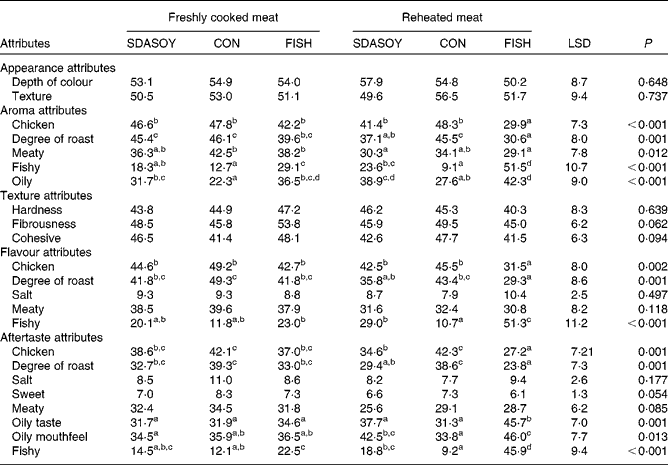
SDASOY, oil derived from soyabeans genetically engineered to produce relatively high concentrations of stearidonic acid (SDA); CON, oil derived from a near-isogenic conventional soyabean variety that did not contain SDA; FISH, feed-grade blended fish oil; LSD, least significant difference.
a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Discussion
The n-3 (and C18 : 3n-6) PUFA concentrations of the diets largely reflected the composition of the oils used in their manufacture, whereas the C18 : 2n-6 concentration of the diets was generally greater than would have been expected, suggesting that the other feed ingredients used in the diet contributed relatively large amounts of C18 : 2 but virtually no n-3 fatty acids. Some traces of C18 : 3n-6 must have been present in other feeds, as all diets contained some C18 : 3n-6, although this fatty acid was undetectable in CON.
The performance of the birds was within the expected range of the breed standard(Reference Aviagen17), and so the study did replicate commercial performance. Feeding SDASOY had no effect on bird performance or carcass composition. Compared with the birds fed CON or SDASOY, the birds fed fish oil (FISH) did not produce as much saleable meat, which was largely a reflection of their reduced intake of feed.
Although birds fed the CON diet consumed over 17 g of LNA each, this did not result in the accumulation of LC n-3 PUFA in the edible tissues to any nutritionally meaningful extent, which is in accordance with the findings of both López-Ferrer et al. (Reference López-Ferrer, Baucells and Barroeta12) and Rymer & Givens(Reference Rymer and Givens7). However, since the CON diet contained no detectable amounts of SDA or LC n-3 PUFA, the (limited) accumulation of these fatty acids in the edible tissues must have come from the desaturation and elongation of dietary LNA. The accumulation of SDA, EPA, DPA and DHA in the edible tissues of CON birds accounted for 1·3, 0·9, 1·3 and 0·8 %, respectively, of dietary LNA consumed. Replacing soya oil (CON or SDASOY) with FISH, as expected, significantly increased the tissue concentration of LC n-3 PUFA. This was because of the dietary supply of preformed LC n-3 PUFA, and this approach to enriching poultry meat with LC n-3 PUFA has been well reported(Reference Hulan, Ackman and Ratnayake3–Reference Mirghelenj, Golian and Taghizadeh8). With SDASOY, an increase in the LC n-3 PUFA content of the tissues was observed, although not to the same extent as was observed with FISH. If it is assumed that the LNA consumed by birds fed either CON or SDASOY was converted with equal efficiency, and that any other deposited SDA and LC n-3 PUFA in tissues from birds fed SDASOY was derived from dietary SDA, then dietary SDA accounted for the accumulation of 9389, 798, 870 and 173 mg of SDA, EPA, DPA and DHA, respectively. This accumulation accounts for 18·33, 1·56, 1·70 and 0·34 % of the SDA consumed by birds fed SDASOY. The conversion of LNA to SDA was probably somewhat inhibited by the dietary supply of SDA, but even if the contribution of LNA to SDA and LC n-3 PUFA is assumed to be zero, the conversion and accumulation efficiency of SDA to EPA, DPA and DHA is only 1·9, 2·2 and 0·6 %, respectively. The conversion (and accumulation in the edible tissues) efficiency of SDA to LC n-3 PUFA does not therefore appear to be very different from that of LNA.
The accumulation of SDA in the birds' tissues appears to be in contrast to the metabolism of SDA by human subjects(Reference James, Ursin and Cleland18). Human subjects do not appear to accumulate SDA in their tissues(Reference James, Ursin and Cleland18, Reference Miles, Tapati and Dooper19), but the birds in this experiment and that reported by Kitessa & Young(Reference Kitessa and Young14) did accumulate considerable amounts of SDA. This accumulation of dietary SDA has also been observed in fish(Reference Bell, Strachan and Good20, Reference Miller, Nichols and Carter21). Approximately, 19 % of the SDA consumed accumulated in the edible tissues (breast muscle, leg muscle and skin from the breast and leg). In the case of birds fed SDASOY, this amounted to 509 and 838 mg/100 g in the breast and leg meat (with skin), respectively. James et al. (Reference James, Ursin and Cleland18) suggested that 3 g SDA was, in human subjects, equivalent to 1 g EPA. On this basis, the SDA accumulated in the breast and leg meat (with skin) of SDASOY-fed birds would supply the equivalent of an additional 170 and 279 mg LC n-3 PUFA/100 g meat, respectively. Even in the much less lipid-rich tissues of skinless breast and leg meat, the potential contribution from SDA from birds fed SDASOY would provide the equivalent of 164 and 288 mg LC n-3 PUFA/100 g meat, respectively, if the estimate of James et al. (Reference James, Ursin and Cleland18) is correct.
The amount of SDA that could be fed to birds in the present study (approximately 51 g over a mean 32 d feeding period) was much greater than that could be achieved by Kitessa & Young(Reference Kitessa and Young14) when they fed echium oil (approximately 15 g over a 34 d feeding period). The inclusion rate of oil was higher in the present study compared with that of Kitessa & Young(Reference Kitessa and Young14), but SDASOY was also a much richer source of SDA (approximately 24 %) than echium oil (approximately 12 %). The large amount of SDA that was fed in the present study when SDASOY was fed did mean that there was a significant increase in the pool size of LC n-3 PUFA in the meat and skin of SDASOY-fed birds compared with CON. The amount of LC n-3 PUFA supplied in 100 g of either breast or leg meat (with skin) from birds fed SDASOY would meet 30 or 47 %, respectively, of the UK minimum daily recommended intake(1), while skinless breast or leg meat would provide 19 or 31 %. In the study reported by Kitessa & Young(Reference Kitessa and Young14), the skinless breast and leg meat that was produced would supply 2 and 30 % of the daily recommended intake of 450 mg. The difference in accumulation of LC n-3 PUFA between these two studies was particularly marked in the leaner skinless breast meat.
The composition of LC n-3 PUFA in the broiler tissues was affected by the diet. While up to 43 % of the LC n-3 PUFA in the meat (with skin) from birds fed FISH was DHA, the corresponding estimate for CON was 26 % and for SDASOY was 14 %. DPA, on the other hand, accounted for up to 47 % of the LC n-3 PUFA in birds fed SDASOY, but it accounted for only 24 % in birds fed FISH. The lack of accumulation of DHA in poultry tissues in response to feeding SDA was also observed by Kitessa & Young(Reference Kitessa and Young14), but the accumulation of DPA in addition to EPA may be of nutritional significance. The consumption of meat rather than fish results in an increased consumption of DPA(Reference Howe, Meyer and Record22), but the implications of this for cardiovascular health are not clear. There is some evidence that DPA is more beneficial than EPA in protecting against peripheral arterial disease(Reference Leng, Horrobin and Fowkes23) and that a higher plasma concentration of DPA (but not DHA) was associated with a lower risk of non-fatal myocardial infarction(Reference Sun, Ma and Campos24). Short-term supplementation with DPA in rats was observed to increase the concentration of DHA in the liver, and the concentration of EPA in the liver, heart and skeletal muscle(Reference Kaur, Begg and Barr25). However, the relative merits of consuming chicken meat enriched with DPA rather than DHA (or not enriched with any LC n-3 PUFA) need to be evaluated.
Sensory qualities
The great advantage of the meat from SDA birds compared with that from FISH birds was its improved sensory attributes, although this was largely because of the lower LC n-3 PUFA content of the SDA meat. The negative impact of fish oil in the poultry diet on the sensory characteristics of poultry meat is well documented(Reference Carrick and Hauge26, Reference Meynier, Genot and Gandemer27), particularly when the meat is cooked, refrigerated and then reheated(Reference O'Keefe, Proudfoot and Ackman15, Reference Wilson, Pearson and Shorland28). The fishy notes detectable in the meat from birds fed fish oil can be partly overcome by supplementing the diet with antioxidants such as vitamin E(Reference Nam, Lee and Min29), but even at relatively high doses (in excess of 100 mg dl-α-tocopherol/kg), fishy flavours may still be detected when the meat has been reheated(Reference Rymer and Givens30). Although the aroma, flavour and aftertaste of skinless, freshly cooked breast meat were not significantly affected by dietary treatment, the same was not the case for the more lipid-rich leg meat. Differences that were perceived in the attributes of the breast meat were related more to birds fed CON, but were because of perceived textural differences. This may be a consequence of differences in the melting points of different fatty acids, and the differences in fatty acid profiles (notably the higher concentration of C18 : 2n-6 in the CON meat) affecting the meat's texture.
The sensory attributes of the more lipid-rich leg meat, however, were significantly affected by the diet that the birds had been fed. Significant fishy aromas, flavours and aftertastes were perceived in both the freshly cooked and reheated meat, and these were most marked in the meat from birds fed FISH (which had the highest LC n-3 PUFA content). Although these fishy attributes were also detected in the meat from birds fed SDASOY, the scores were lower, as the LC n-3 PUFA content of the SDASOY meat was lower. In the freshly cooked SDASOY meat, fishy scores were not significantly different from the CON meat. The reheated SDASOY meat had a more fishy aroma and flavour than the reheated CON meat (but was not significantly different from the freshly cooked SDASOY meat), and its fishy aftertaste was not significantly different from the freshly cooked CON meat. The reheated FISH meat, on the other hand, had significantly higher scores for all three ‘fishy’ attributes (aroma, flavour and aftertaste). A major benefit of birds accumulating dietary SDA as SDA (rather than as LC n-3 PUFA) may therefore be the greater oxidative stability this confers to the meat because of the lower peroxidisability index(Reference Arakawa and Sagai31) of SDA compared with LC n-3 PUFA. Enriching meat by feeding FISH (and therefore accumulating LC n-3 PUFA), on the other hand, resulted in a much greater negative impact on the sensory qualities of the meat. Skinless meat was used in the sensory analysis, and it seems likely that fishy attributes would be more noticeable in the meat with skin because of its much higher lipid content. For such meat, more protection through increased concentrations of vitamin E in the broiler diet may be required (and effective), although this would need to be determined by further research.
Conclusions
Broilers fed the diets supplemented with oil from soyabeans genetically modified to produce relatively high concentrations of SDA produced meat with increased concentrations of SDA, EPA and DPA, but not DHA (except in the skinless breast meat). There was no evidence that birds converted SDA to LC n-3 PUFA any more efficiently than LNA, but the use of an SDA-containing oil derived from a genetically engineered soya plant enabled much higher doses of SDA to be fed than would be the case if existing conventional sources (such as echium) were used. Although feeding the SDA-rich oil to birds had some negative effects on the sensory quality of the meat, these effects were much less marked than when fish oil was fed to the birds because of the lower LC n-3 PUFA content of the meat from birds fed the SDA-rich oil.
Acknowledgements
The present study was funded by Monsanto Company. We are grateful to Mr Colin Green for his assistance during the feeding experiment and slaughter of the birds, and to Mr Ron Brown for the analysis of the fatty acids. We are also grateful to Sensory Dimensions, for conducting the sensory analysis of the samples. C. R. designed the experiment, analysed the data and drafted the manuscript. G. F. H. oversaw the experiment. Both G. F. H. and D. I. G. helped with the design and analysis of the data. Neither C. R. nor D. I. G. has any conflict of interest to disclose. G. F. H. works for Monsanto Company, which funded the work.















