β-Cryptoxanthin is a provitamin A carotenoid found in high concentrations in tangerines, oranges, papayas and red sweet peppers( Reference Turner, Burri, La Frano and Yamaguchi 1 ). In addition to its vitamin A-forming capabilities, β-cryptoxanthin may have other beneficial functions. It possesses antioxidant activity( Reference Fu, Xie and Fan 2 ), and animal studies have indicated an ability to reduce tumorigenesis in several different organs( Reference Tanaka, Tanaka and Kuno 3 – Reference Iskandar, Liu and Smith 6 ). Furthermore, β-cryptoxanthin has been postulated to have a role in bone formation( Reference Yamaguchi 7 ).
Although it is one of the most commonly consumed carotenoids( Reference Turner, Burri, La Frano and Yamaguchi 1 ), there is relatively little known about its metabolism. β-Cryptoxanthin can effectively improve long-term vitamin A status in animals( Reference Davis, Jing and Howe 8 ), and probably in humans( Reference Turner, Burri and Jamil 9 ). Studies on comparisons of estimated dietary intakes with plasma concentrations have shown that β-cryptoxanthin appears to have greater bioavailability than α- and β-carotene in humans( Reference Burri, Chang and Neidlinger 10 , Reference Burri, Chang and Turner 11 ). β-Cryptoxanthin micellerisation in the intestine has been shown to be 3-fold higher than β-carotene( Reference Dhuique-Mayer, Borel and Reboul 12 ), presumably due to its higher polarity. Also, it is more effectively dissolved in lipid droplets in the chromoplasts of fruits than carotenoids bound to the chloroplasts of green leafy vegetables( Reference Castenmiller and West 13 – Reference de Pee, West and Permaesih 15 ).
A whole-body assessment of β-cryptoxanthin absorption, including the testing of multiple biological tissues in response to its increased intake, has not yet been performed. Such a study can provide better understanding of its metabolism and where it may have functional benefits. To date, most research on β-cryptoxanthin has used animal models that do not metabolise carotenoids similarly to humans( Reference Tomita, Senanayake and Yosioka 16 , Reference Surai, Royle and Sparks 17 ). It is important to study an appropriate small animal model of carotenoid metabolism, whose results can be more easily translated to humans. The Mongolian gerbil appears to be the best small animal model available for human provitamin A carotenoid metabolism( Reference House, Apgar and Smith 18 , Reference Lee, Lederman and Hofmann 19 ), primarily because they absorb carotenoids intact and are able to convert provitamin A carotenoids to vitamin A( Reference Pollack, Campbell and Potter 20 ).
The purpose of the present study was to gain insight into the whole-body tissue distribution and response to different levels of β-cryptoxanthin consumption in the gerbil. The objectives of the study were to test fourteen tissues and blood from Mongolian gerbils fed three different concentrations of β-cryptoxanthin, to identify locations of interest, and the effect of these dosages on the distribution of β-cryptoxanthin in tissues.
Materials and methods
Chemicals and reagents
All-trans-retinol, all-trans-retinyl palmitate, all-trans-β-carotene, all-trans-retinal, β-ionone, pyrogallate and anhydrous sodium borohydride were purchased from Sigma-Aldrich Company. All-trans-β-cryptoxanthin and lycopene were purchased from Chromadex, and all-trans-α-carotene, all-trans-lutein and all-trans-3,4-didehydroretinol were purchased from Santa Cruz Biochemicals. 3-Hydroxyretinal was purchased from Toronto Research Chemicals, and β-apo-10-carotenal was purchased from Carotenature. Hexane was purchased from Burdick and Jackson Laboratories, Inc. All other chemicals were purchased from Thermo Fisher Scientific. All solvents were of HPLC grade.
Both retinal-O-ethyloxime and 3-hydroxyretinol standards were not commercially available for purchase and required synthesis. Retinal-O-ethyloxime was synthesised as described previously( Reference Vankuijk, Handelman and Dratz 21 ). 3-Hydroxyretinol was synthesised from 3-hydroxyretinal. Briefly, 1 mg of 3-hydroxyretinal in ethanol was reduced to 3-hydroxyretinol by the addition of 12 mg sodium borohydride. Conversion appeared to be complete (100 %), with purity confirmed by proton NMR spectrometry and HPLC.
Animals
Female Mongolian gerbils (Meriones unguiculatus, n 29), age 34 d, were purchased from Charles River Laboratories. Upon arrival, gerbils were housed in individual plastic cages in a temperature-controlled room (25°C) with a diurnal cycle of 12 h light–12 h dark. Gerbils were weighed daily. After the acclimatisation period, gerbils were fed a vitamin A-deficient diet. After 5 d, five gerbils with extreme weights were euthanised for baseline test measurements. Gerbils were euthanised by being placed in a CO2 chamber, followed by exsanguination. The remaining gerbils were separated into three weight-matched groups (eight gerbils per group; Table 1). Gerbils ate the same diet throughout the study, supplemented with one of three β-cryptoxanthin treatments. During the 21 d treatment period, gerbils were weighed every 2nd day. During the last 2 d of the experiment, two gerbils from each group were transferred into individual metabolism cages, and urine and faecal samples were collected every 6 h.
Table 1 Initial and final gerbil group weights† (Mean values with their standard errors, n 5 (baseline) or n 8 (treatment groups))
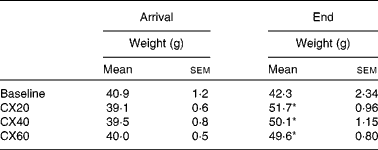
CX20, gerbil group fed 20 μg β-cryptoxanthin/d; CX40, group fed 40 μg β-cryptoxanthin/d; CX60, group fed 60 μg β-cryptoxanthin/d.
* Mean values were significantly different from those of the baseline group (P< 0·05).
† Gerbils began the depletion diet when 51 d old. The baseline group was euthanised at 55 d and the experimental groups at 75 d.
After 3 weeks of treatment, gerbils were euthanised, as described previously, 20–24 h after their last treatment dose in order to allow for complete absorption and circulation of β-cryptoxanthin in the body. Blood was collected via cardiac puncture, and tissue samples were dissected immediately and stored at − 70°C until retinoid and carotenoid analysis. All animal handling procedures were approved by the University of California, Davis Institutional Animal Care and Use Committee (approval 13 104).
Diets
Before the study, while at Charles River Laboratories, gerbils consumed the Purina 5L2F 18 % Rodent-Kingston diet (Purina). Upon arrival at the Western Human Nutrition Research Center, the gerbils began a 17 d acclimatisation period during which they consumed the Purina 5001 Laboratory Rodent Diet (Purina) ad libitum. Once the study began, the gerbils consumed a diet void of vitamin A and provitamin A carotenoids (Harlan Teklad TD.09 084 Gerbil Vitamin A Deficient Diet; Harlan Teklad; Table 2) and water ad libitum.
Table 2 Proximate composition of the vitamin A-deficient diet* fed to gerbils during the study
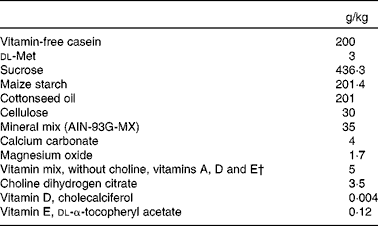
AIN, American Institute of Nutrition.
* Harlan Teklad TD.09 084 Gerbil Vitamin A Deficient Diet.
† Vitamin mix provided (mg/kg feed): biotin, 0·4; calcium pantothenate, 66·1; folic acid, 2; inositol, 110·1; menadione, 49·6; niacin, 99·1; p-aminobenzoic acid, 110·1; pyrodoxine-HCl, 22; riboflavin, 22; thiamin-HCl, 22; vitamin B12, 29·7; ascorbic acid (97·5 %), 1016·6.
All gerbils were fed a 246 μl dose of tangerine concentrate (kindly donated by Ventura Coastal; lot no. VP71155) containing 20 μg/d of β-cryptoxanthin and smaller amounts ( < 1 μg/d) of other provitamin A carotenoids. The concentrate was made from washed, sanitised and pasteurised tangerine juice with most pulp removed and oils extracted. BRIX was 64·97 (w/w), pH 4·88, % oil 0 and RGB angle hue (colour) 41·4.
It was fed with 20 μl of extra-virgin olive oil (Tantillo; T.M. Imports) using a Gilson positive-displacement pipette (Rainin Instruments). In addition, two treatment groups (CX40 and CX60) also consumed 20 and 40 μg/d, respectively, of β-cryptoxanthin standard (Chromadex) fed directly via the displacement pipette.
Carotenoid and vitamin A composition of the diet
Carotenoids were extracted from the tangerine concentrate using methods modified from Breithaupt et al. ( Reference Breithaupt, Weller and Wolters 22 ) and Rodriguez-Amaya and Kimura( Reference Rodriguez-Amaya and Kimura 23 ).
HPLC analysis of the tangerine concentrate was performed on an Agilent 1100 system (Agilent Technologies) using a gradient method( Reference Rajendran, Pu and Chen 24 ). Carotenoids were separated using a YMC C30, 5 μm, 4·6 × 250 mm reverse-phase column (Waters Corporation).
arotenoids and retinoids in the feed were extracted using a method similar to that of Howe et al. ( Reference Howe, Maziya-Dixon and Tanumihardjo 25 ). Carotenoids in the gerbil feed, blood and tissues were measured by a reverse-phase isocratic method( Reference Turner and Burri 26 ) on an Agilent 1100 system (Agilent Technologies). Carotenoids and retinoids were separated using a 3 × 125 mm, 3 μm, Waters Spherisorb ODS2 column (Waters Corporation).
Most retinoids were analysed at 325 nm and carotenoids at 452 nm. The internal standard retinal-O-ethyloxime (added for quality control) and 3,4-didehydroretinol were analysed at 357 nm. Carotenoid standards were prepared in hexane–methylene chloride (4:1), while retinol and retinal-O-ethyloxime were prepared in methanol and retinyl palmitate in hexane. Standard concentrations were verified on a spectrophotometer (Spectronic Genesys 5; Milton Roy Company). On each test day, inter-assay precision was evaluated using a pooled plasma sample purchased from UTAK. The inter-assay precision was 4·5 % for retinol and 5·9 % for β-cryptoxanthin.
The purity of the β-cryptoxanthin standards fed to the gerbils was monitored by HPLC and determined to be >99 % all-trans-β-cryptoxanthin. Concentrations were verified using a spectrophotometer.
Experimental design
After acclimatisation, gerbils were randomly assigned to one of three treatment groups receiving 20, 40 or 60 μg β-cryptoxanthin/d (CX20, CX40 and CX60, respectively; Table 3). All gerbils received 246 μl tangerine concentrate containing 20 μg β-cryptoxanthin/d and small amounts of β- and α-carotenes ( < 1 μg/d). To test the effects of higher β-cryptoxanthin concentrations, we added pure β-cryptoxanthin to the juice. Gerbils in groups CX40 and CX60 received an additional 20 or 40 μg β-cryptoxanthin/d dissolved in ethanol, respectively, for 21 d. We collected samples from fourteen tissues and blood.
Table 3 Composition of vitamin A and carotenoids from the pre-study and experimental diets* (Mean values with their standard errors, n 3 (pre-study diets) or n 6 (experimental diet))
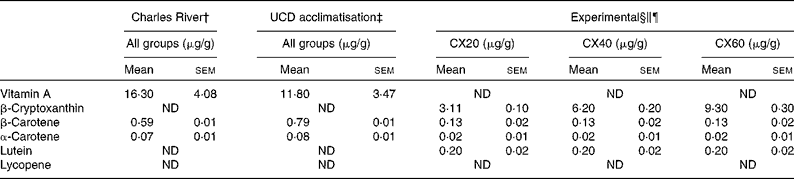
UCD, University of California, Davis; CX20, gerbil group fed 20 μg β-cryptoxanthin/d; CX40, gerbil group fed 40 μg β-cryptoxanthin/d; CX60, gerbil group fed 60 μg β-cryptoxanthin/d; ND, not detected.
* β-Cryptoxanthin standard concentrations were monitored throughout the study.
† Purina 5L2F 18 % Rodent-Kingston diet. Gerbils consumed this diet at Charles River Laboratories, before their arrival (34 d).
‡ Purina 5001 Laboratory Rodent diet. Gerbils consumed this diet during a 17 d acclimatisation period.
§ Calculation of μg/g for the experimental diets based on the combined consumption of tangerine concentrate and oil, and the National Research Council estimation of 6 g feed consumed per d.
∥ Experimental diet consisted of 246 μl tangerine concentrate and 20 μl oil, plus a Harlan Teklad TD.09 084 Vitamin A Deficient Gerbil Diet for all the groups.
¶ In addition to the experimental diet, groups CX40 and CX60 received 20 and 40 μg β-cryptoxanthin standard, respectively.
Blood and tissue extraction and analysis
Since systematic quantification errors are often found when using varying volumes of blood( Reference Valentine and Tanumihardjo 27 ), retinoids and carotenoids were extracted from a standardised volume of 400 μl whole blood. Methanol (1 ml) with 3 % pyrogallate and the internal standard retinal-O-ethyloxime as a control for recovery was added to the whole blood and vortexed for 30 s. Thereafter, 300 μl deionised water and 2 ml hexane were added, and the mixture was vortexed for an additional 1 min before being centrifuged for 5 min at 2500 rpm at 4°C. The hexane layer was collected, and the extraction procedure repeated. The sample was reconstituted in a 100 μl mobile phase. Blood was analysed in duplicate. Blood was not saponified since no esters were detected.
All tissues were extracted using a different procedure. Precautions were taken to obtain the most possible representative tissue samples by removing blemishes from large organs. Since most organs were small and we expected low β-cryptoxanthin concentrations in many of them, the entire organs were extracted and single 70 μl injections were performed. The exceptions were liver, adipose and the small and large intestine, where portions of organs were dissected from the same regions of these organs. For the liver, portions of the upper and lower liver were dissected and mixed, and duplicate injections of 5 μl were performed. A representative portion of the abdominal adipose was dissected and extracted as a whole. For the small and large intestine, each had portions from both ends and the middle that were collected for extraction.
The range of tissue sample weights was as follows: liver, 0·060–0·092 g; abdominal adipose, 0·017–0·29 g; small intestine, 0·27–0·96 g; large intestine, 0·19–0·46 g. Whole-organ weights were as follows: kidneys, 0·17–0·33 g; adrenals, 0·013–0·055 g; pancreas, 0·032–0·21 g; heart, 0·18–0·31 g; lungs, 0·17–0·41 g; spleen, 0·033–0·10 g; eyes, 0·16–0·24 g; brain, 0·35–0·94 g; stomach, 0·094–0·48 g; caecum, 0·14–0·32 g. There were no significant differences in tissue weights between the groups.
Tissue samples were extracted using a different procedure from that used for blood. Tissues were saponified to reduce retinyl esters. Samples were ground with anhydrous sodium sulphate (0·2–1 g), mixed with 1–1·5 ml methanol containing 2 % pyrogallate and the internal standard retinal-O-ethyloxime, and vortexed for 30 s. (The amount of anhydrous sodium sulphate and solvents used were adjusted based on the sample size.) Subsequently, samples were saponified with an equal volume of potassium hydroxide (20 % in water) and vortexed for 5 min before being placed in a shaking water-bath (37°C) for 1 h. This led to the conversion of most of the retinyl esters to retinol, simplifying our chromatograms to allow for the detection of other β-cryptoxanthin metabolites. Henceforth, we use the term ‘vitamin A’ to refer to the retinol and retinyl ester residues left after saponification. Less than 2·5 % of the saponified vitamin A remained in the form of retinyl esters. Then, 200–400 μl DI water and 3 ml hexane were added, and the mixture was vortexed for an additional 2 min before being centrifuged for 5 min at 2500 rpm and 4°C. The hexane layer was extracted twice and filtered through a 0·2 μm Acrodisc filter into a separate test-tube under N2 gas. The sample was dried completely, reconstituted in the mobile phase and analysed as described previously.
A gradient HPLC method was performed on an Agilent 1200 system (Agilent Technologies) to test for the presence of β-cryptoxanthin apocarotenoids including β-apo-10-carotenal and 3-hydroxy-β-apo-10-carotenal in liver tissue. Briefly, representative samples from each gerbil liver were combined to form a 1 g sample of liver for each test group. This sample was extracted using the procedure for tissue extraction described above. Compounds were separated using a Restek Viva reverse-phase column (C18, 5 μm, 5 × 250 mm; Restek) at room temperature by a previously described method( Reference Mein, Dolnikowski and Ernst 28 ).
A gradient HPLC method was performed on an Agilent 1100 system (Agilent Technologies) to test for the presence of other potential β-cryptoxanthin metabolites (3-hydroxyretinol and 3,4-didehydroretinol) in liver tissue. Briefly, a 0·2 g sample of liver from each group was extracted using the procedure for tissue extraction, as described previously. Compounds were separated using a YMC C30, S-5, 4·6 × 250 mm column (Waters Corporation), as described previously( Reference La Frano and Burri 29 ).
Statistical analyses
To approximate the total amount of β-cryptoxanthin and vitamin A in gerbil blood, the weight (g) of each gerbil was multiplied by the blood volume approximation (72 ml/kg), estimated from a healthy gerbil sample by the Iowa State University Institutional Animal Care and Use Committee (IACUC)( 30 ). An estimate was not available for the total weight of adipose tissue from this reference, so we used the data from a study that tested normal-weight, similarly aged, female gerbils consuming a regular diet as a reference for the gerbil groups( Reference Xu and Wang 31 ).
Outcome variables were assessed for conformity to a normal distribution by the Shapiro–Wilk test and transformed, if necessary, using log or reciprocal values. If a suitable transformation could not be found, analyses were performed on the ranks of the variables using the Kruskal–Wallis test or Spearman's rank correlation coefficient.
Tissue concentrations were compared using both nmol/g and nmol/organ. Differences in β-cryptoxanthin concentrations, vitamin A concentrations, and gerbil and organ weights were compared between groups using ANOVA, followed by Tukey's test for pairwise comparisons. Pearson's or Spearman's correlations were calculated to determine the associations between tissues, compounds and the β-cryptoxanthin dose.
A post hoc analysis was performed to further investigate trends in the tissue distribution of β-cryptoxanthin and vitamin A. The preferential accumulation of both the compounds in specific tissues was assessed using Pearson's or Spearman's rank correlation tests.
Vitamin A (retinol and retinyl ester residues) was present in all tissues at baseline, and thus the baseline group was included in ANOVA for vitamin A concentrations. The exceptions were the blood, spleen and caecum whose baseline group tissues were not collected. Values are presented as means with their standard errors. P values < 0·05 were considered as statistically significant for testing the effects of concentration. However, when comparisons between tissues were made, we used a significance level of 0·01 because of the large number of correlations made. Statistical analyses were performed using Statistical Analysis Systems statistical software (Windows version 9.3; SAS Institute, Inc.).
Results
Body weight
Gerbil weights at the beginning and end of the experiment are shown in Table 1. The weight gain of the treatment groups CX20, CX40 and CX60 after 21 d was 7·1 (sem 1·3), 5·7 (sem 1·3) and 5·2 (sem 0·8) g, respectively. The three treatment groups were not significantly different from each other.
Feed contents
Both the pre-study diet consumed by gerbils while at Charles River Laboratories and the diet consumed during the acclimatisation period contained high concentrations of vitamin A, in the form of retinyl acetate, and minimal amounts of carotenoids (Table 3). We measured the retinoid and carotenoid content of the diet consumed while at Charles River Laboratories, as well as the acclimatisation diet consumed when the gerbils arrived at our facility. It should be noted that we tested a small sample of the diet fed to the gerbils. Purina specification stated that the Charles River and acclimatisation diets contained 13·2 μg vitamin A/g and 4·5 μg/g, respectively. The vitamin A-deficient diet was confirmed to contain undetectable retinoid or carotenoid concentrations. Thus, the sole source of carotenoids in the vitamin A-deficient diet was the 246 μl dose of tangerine concentrate and β-cryptoxanthin supplements provided. Specifically, the tangerine concentrate contained 81·3 (sem 2·6) μg/g of β-cryptoxanthin, 3·5 (sem 0·4) μg/g of β-carotene, 0·5 (sem 0·04) μg/g of α-carotene and 6·0 (sem 0·5) μg/g of lutein. Thus, β-cryptoxanthin represented >95 % of the provitamin A carotenoid concentrations in the tangerine concentrate.
β-Cryptoxanthin and vitamin A tissue concentrations
The study measured β-cryptoxanthin concentrations in fourteen tissues and blood. Organ weights did not differ with treatment. The baseline group had a lower adipose weight because they were euthanised at a younger age and were smaller (Tables 1 and 4). Not surprisingly, statistical analyses using both nmol/organ and nmol/g showed similar statistical differences (Tables 5 and 6).
Table 4 Weight of gerbil organs (g) (Mean values with their standard errors, n 5 (baseline) or n 8 (treatment groups))

CX20, gerbil group fed 20 μg β-cryptoxanthin/d; CX40, gerbil group fed 40 μg β-cryptoxanthin/d; CX60, gerbil group fed 60 μg β-cryptoxanthin/d; NT, not tested.
* Mean value was significantly different from that at baseline (P< 0·05).
† Adipose calculation is based on the data showing 12·9 % total body fat in similar gerbils( Reference Xu and Wang 31 ).
‡ Blood volume estimate based on the Iowa State University Institutional Animal Care and Use Committee (IACUC) calculation( 30 ).
Table 5 Total and per g weight concentrations of β-cryptoxanthin in the plasma and tissue of gerbils at baseline or fed 20, 40 or 60 μg β-cryptoxanthin (Mean values with their standard errors, n 5 (baseline and all caecal samples) or n 8 (treatment groups))

CX20, gerbil group fed 20 μg β-cryptoxanthin/d; CX40, gerbil group fed 40 μg β-cryptoxanthin/d; CX60, gerbil group fed 60 μg β-cryptoxanthin/d; ND, not detected; NT, not tested.
a,b,cMean values with unlike superscript letters were significantly different (P< 0·05).
* All baseline group tissues measured were significantly different from the treatment groups (P< 0·05).
† Group CX60 was significantly different from all the other groups.
Table 6 Total and per g weight concentrations of vitamin A in the plasma and tissue of gerbils at baseline or fed 20, 40 or 60 μg β-cryptoxanthin* (Mean values with their standard errors, n 5 (baseline and all caecal samples) or n 8 (treatment groups))

CX20, gerbil group fed 20 μg β-cryptoxanthin/d; CX40, gerbil group fed 40 μg β-cryptoxanthin/d; CX60, gerbil group fed 60 μg β-cryptoxanthin/d; NT, not tested.
a,bMean values with unlike superscript letters were significantly different (P< 0·05).
* Vitamin A concentration includes retinol and saponified retinyl ester residues.
β-Cryptoxanthin was not present in any of the baseline tissues tested. After the treatment, β-cryptoxanthin increased in all the groups; however, there were significant differences between the groups in β-cryptoxanthin concentrations per organ (nmol β-cryptoxanthin/organ) and per g observed in the liver, kidneys, pancreas, spleen, small intestine and large intestine (Tables 5 and 6).
When we compared β-cryptoxanthin tissue concentrations with each other, correlations were observed between the liver and kidneys (P< 0·0001), the liver and large intestine (P< 0·0001), the kidneys and spleen (P= 0·0058) and the kidneys and large intestine (P= 0·0002).
β-Cryptoxanthin concentrations in many tissues increased with the increasing amounts of β-cryptoxanthin in the diet, including the liver (P= 0·0002), kidneys (P< 0·0001), pancreas (P< 0·0001), small intestine (P= 0·023) and large intestine (P= 0·0003).
Most β-cryptoxanthin was present in the liver. Other important storage sites were adipose tissue and blood. However, it appears that β-cryptoxanthin may be highly concentrated in certain tissues, regardless of organ size. As shown in Table 5, there were accumulations in the adrenal glands and spleen.
The primary route of excretion was via the faeces, as is expected for carotenoids( Reference Preedy 32 ). A small amount of β-cryptoxanthin was also excreted in the urine. There was a significant difference in faecal excretion between the group fed the greatest amount of β-cryptoxanthin and that fed the least (P= 0·021; Table 7). There were no significant differences between the groups for urine excretion.
Table 7 Faeces and urine excretion per d from gerbils fed 20, 40 or 60 μg β-cryptoxanthin (Mean values with their standard errors, n 8)
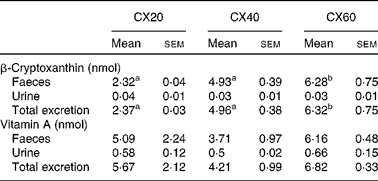
CX20, gerbil group fed 20 μg β-cryptoxanthin/d; CX40, gerbil group fed 40 μg β-cryptoxanthin/d; CX60, gerbil group fed 60 μg β-cryptoxanthin/d.
a,bMean values with unlike superscript letters were significantly different (P< 0·05).
There were significant differences between the groups for total vitamin A concentrations (nmol/organ) in the eyes, lungs and large intestine (Table 5). Correlations for total vitamin A concentrations in tissues were observed between the adrenal gland and large intestine (P= 0·0048), the heart and pancreas (P= 0·0006) and the caecum and kidneys (P= 0·002). As β-cryptoxanthin intake increased, a concomitant rise in vitamin A concentrations per organ was observed in the caecum (P= 0·032) and large intestine (P= 0·0014).
Overall, 92 % of the vitamin A measured in the tissues that we sampled was stored in the liver. Vitamin A was concentrated in the other tissues such as the lungs and eyes, and, to a lesser extent, the adipose tissue and adrenal glands. There were no significant differences between the groups in terms of faecal and urine excretion of vitamin A (Table 7).
Correlation tests were performed to identify the tissues, in which both β-cryptoxanthin and vitamin A concentrations appeared to be preferentially accumulated. These tissues included the blood (r 0·61; P= 0·0015), brain (r 0·68; P= 0·0003), adipose (P= 0·022), caecum (r 0·63; P= 0·011), small intestine (r 0·91; P< 0·0001) and large intestine (r 0·76; P< 0·0001).
The gerbil liver was tested for the presence of several potential β-cryptoxanthin metabolites. Unfortunately, β-apo-10′-carotenal, β-ionone, 3,4-didehydroretinol and 3-hydroxyretinol were not detected. The limits of detection for β-apo-10′-carotenal, β-ionone, 3,4-didehydroretinol and 3-hydroxyretinol were 12·7, 1·45, 1·51 and 5·07 nmol/g (or 4·78, 0·28, 0·43 and 1·54 ng/g), respectively.
Discussion
The present study is the first to investigate the tissue distribution and concentration response to β-cryptoxanthin intake in a recognised small animal model for human provitamin A carotenoid metabolism. Few studies have detected the presence of β-cryptoxanthin in tissues, and most of these have used an unsuitable animal model for human metabolism, such as gulls( Reference Surai, Royle and Sparks 17 ), mice( Reference Tomita, Senanayake and Yosioka 16 ) and rats( Reference Breithaupt, Yahia and Velazquez 33 ). However, studies using suitable animal models for human carotenoid metabolism, such as gerbils and ferrets, have detected β-cryptoxanthin in the lungs and liver( Reference Liu, Bronson and Russell 5 , Reference Arscott, Howe and Davis 34 ). Human studies have detected β-cryptoxanthin in the blood, brain and adipose tissue( Reference Wingerath, Stahl and Sies 35 – Reference Chung, Ferreira and Epstein 38 ).
As expected, we found β-cryptoxanthin concentrations in most tissues of all the treatment groups after feeding with tangerine concentrate. This appears to be the first report of β-cryptoxanthin in most tissues. However, we can compare the present results with those for other carotenoids, such as β-carotene. Liver and adipose tissue are known to be the two major storage sites of these other carotenoids( Reference Chung, Ferreira and Epstein 38 , Reference Otten, Hellwig and Meyers 39 ). Liver and adipose tissue were the main storage organs for all-trans-β-cryptoxanthin, accounting for 59·8 and 36·4 % of the total detected amounts for the tissues surveyed, respectively. The present results are similar to other studies indicating that β-carotene is primarily stored in the liver in animals( Reference Gugger, Bierer and Henze 40 , Reference Ribaya-mercado, Holmgren and Fox 41 ), but primarily stored in the adipose tissue in humans( Reference Bendich and Olson 42 , Reference Olson 43 ). Unfortunately, little is known about carotenoid distribution in other tissues, or the factors that influence this distribution. Statistically significant increases in tissue concentrations with higher β-cryptoxanthin intake were observed in many of the tissues that we studied, including the liver and kidneys, as well as the components of the digestive tract such as the small and large intestine. β-Cryptoxanthin concentrations increased in adipose tissue over baseline, but the changes were not significant. This may be an artifact of our sampling method since we did not excise and measure all adipose stores in the gerbil. Only abdominal adipose tissue was sampled in the present study, and adipose carotenoid composition can vary with the area of the body from which it was obtained( Reference Chung, Ferreira and Epstein 38 ). Thus, our values may not be representative of all adipose tissue. Alternatively, these results may suggest that β-cryptoxanthin uptake into adipose tissue is influenced by physiological mechanisms. For example, β-cryptoxanthin may be stored first in the liver, where its release is controlled. Alternatively, its absorption into adipose tissue may be saturable.
The apparent lack of a response to dietary intake for vitamin A might be due to the consumption of high concentrations of vitamin A by the gerbils in their pre-study and the acclimatisation period feed. Vitamin A concentrations were high in gerbil tissues at the beginning of the study and remained high in all the groups despite the fact that the study diet contained no preformed vitamin A. This might mean that the intake of β-cryptoxanthin was sufficient to sustain vitamin A tissue concentrations. However, it may also have been a result of the efficient homeostatic control of vitamin A stores in the body( Reference Tanumihardjo 44 ). The vitamin A response tested in the present study included retinol and retinyl esters, but did not measure retinoic acid, a metabolite of β-cryptoxanthin, with biological activity of vitamin A found in low concentrations in biological tissues( Reference Ribaya-mercado, Holmgren and Fox 41 , Reference Bendich and Olson 42 ). Greater liver vitamin A stores in Mongolian gerbils are associated with decreased vitamin A bioconversion and consequently increased absorption of intact β-cryptoxanthin( Reference Tanumihardjo 45 ). This occurred when gerbil retinol stores were 400 nmol/g( Reference Dosti, Mills and Simon 46 , Reference Howe and Tanumihardjo 47 ), an amount 6-fold less than that which was found in our gerbils.
The presence of β-cryptoxanthin in the entire digestive tract supports studies indicating that carotenoid absorption occurs not only in the small intestine, but in the caecum and large intestine as well (Fig. 1)( Reference Goñi, Serrano and Saura-Calixto 48 , Reference Escaron and Tanumihardjo 49 ). The present results suggest that β-cryptoxanthin concentrations in these tissues are saturable (Fig. 2). β-Cryptoxanthin concentrations were not significantly different between the CX40 and CX60 groups except in the pancreas and intestines. In these tissues, there was a significant increase between the CX40 and CX60 groups. Although intestinal absorption of carotenoids had long been considered to occur by passive diffusion, research has now indicated that β-cryptoxanthin absorption may be facilitated by interaction with the enterocyte apical membrane transporter scavenger receptor class B type I protein, an epithelial transporter also involved in cholesterol uptake( Reference During, Dawson and Harrison 50 ). Saturation has been shown to occur in Caco-2 monolayers at concentrations equivalent to a 100 mg dose of β-carotene in human subjects( Reference During, Hussain and Morel 51 ). When we translated the amounts of β-cryptoxanthin consumed by the gerbils to humans based on their body weights, we estimated that the CX20, CX40 and CX60 groups would have consumed 31, 61 and 92 mg β-cryptoxanthin, respectively. The present results suggest that β-cryptoxanthin may be saturable up to 61 mg and absorbed by passive diffusion at higher concentrations. However, since the average daily intake of β-carotene in humans is 1–2 mg( Reference Grune, Lietz and Palou 52 ), the consumption of doses comparable with that which we tested is uncommon.
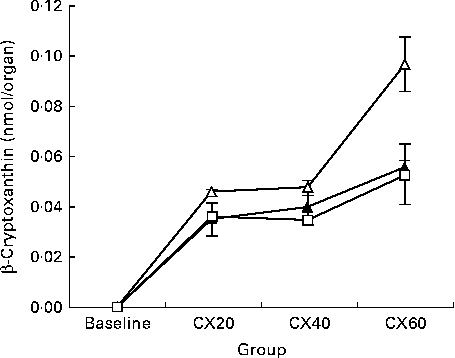
Fig. 1 Change in tissue concentration with varying intake of β-cryptoxanthin in the small intestine (![]() ), caecum (
), caecum (![]() ) and large intestine (
) and large intestine (![]() ). Values are means for baseline (n 5) and for treatment groups (n 8), with their standard errors represented by vertical bars. CX20, gerbil group fed 20 μg β-cryptoxanthin/d; CX40, gerbil group fed 40 μg β-cryptoxanthin/d; CX60, gerbil group fed 60 μg β-cryptoxanthin/d.
). Values are means for baseline (n 5) and for treatment groups (n 8), with their standard errors represented by vertical bars. CX20, gerbil group fed 20 μg β-cryptoxanthin/d; CX40, gerbil group fed 40 μg β-cryptoxanthin/d; CX60, gerbil group fed 60 μg β-cryptoxanthin/d.
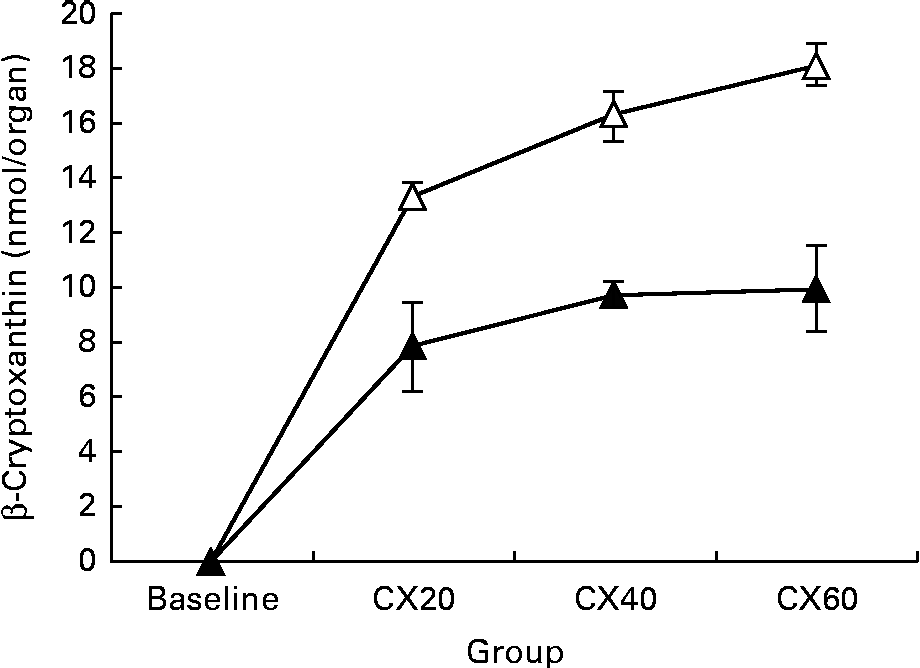
Fig. 2 Change in tissue concentration with varying intake of β-cryptoxanthin in the liver (![]() ) and adipose (
) and adipose (![]() ). Values are means (n 5) for baseline and (n 8) for treatment groups, with their standard errors represented by vertical bars. CX20, gerbil group fed 20 μg β-cryptoxanthin/d; CX40, gerbil group fed 40 μg β-cryptoxanthin/d; CX60, gerbil group fed 60 μg β-cryptoxanthin/d.
). Values are means (n 5) for baseline and (n 8) for treatment groups, with their standard errors represented by vertical bars. CX20, gerbil group fed 20 μg β-cryptoxanthin/d; CX40, gerbil group fed 40 μg β-cryptoxanthin/d; CX60, gerbil group fed 60 μg β-cryptoxanthin/d.
Over 90 % of β-cryptoxanthin excretion (probably a combination of unabsorbed and recycled β-cryptoxanthin) was through the faeces. This has also been observed for other carotenoids such as β-carotene and lutein, where most excretion occurs in the faeces( Reference Ho, de Moura and Kim 53 ). An increase in the excretion of β-cryptoxanthin in the faeces was observed as the intake of β-cryptoxanthin increased.
Interesting observations were the absence of detectable concentrations of β-cryptoxanthin in the brain and eyes and its presence in only the highest dose group within the pancreas. It is possible that β-cryptoxanthin concentrations in the gerbil brain were lower than our limits of detection, since it has previously been identified in the human brain( Reference Craft, Haitema and Garnett 37 ). However, there is no evidence in the past studies of β-cryptoxanthin in the eyes. This may suggest that not all xanthophylls are absorbed in ocular tissues or affect ocular function( Reference Ahmed, Lott and Marcus 54 ). Thus, there may be an active transport in the eye specific for lutein and zeaxanthin, although recent research has suggested that astaxanthin may have a role as well( Reference Cort, Ozturk and Akpinar 55 ). The presence of β-cryptoxanthin in the pancreas at only high dosages may indicate that this is not a common storage area for β-cryptoxanthin and, instead, is only utilised during periods of excess intake.
The presence of retinoids in all of the tissues tested was expected( Reference D'Ambrosio, Clugston and Blaner 56 ). Vitamin A is a ubiquitous compound in the body, with functions such as gene expression, vision and immunity in numerous tissues. Vitamin A stores did not decrease significantly in the present study despite the fact that gerbils were fed a vitamin A-depleted diet for 21 d. This is probably due to the long half-life of vitamin A in the body. However, β-cryptoxanthin might have helped maintain vitamin A stores, since it can form vitamin A. The treatment group that received the greatest amount of β-cryptoxanthin maintained liver vitamin A stores, while the groups that received 20 and 40 μg β-cryptoxanthin appeared to lose 14·2 and 3·1 % of their vitamin A stores, respectively. The slight decrease of vitamin A in the CX20 group may have been due to the formation of active retinol and retinoic acid metabolites in the body, although one study has reported that β-cryptoxanthin might also be capable of activating retinoic acid receptors( Reference Matsumoto, Mizukami and Mizuno 57 ). Thus, the present results support other studies( Reference Davis, Jing and Howe 8 , Reference Arscott, Howe and Davis 34 , Reference Mills, Simon and Tanumihardjo 58 ) showing that β-cryptoxanthin might maintain or improve vitamin A stores in gerbils. Vitamin A was maintained or increased in several other tissues as well.
β-Cryptoxanthin apocarotenoids, such as β-apo-10′-carotenal and 3-OH-β-apo-10′-carotenal, have been detected in tissue cultures( Reference Mein, Dolnikowski and Ernst 28 ). We attempted to detect the presence of some of their known metabolites in the gerbil liver. However, we did not detect these compounds in our samples. It may be that the size of our sample was too small, or that these metabolites may have been present only briefly after every meal. The potential β-cryptoxanthin metabolites 3-hydroxyretinol and 3,4-didehydroretinol were also not detected. These compounds are typically seen in freshwater fish( Reference La Frano and Burri 29 , Reference Barua, Das and Verma 59 , Reference Doyon, Boileau and Fortin 60 ), which synthesise them from lutein- and cryptoxanthin-containing algae, or when they are consumed directly( Reference Tanumihardjo, Muherdiyantiningsih and Permaesih 61 ). These metabolites have only been previously detected in animals other than fish after they were consumed directly.
The results of the present study indicate that β-cryptoxanthin is present in numerous tissues within the body, and that it appears to be absorbed at least in part by an active transport mechanism. However, β-cryptoxanthin metabolites other than vitamin A might be rare or transitory. Future studies, including those utilising isotopic tracers, to investigate the metabolism of β-cryptoxanthin with respect to retinol, retinoic acid and apocarotenoids are necessary. These studies might use a longer depletion period for vitamin A, or a negative control group, all of which would increase our insight into the absorption and metabolism of this carotenoid.
Acknowledgements
We thank Chloe Dupertuis and Terry Neidlinger of the USDA WHNRC for their laboratory assistance during the study, Reina Engle-Stone of the UC Davis Department of Nutrition and John Newman and Theresa Pedersen of the USDA WHNRC for assistance with the procedure and sample analysis planning, Sherri Goss of the UC Davis Campus Veterinary Assistance for gerbil handling instruction, Rick Torres of Ventura Coastal for providing the tangerine concentrate used in the study, and Lacey Baldiviez and Jan Peerson of the UC Davis Department of Nutrition for assistance with the statistical analyses of the data.
The present study was funded by the USDA WHNRC in-house funds and a Henry A. Jastro Research Scholarship Award, University of California, Davis. The funder did not contribute to the study design, management, analysis or interpretation of this article.
The authors' contributions were as follows: M. R. L. F. conducted the research, analysed the data and wrote the paper; C. Z. assisted with the extraction of tissues and performed the analysis of the data; B. J. B. designed the research, analysed the data, wrote the paper and had primary responsibility for the final content. All authors read and approved the final version of the paper.
The authors have no conflicts of interest.











