Protein detection is critical in many biomedical and clinical applications, including disease diagnosis and food allergen detection. Wesley Wong and colleagues at Harvard Medical School and Boston Children’s Hospital recently reported in Proceedings of the National Academy of Sciences (doi:10.1073/pnas.1708148114) that structural changes in antibody-conjugated DNA nanoswitches can provide a cheaper, faster, and more sensitive method for protein detection than the gold standard, enzyme-linked immunosorbent assay (ELISA). They also showed that in their nanoswitch-linked immunosorbent assay (NLISA), results can be read off of mobile-imaging platforms to facilitate remote detection.
The motivation for their work stems from wanting to improve sensitivity, specificity, speed, and costs and to avoid the many washing steps in current ELISA assays. First author Clinton Hansen stressed that “unlike ELISA, no enzymes are required with the nanoswitch reagents which are composed solely of DNA and antibodies that can be lyophilized to enable a long shelf life under ambient or refrigerated conditions.”
To create a NLISA, Hansen and co-workers had to first conjugate antibodies to short polymers of nucleic acid known as oligonucleotides, which have complementary sequences to parts of a linear DNA molecule. This was followed by coupling the antibody-oligonucleotide to linear DNA to create the nanoswitch; see (a) in the figure. The binding of the nanoswitch to their target antigen analytes causes the linear DNA to develop a looped conformation; see (b) in the figure.
Next, the team made use of gel electrophoresis, a widely used technique where negatively charged DNA molecules are separated based on size and conformation in an electric field; see (b) in the figure. Linear, unbound DNA passes through a porous gel faster than analyte-bound looped DNA, allowing DNA separation. The intensities of the separated bands can be quantified to determine the concentrations of the analytes. The limit of detection (LOD) defined as the concentration that exceeds 3 standard deviations of the background signal for the DNA nanoswitches was found to be 0.4–1.5 femto (10–15)-Molar (fM).
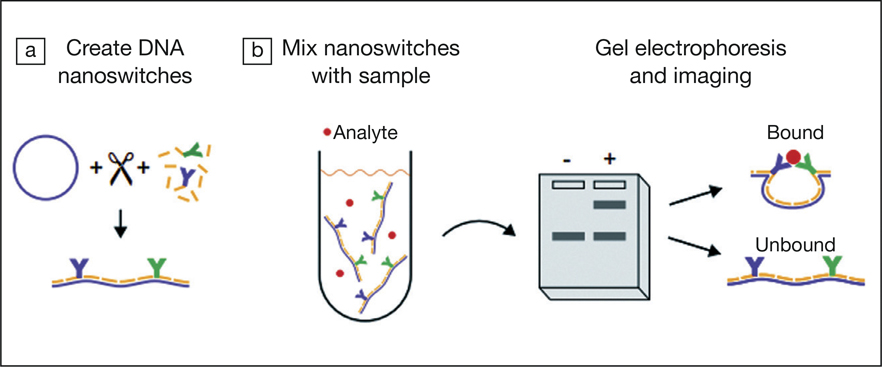
Schematic of a nanoswitch-linked immunosorbent assay. (a) DNA nanoswitches are first made with enzymatic digestion of circular DNA into linear DNA. This is followed by conjugating oligonucleotide antibodies to the digested, linear DNA. (b) Nanoswitches bind to antigen analytes in samples causing conformational changes in DNA to form looped structures. With gel electrophoresis, linear and looped DNA can be separated by size, allowing protein detection and quantification. Credit: PNAS.
The researchers demonstrated the utility of NLISA with well-characterized binding between biotinylated oligonucleotide and streptavidin and achieved a LOD at 9–22 fM. They then applied their technology for detection in urine and serum, which are the most common biological fluids used for diagnostics. The researchers synthesized nanoswitches by conjugating antibodies, which bind to prostate-specific antigen (PSA), to oligonucleotides. PSA is commonly used to determine if a patient suffers from a prostate disease like cancer, although its utility as a diagnostic marker is controversial. Compared to a commercial PSA test kit (SimpleStep) with a LOD of 220 fM, the team achieved a LOD eight times lower with the same serum volume. The time needed for PSA detection using NLISA is also halved compared to the commercial kit. The team additionally highlighted that the performance of NLISA can be tailored to its application, specifically “if increased sensitivity is required, we can perform NLISA with a longer incubation time.”
The research group also presented data showing lower cross-reactivity using NLISA compared to ELISA. The higher specificity of NLISA originates from the weak binding of the antibodies to off-target antigen analytes in the nanoswitches, forming loose DNA loops. As a result, these loops tend to unfold and revert to a linear conformation when they are being separated during gel electrophoresis.
Donghyuk Kim at the University of California, Los Angeles, who was not involved in this study, commented that the technology is “clever” as “the only chemistry is the target-specific antigen-antibody binding in their system which removes any need for washing steps and thermal controls.” However, he notes that the presented sensitivity is dependent on the analysis by the researchers, and hopes that they provide a more straightforward analysis method.
Kim, who also developed a protein detection assay with comparable LOD (ACS Nano, doi:10.1021/acsnano.6b02060), hopes to see how the NLISA platform can be “multiplexed by varying loop structures to detect several biomarkers” as many diseases are complex and cannot be well represented with a single marker.
Wong and his team suggest that “with the proper engineering efforts, we could also envision our technology embedded into a small handheld device to enable clinical-grade point-of-care detection in both the developed and developing world.”


