INTRODUCTION
Carriage of methicillin-resistant Staphylococcus aureus (MRSA) in healthy animals has been of concern since transmission of MRSA between humans and animals was first suspected more than 20 years ago. Historically, a healthy cat carrying MRSA on its skin and paws was the first animal to be implicated in reverse zoonotic spread of MRSA to vulnerable patients [Reference Scott1]. Since then, other case reports have supported the concept of carrier animals as vectors for recurrent human infection [Reference Manian2] or promoting recurrent MRSA colonization in their owners [Reference Cefai, Ashurst and Owens3]. Although other causes, such as contaminated environments, were not directly investigated in these settings, human re-infections and re-colonization ceased after elimination or decontamination of the suspected animal carriers combined with routine treatment and hygiene measures.
In humans, carriage of MRSA is a key factor in the success of this opportunistic pathogen; first, by increasing the risk of subsequent MRSA infection for the carrier individual and second, by contributing to its spread [Reference Casewell and Hill4, Reference Davis5]. Consequently, much research has focused on the prevalence of human MRSA carriage, at least in risk groups, on the identification of risk factors for MRSA carriage and on treatment strategies [Reference Albrich and Harbarth6].
In contrast, little is known about MRSA carriage in companion animals even though such carriage may have an impact on animal and human health. For example, carrier animals may be at risk of subsequent MRSA infection themselves [Reference Weese and Lefebvre7] or they may spread MRSA, particularly into veterinary environments, thus putting other animals at risk of infection. Furthermore, most importantly, MRSA carriage in dogs, cats and horses can present a risk for human health as close contact between companion animals and their owners provides opportunity for exchange of pathogens. This may be the case with friendly contact [Reference Scott1, Reference Manian2], and also after animal bites [Reference Oehler8], or at healthcare and nursing facilities where pets visit for companionship [Reference Lefebvre9].
Following the initial case reports, various cross-sectional studies have failed to isolate MRSA from healthy companion animals in Slovenia [Reference Vengust10], Denmark [Reference Bagcigil11], The Netherlands [Reference Busscher, van Duijkeren and Sloet Oldruitenborgh-Oosterbaan12], USA [Reference Hanselman, Kruth and Weese13] and Canada [Reference Burton14, Reference Weese15]. Other studies have reported infrequent MRSA isolation (0·5–2%) from similar populations in Brazil and Hong Kong [Reference Lilenbaum, Nunes and Azeredo16, Reference Boost, O'Donoghue and James17]. In contrast, among animals admitted to referral hospitals, carriage was recognized in up to 20% of dogs hospitalized during outbreak conditions in a Canadian veterinary teaching hospital [Reference Weese18].
In the UK, only three reports have been published to date on the frequency of MRSA carriage in healthy companion animals. No MRSA was isolated from 40 horses, 22 dogs and 24 cats in the Liverpool area [Reference Baptiste19] and from 50 therapy dogs in Scotland [Reference Leslie20]. In contrast, 8% of dogs in a rescue kennel carried MRSA, at least transiently, during an MRSA outbreak [Reference Loeffler21]. Beyond that, a Royal Veterinary College (RVC), London, pilot study in 2004 had identified a surprisingly high carriage rate of 9% in dogs referred to the small animal teaching hospital for non-MRSA disease [Reference Loeffler22]. Similarly, 16% of horses hospitalized during an MRSA outbreak tested positive in the Liverpool university equine referral hospital [Reference Baptiste19]. More information is needed, particularly in the UK where pets, horses and MRSA are frequent, to help to assess the risk that animal contact may pose to humans.
In people, risk factors for MRSA colonization or carriage have been investigated mainly in patients admitted to healthcare facilities. These include antimicrobial therapy, hospitalization and HIV infection [Reference Abudu23–Reference Jernigan25]; bacterial interference at carriage sites and host-specific characteristics selecting for specific lineages have also been proposed as risk factors for human nasal carriage of MRSA [Reference Dall'Antonia26].
For dogs and cats, such predisposing factors have not been reported to date; however, in horses, previous MRSA carriage, antimicrobial therapy, living at an MRSA-positive farm and hospital-specific interventions predisposed to nosocomial MRSA carriage [Reference Weese and Lefebvre7, Reference Weese27]. Due to the zoonotic nature of MRSA and the close relationship between people and pets and horses, knowledge of risk factors for carriage could aid infection control measures both in human and veterinary medical fields.
This study determined prevalence estimates for MRSA carriage in a convenience sample from purposely selected populations of companion animals in the Greater London area and compared healthy animals to those presenting for first-opinion veterinary care. Risk factors for MRSA carriage were investigated in animals presenting for veterinary care.
MATERIALS AND METHODS
Study design
A survey of dogs, cats and horses living in the community in the Greater London area was conducted between January 2007 and October 2008 in order to estimate the prevalence of MRSA carriage. A convenience sample was taken of animals stratified by host species and within that, by health status. The ‘healthy animals’ category included rescue dogs and cats and working horses considered fit for re-homing or work which were free of non-neoplastic skin disease and wounds, did not suffer from health problems other than chronic osteoarthritis, controlled heart or thyroid disease and urinary incontinence, and had not received any medication or veterinary treatment except routine examination, neutering, vaccination or parasiticides on site by the facility veterinary surgeon. The ‘veterinary treatment animals’ category was based on privately owned dogs, cats and horses presenting to first-opinion veterinary surgeons for preventive, medical or surgical care. Animal facilities and veterinary establishments with large catchment areas and case-loads were selected purposely. Animals were sampled at one rescue kennel (Dogs Trust, Rehoming Centre, Harefield, London), one cattery [Royal Society for the Protection against Cruelty to Animals (RSPCA) Animal Rehoming Centre, Southridge, Hertfordshire] and at two military stables (Household Cavalry Mounted Regiment, Knightsbridge, and King's Troop Royal Horse Artillery, St John's Wood, London). Rescue dogs and cats were housed in pairs unless their behaviour allowed individual kennelling only; working horses were kept in individual boxes with rare opportunity for direct animal contact other than with their box neighbours and occasionally during training or work. ‘Veterinary treatment group’ animals had presented for first-opinion veterinary care to one of two clinics [RSPCA London North (Harmsworth Memorial) Animal Hospital, London; RVC equine first-opinion clinic, Hertfordshire]. Information on health and veterinary care for these animals was gathered from their medical histories covering the 6-month period prior to sampling. Numbers of animals to be sampled were calculated by assuming an MRSA carriage of 3·5% and 5% in ‘healthy’ and ‘veterinary treatment’ animals, respectively, from previous reports [2% error, 95% confidence interval (CI)] (WinEpiscope 2.0) [Reference Lilenbaum, Nunes and Azeredo16, Reference Baptiste19, Reference Loeffler22]. Estimated population sizes are shown in Table 1. All animals present on the sampling days at the selected facilities were sampled unless owners or animals did not allow swabbing.
Table 1. Prevalence estimates for MRSA carriage in selected populations of companion animals in the Greater London area
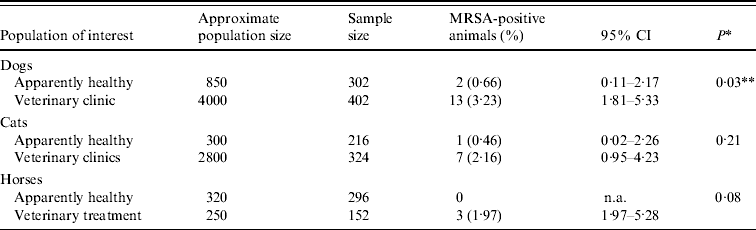
CI, Confidence interval; n.a., not available.
* Comparison between subpopulations of healthy vs. veterinary treatment animals using Fisher's exact test.
** Statistically significant at P<0·05.
Ethics
Written consent was obtained from owners for all privately owned animals and from facility managers for rescue and working animals prior to sampling. The study protocol, consent forms, information sheets for animal owners and recommendations on management of carrier animals had been approved by the Royal Veterinary College Ethics and Welfare Committee. A positive MRSA result was reported to the animal owner or the facility manager. The zoonotic potential was explained and owners were advised to inform their medical practitioner of their animal's carrier status. Follow-up sampling was offered and decontamination recommended if the follow-up swab was positive. In the absence of published controlled data on decolonization in animals, topical antimicrobial therapy with proven efficacy against S. pseudintermedius (chlorhexidine body washes and fusidic acid application to mucocutaneous junctions) [Reference Bond28, Reference Saijonmaa-Koulumies, Parsons and Lloyd29] was prescribed until the first negative result from weekly follow-up sampling was available (therapy withheld 24 h prior to sample collection). Follow-up samples were taken as described below for the initial screening swabs, but in contrast to the screening swabs, follow-up swabs were not pooled but analysed individually per site. Increased hygiene measures and isolation, where practical, were recommended and re-homing of rescue animals was postponed until MRSA could no longer be isolated from the pet.
Sampling and microbiology
Cotton swabs moistened in sterile saline were rolled over mucosae or skin in one nostril, the mouth, one axilla and the perianal area for 3–5 s. The four swabs were pooled and incubated at 37°C for 48 h in tryptone soya broth (Oxoid, UK) with 10% sodium chloride (Sigma-Aldrich, UK) for selective enrichment. Staphylococci were isolated on sheep blood agar (Oxoid), screened for oxacillin-resistance on mannitol-salt agar (Oxoid) with 6 mg/l oxacillin (Sigma-Aldrich) and staphylococcal groups [coagulase-negative staphylococci (CNS), coagulase-positive staphylococci (CPS)] and S. aureus isolates were determined by standard morphological and biochemical tests. CPS were identified based on a positive DNase test plus either a positive slide coagulation test with dog plasma or a positive tube coagulase test using rabbit plasma. CNS were not speciated; those that had grown on mannitol-salt agar with oxacillin (and were subsequently DNase- and coagulase-negative when tested from blood agar subcultures) were recorded as methicillin-resistant coagulase-negative staphylococci (MR-CNS).
All S. aureus isolates were confirmed by additional biochemical (Voges–Proskauer reaction) and genetic tests (presence of S. aureus specific nuc) as described previously and all phenotypically MR-CPS were investigated for the presence of mecA [Reference Loeffler21]. S. aureus lineages were determined by characterizing the gene for the specificity subunit (hsdS) of the S. aureus lineage-specific restriction modification (RM) enzyme system. The hsdS of one equine isolate could not be typed by this method and was further investigated by PFGE and spa-typing [Reference Loeffler30].
Statistical analysis and risk factors
Data on animal signalment, veterinary treatment and swab results were recorded; they were compared between groups and subgroups by two-tailed Fisher's exact or Mann–Whitney U tests using SPSS software version 17.0 for Windows. The odds ratio (OR) and its 95% confidence interval was used to compare the odds of being a MRSA carrier between healthy animals and veterinary treatment group with P⩽0·05 indicating statistical significance.
Signalment data, where of sufficient quality, were compared between ‘healthy’ and ‘veterinary treatment’ groups within each species. Ages for rescue dogs and cats were approximated based on physical appearance and dental status and categorized into one of three age groups at the time of sampling (<6 months, 6–24 months, >24 months) [Reference Schummer, Habermehl, Nickel, Schummer and Seiferle31].
The effect of individual animal characteristics and veterinary treatment-related variables on MRSA carriage was examined within the ‘veterinary treatment’ groups by exact logistic regression analysis using SAS for Windows version 9.2 (SAS Institute, USA). Data from dogs and cats were combined as ‘pets’ based on the assumption that dogs and cats visit the same veterinary premises, share their living environment with humans and are likely to undergo similar veterinary treatment procedures with regard to diagnostic tests, surgical procedures and indications for antimicrobial prescribing. Horses were analysed separately. Fourteen nominal- and one ordinal-scale (age) potential risk-factor variables, similar to those reported as risk factors for human MRSA carriage [Reference Hidron24, Reference Jernigan25] (Table 2), were screened for their association with MRSA carriage. In addition, ‘species’ (dog or cat) was included in the univariable analysis within the pet group. Those variables, significant at P⩽0·2, were included in a multivariable model. Forward stepwise variable selection based on the likelihood ratio statistic using P⩽0·05 for entry was used to identify the final multivariable logistic regression model.
Table 2. Univariable screening of putative risk factor variables between MRSA carrier animals and non-carrier animals in 878 companion animals presenting for veterinary treatment
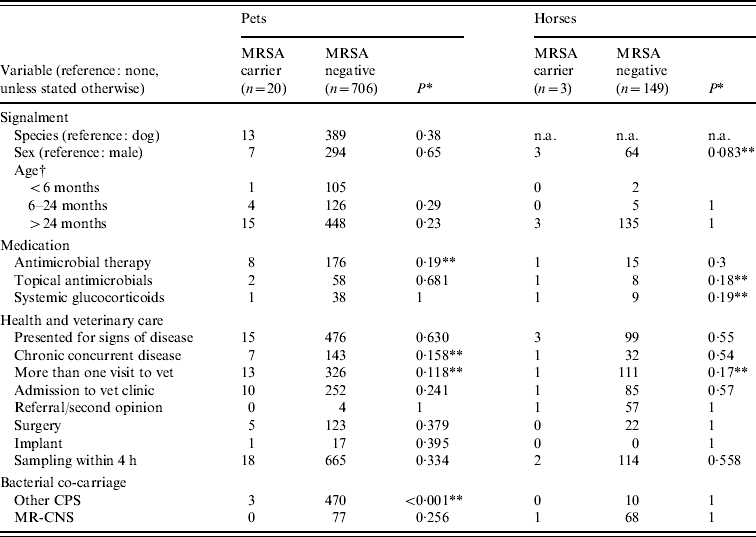
CPS, Coagulase-positive staphylococci; MR-CNS, methicillin-resistant coagulase-negative staphylococci; n.a., not available.
† Compared by univariable logistic regression.
* Fisher's exact test.
** Significant at P<0·2.
Apparently healthy animals were sampled irrespective of their length of stay at the facility; those presenting for veterinary treatment were grouped into animals sampled within or after 4 h of arrival at the veterinary clinic. Reasons for veterinary visits were categorized into preventive measures (e.g. vaccination or claw clipping) and clinical presentations (e.g. vomiting, pruritus). Animal health characteristics and veterinary interventions were recorded from the medical histories. Additional potential risk factors investigated were the isolation of non-MRSA CPS and of MR-CNS from carriage sites.
Strict hand hygiene and voluntary self-sampling for nasal MRSA carriage were implemented by the investigators to avoid contamination of samples.
RESULTS
MRSA prevalence
A total of 1692 companion animals were sampled. Available signalment data of the 814 healthy rescue and working animals and of the 878 animals presenting for veterinary treatment are summarized in Table 3. The 704 dogs, 540 cats and 448 horses represented about 15%, 17% and 78%, respectively, of the total populations housed or seen at the centres. At the clinics, 799 animals (91·0%) were sampled within 4 h of arrival, another 56 (6·4%) were sampled within 48 h of arrival while the remaining 23 (2·6%) had been hospitalized for at least 2 days.
Table 3. Available signalment of 1692 animals sampled for MRSA carriage
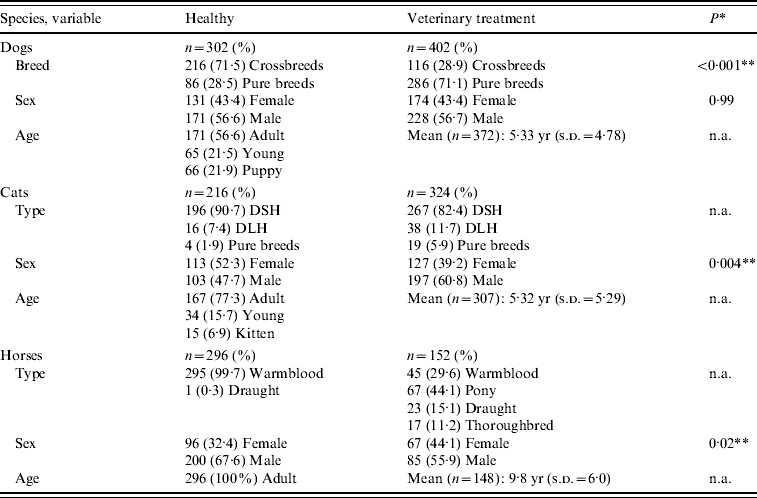
DSH, Domestic shorthaired cat; DLH, domestic longhaired cat; s.d., standard deviation; n.a., not available.
* Fisher's exact test.
** Statistically significant at P<0·05.
MRSA carriage was identified in 26 animals (1·53%, 95% CI 0·95–2·13), including 15 (2·13%, 95% CI 1·06–3·2) of all dogs, eight (1·48%, 95% CI 0·46–2·5) of all cats and three (0·67%, 95% CI −0·09 to 1·43) of all horses with the individual subgroups detailed in Table 1. There was no difference in carriage proportions between the three host species. However, only three carriers (two dogs, one cat) were identified in the healthy populations, and animals presenting for veterinary treatment had a seven times increased odds of carrying MRSA than apparently healthy animals (2·62% vs. 0·37%; OR 7·27, 95% CI 2·18–24·31, P<0·001). Following stratification by species, the same trend was seen for each species individually but the association was only statistically significant for dogs (OR 5·01, 95% CI 1·12–22·38, P=0·03). Non-S. aureus CPS with methicillin resistance were not identified.
Follow-up
The two healthy carrier rescue dogs were adult, neutered male crossbreeds. One had been at the facility for 3 weeks, the other for 4 days prior to swabbing. They were housed in different kennels on the same corridor and each shared their kennel with one other dog, both of which were negative for MRSA. Follow-up swabs from both dogs taken 1 week later did not grow any staphylococci.
The 13 carrier dogs in the veterinary treatment group included five females and eight males of eight different breeds and three crossbreeds, aged between 10 months and 12 years (mean 5·4 years), weighing between 3 and 39 kg (mean 15·2 kg). Six dogs had presented with skin disease (five as outpatients with MRSA-unrelated dermatitis and one hospitalized for treatment of demodicosis); the other seven had been seen as outpatients for MRSA-unrelated disease or preventive interventions (claw clipping, vaccination). Follow-up swabs were taken from 12 dogs while one owner could not be contacted. Ten dogs were negative at the first follow-up sampling without antimicrobial therapy. Two had remained positive after 1 week (one dog in the nostrils, the other in mouth and around the anus). Those two dogs received decolonization therapy and sampled negative another week later.
All eight feline carriers were domestic shorthaired animals including three female and four male neutered adults and one entire male kitten. One cat presented for vaccination, the others for MRSA-unrelated disease or interventions (blood sampling, examination after trauma, tumour excision). Follow-up swabs were taken from seven cats (one not returned by its owner) 10–15 days after the first sampling (delay due to non-compliance) and all were negative for MRSA.
The three horses carrying MRSA were adult mares, one crossbred draught horse, one Welsh pony and a European warmblood; one was seen for sweet itch, the others for lameness. The follow-up swab from the sweet-itch patient was negative while follow-up samples were not available for the other two horses.
Typing
Twenty-nine MRSA (26 initial carriage isolates plus three from follow-up swabs) from dogs, cats and horses were investigated for their S. aureus lineage identity. Sixteen dog isolates, 13 from initial screening samples and three from first follow-up swabs and all eight feline MRSA belonged to clonal cluster (CC) 22; the remaining two canine isolates were of lineage CC30/sequence type (ST) 36. The three equine MRSA belonged to three different lineages; one was identified as CC22, the second as CC8/ST239 while the third isolate was negative on all three RM assays; it was subsequently identified as a strain compatible with ST398 [Reference Loeffler30].
Risk factors
Investigation of individual putative risk factors within the veterinary treatment groups (pets and horses) by univariable screening showed that four variables in each of the two host species groups were associated with MRSA carrier status (Table 2). In the univariable screening using P⩽0·2, ‘antimicrobial therapy’, ‘concurrent chronic disease’, ‘more than 1 visit to veterinary clinics’ and ‘concurrent carriage of CPS’ were associated with MRSA carriage in pets; ‘sex’, ‘topical antimicrobial therapy’, ‘systemic glucocorticoids’ and ‘only one visit by a veterinary surgeon’ were significant in horses. In the subsequent multivariable analysis, only ‘concurrent carriage of CPS’ was retained for pets in the model (OR 0·088, 95% CI 0·016–0·31, P<0·001). For horses, none of the four variables included was retained in the final multivariable model.
Analyses of ‘concurrent carriage of CPS’ separately for dogs and cats showed that its negative association with MRSA carriage at univariable level was highly significant in dogs (P<0·001, Fisher's exact test) but not in cats (P=0·71).
DISCUSSION
The findings from this study, the largest survey on MRSA carriage in healthy companion animals in the UK published to date, indicated that MRSA carriage in the selected populations of companion animals in the Greater London area was low.
The low overall MRSA carriage of 1·5% in the sampled companion animals is encouraging but must not lead to complacency among veterinary professionals. In fact, MRSA was found in five of the six studied populations which highlights that animals should be considered in infection control strategies where vulnerable human patients are involved and where human infections are recurrent [Reference Manian2]. In humans, MRSA carriage in the community is thought to have remained low between 0·5 and 2% despite a substantial rise in hospital-associated MRSA [Reference Abudu23, Reference Grundmann32, Reference Maudsley33]. This emphasizes that MRSA requires the selective advantage of healthcare environments and a similar scenario may exist in veterinary hospitals. One Swiss study demonstrated that staphylococci isolated from horses several days after hospitalization carried significantly more resistance genes than those isolated pre-admission [Reference Schnellmann34]. In addition, an occupational risk for MRSA carriage has recently been identified for UK veterinary staff [Reference Loeffler35]. Thus, efforts must continue to reduce such selective advantage for multidrug-resistant pathogens by conscientious use of antimicrobial agents and excellent hygiene in veterinary practices.
Previously, most studies investigated MRSA animal carriage in connection with outbreaks or in veterinary hospitals. Carriage rates were between 5% and 13% and raised concern over pets and horses as reservoirs for MRSA [Reference Weese15, Reference Baptiste19, Reference Loeffler22, Reference Faires, Tater and Weese36]. However, screening of animals that had little or no association with known MRSA infection yielded varied results. MRSA was reported in 0·5–4% of healthy companion animals, typically from countries with a high prevalence of human MRSA [Reference Hanselman, Kruth and Weese13, Reference Lilenbaum, Nunes and Azeredo16, Reference Boost, O'Donoghue and James17]; others found no MRSA [Reference Vengust10–Reference Busscher, van Duijkeren and Sloet Oldruitenborgh-Oosterbaan12]. Thus, the presented results help to confirm that MRSA carriage in companion animals in the community is indeed low even in the UK where MRSA is endemic in human hospitals.
While false-positive isolation was highly unlikely following the genetic confirmation of MRSA, false-negative results cannot be ruled out. Salt enrichment, the use of moistened swabs and sampling of four different sites were chosen to achieve a high sensitivity of test methods [Reference Noble, Somerville, Noble and Somerville37]. An underestimation of MRSA may have resulted from the use of high salt concentrations (10%) and oxacillin rather than cefoxitin discs [Reference Pottumarthy38]. However, the methods were designed with the objective of sampling all CPS from animals which is less reliably done with methods purely extrapolated from human S. aureus studies [Reference Bemis39]. Swabs from the four carriage sites were pooled per animal to allow sampling of large populations in a labour- and cost-efficient manner. Pooling is likely to be of value in animal studies where the best site for sampling remains unknown and where animal cooperation during sampling may be inconsistent.
In addition, the purposive selection of populations may have influenced carriage rates. As the results from this study were not based on random selection of animals from the three species, comparisons conducted between populations within this study should consider potential biases in risk factors, and particular care needs to be taken when generalizing the findings to the Greater London area and beyond. Still, the study provides baseline information that will be useful for informing policy development as well as design of further studies.
Surprisingly, risk factors ‘classical’ for human MRSA carriage such as antimicrobial use or hospitalization were not associated with MRSA carriage in pets or horses. As sample sizes were originally calculated for the purpose of estimating prevalence of MRSA carriage and not for group comparison, the statistical power of this analysis may be low. Exact logistic regression was chosen to allow for the small number of positive animals, and dogs and cats were combined to increase power. However, no convincing trends for predisposing risk factors were apparent. This is in contrast to the only carriage risk-factor study published on companion animals to date where antimicrobial use within 30 days of admission to an equine referral hospital predisposed horses to nosocomial MRSA colonization [Reference Weese and Lefebvre7, Reference Weese27]. While antimicrobial therapy is expected to favour true MRSA colonization by suppressing any susceptible competing bacterial microflora, it is less likely to influence contamination of animals. Contamination would be predominantly influenced by the extent of MRSA exposure, most likely through contact with human carriers and contaminated environments [Reference Manian2, Reference Casewell and Hill4]. Thus, the findings provide further support to the concept that companion animals are vectors rather than reservoirs for MRSA and suggest that MRSA control, at least in dogs and cats, may be achieved with simple but well designed hygiene strategies.
The results also indicate a protective effect of concurrent carriage of non-MRSA CPS but only in dogs. A protective effect of methicillin-susceptible S. aureus against MRSA colonization has been suggested in humans previously and further research in this area is warranted [Reference Dall'Antonia26].
All pet MRSA carriage was of dominant human healthcare-associated S. aureus lineages, which suggests that their origins are in human hospitals. In horses, only one healthcare-associated CC22 was found, while the CC8 MRSA was in line with previous reports from other countries which proposed this lineage as typically equine associated [Reference Weese40]. The third isolate, compatible with MRSA ST398, was probably associated with importation from continental Europe [Reference Loeffler30]. In view of the recent isolation of MRSA ST398 from 9% and 11% of equine hospital admissions in continental Europe [Reference van den Eede41, Reference van Duijkeren42], monitoring of carriage in horses should continue.
The infrequent isolation of MRSA from healthy companion animals in the selected populations indicated that the risk to human health from companion animal MRSA carriage is likely to be small, at least for immunocompetent people, and that it should not overshadow the benefit gained from companionship with animals [Reference Friedmann and Son43]. The protective effect of other staphylococci at carriage sites, in the absence of other risk factors for carriage, warrants further investigation.
ACKNOWLEDGEMENTS
This work was funded by the PetPlan Charitable Trust (Grant 06-15). The authors are grateful to David Grant (RSPCA), Chris Laurence (Dogs Trust), Major Beverley DeRenzy Channer and Major Rosalind Jennings (Household Cavalry Mounted Regiment & The King's Troop, Royal Horse Artillery) for their support with the study.
DECLARATION OF INTEREST
None.





