Twins are usually classified as monozygotic (MZ) or dizygotic (DZ). MZ twins arise from cleavage of a single zygote and therefore share 100% of their DNA (Sadler, Reference Sadler2003; Townsend et al., Reference Townsend, Hughes, Luciano, Bockmann and Brook2009; Wong et al., Reference Wong, Gottesman and Petronis2005). The timing of cleavage determines the nature of the placental membranes. Early cleavage of the zygote, within the first three days following conception, results in monochorionic (MC) placentation. Later cleavage, from day 4 post-conception, results in dichorionic (DC) placentation (Bebbington, Reference Bebbington2009; Benirschke, Reference Benirschke1995; Boklage, Reference Boklage1981; Hur & Shin, Reference Hur and Shin2007; Nikkels et al., Reference Nikkels, Hack and Gemert2008; Race et al., Reference Race, Townsend and Hughes2005; Salafia & Maas, Reference Salafia and Maas2005). DZ twins, on the other hand, arise from two separate zygotes and share on average 50% of their DNA. As no cleavage takes place, DZ twins, in most circumstances, are DC (Newman et al., Reference Newman, Freeman and Holzinger1937; Sadler, Reference Sadler2003; Townsend et al., Reference Townsend, Hughes, Luciano, Bockmann and Brook2009; Wong et al., Reference Wong, Gottesman and Petronis2005).
Chorionicity has been suggested to influence prenatal growth and development (Bebbington, Reference Bebbington2009; Gaziano et al., Reference Gaziano, Lia and Kuhlmann2000; Moon et al., Reference Moon, Park, Song, Yang, Kim, Hong and Park2008). MC twin pairs are thought to be predisposed to dominance of one twin over the other through uneven placental blood flow to the twin fetuses as well as placental asymmetry (Benirschke, Reference Benirschke1995; Lewi et al., Reference Lewi, Gucciardo, Huber, Jani, Mieghem, Doné and Deprest2008; Nikkels et al., Reference Nikkels, Hack and Gemert2008; Salafia & Maas, Reference Salafia and Maas2005). This may in turn lead to BWD which, if severe, can result in significant morbidity or mortality of the twin fetuses. One complication particularly associated with MC twin pregnancies is twin-twin transfusion syndrome (TTTS), which arises from unequal sharing of the fetal vasculature due to anastomoses between the twin fetuses in utero (Gaziano et al., Reference Gaziano, Lia and Kuhlmann2000; Moon et al., Reference Moon, Park, Song, Yang, Kim, Hong and Park2008). These anastomoses may be superficial, bidirectional and have low resistance to blood flow; for example, arterio-arterial (AA) or venous-venous anastomoses (VV); or they may be deep, unidirectional and have high resistance to blood flow, for example, arterio-venous (AV) anastomoses (Bebbington, Reference Bebbington2009; Benirschke, Reference Benirschke1995; Fisk et al., Reference Fisk, Duncombe and Sullivan2009; Gaziano et al., Reference Gaziano, Lia and Kuhlmann2000; Nikkels et al., Reference Nikkels, Hack and Gemert2008). The most detrimental outcome from TTTS is believed to arise when there is an AV anastomosis in the absence of an AA or VV anastomosis, particularly if coupled with placental asymmetry (Bebbington, Reference Bebbington2009; Fisk et al., Reference Fisk, Duncombe and Sullivan2009; Gaziano et al., Reference Gaziano, Lia and Kuhlmann2000; Nikkels et al., Reference Nikkels, Hack and Gemert2008). Both fetuses suffer in TTTS and often, if left untreated, the result is death of one or both fetuses (Huber & Hecher, Reference Huber and Hecher2004).
An area that remains largely unexplored is whether prenatal environmental conditions associated with MC twin pregnancies influence dental development. MZMC twins, while genetically identical, have been shown to exhibit significant intra-pair variation in permanent tooth size, which may be an indicator of the stressed prenatal environment to which MC twin fetuses are subjected (Burris & Harris, Reference Burris and Harris2002; Race et al., Reference Race, Townsend and Hughes2005). Race and colleagues (Reference Race, Townsend and Hughes2005) postulated that the effects of chorion type on primary teeth would be greater than the effects on permanent teeth, as primary teeth develop prenatally, a period when the fetus is dependent upon the placenta for survival.
Primary tooth emergence is believed to have a polygenic, multifactorial mode of inheritance (Garn et al., Reference Garn, Lewis and Kerewsky1965; Townsend et al., Reference Townsend, Richards, Hughes, Pinkerton and Schwerdt2005), with estimates of heritability being very high (Hughes et al., Reference Hughes, Bockmann, Seow, Gotjamanos, Gully, Richards and Townsend2007). While the timing of emergence is thought to be most strongly influenced by genetic factors, epigenetic factors, both at the DNA, and at the local tissue level, as well as environmental factors are also thought to play a small but significant role in contributing to observed variation (Townsend et al., Reference Townsend, Hughes, Luciano, Bockmann and Brook2009; Reference Townsend, Richards, Hughes, Pinkerton and Schwerdt2005). Several previous studies have investigated the influence of gestational age, birth weight, and nutritional status on primary tooth emergence, finding that pre-term, low birth weight twins were older at the timing of emergence of the first primary tooth (Jelliffe & Jelliffe, Reference Jelliffe and Jelliffe1973; Lysell et al., Reference Lysell, Magnusson and Thilander1962; Sajjadian et al., Reference Sajjadian, Shajari, Jahadi, Barakat and Sajjadian2010; Seow et al., Reference Seow, Humphrys, Mahanonda and Tudehope1988). However, a clear relationship between chorionicity and the timing of primary tooth emergence is yet to be established.
In this study, we aimed to determine whether significant relationships exist between chorion type, gestational age, birth weight, birth length, and the timing of emergence of the first primary tooth. We also sought to determine whether a significant relationship exists between chorion type and BWD and between BWD and discordance in the timing of emergence of the first primary tooth.
Materials and Methods
The data for this study come from two samples of twins, Australian and Dutch. The Australian data were collected as part of a larger investigation of Australian twins undertaken by the Craniofacial Biology Research Group at the School of Dentistry, The University of Adelaide. The ongoing twin studies in Adelaide aim to utilize robust models to explore the influence of genetic, epigenetic, and environmental factors on various dental traits and facial features (Townsend et al., Reference Townsend, Richards, Hughes, Pinkerton and Schwerdt2005; Reference Townsend, Hughes and Richards2006). The Australian study sample involved 409 pairs of Australian twins of European ancestry, including 198 MZ, 122 dizygotic same-sex (DZSS), and 89 dizygotic opposite-sex (DZOS) twin pairs. The zygosity of the twin pairs was determined by DNA analysis and information on chorion type, gestational age, birth length, and birth weight was collected in two questionnaires administered to parents when twins were three months and two years of age. The questionnaires required parents to record whether the twins had one or two placentas in utero. Chorionicity was then inferred from the parental report. Timing of first primary tooth emergence (PTE) was calculated in days for both chronological age and post-conception age. The validity of parental recordings was confirmed by clinical examination of 10% of the study sample (Hughes et al., Reference Hughes, Bockmann, Seow, Gotjamanos, Gully, Richards and Townsend2007).
The data from the Dutch sample were collected by the Netherlands Twin Registry (NTR) and form part of a larger study of twins that focuses on investigating differences in mental and physical health in children and adults (Boomsma et al., Reference Boomsma, de Gues, Vink, Stubbe, Distel, Hottenga and Willemsen2006, van Beijsterveldt et al., Reference van Beijsterveldt, Groen-Blokhuis, Hottenga, Franić, Hudziak, Lamb and Boomsma2013). The Dutch sample is from a pilot study for record linkage (van Beijsterveldt et al., Reference Van Beijsterveldt, Oberbeek, McMaster, Rozendaal, Glasner, Bartels and Boomsma2015) of twins born in the year 2000. The Dutch study sample involved 301 pairs of Dutch twins of European ancestry, including 127 MZ, 113 DZSS, and 61 DZOS twin pairs. The zygosity of the twin pairs was determined by short nucleotide polymorphism (SNP) genotyping from buccal swabs and information on gestational age, birth length, and birth weight was obtained from a questionnaire administered to the mother of the twins usually a few weeks or months after the birth. Data on chorionicity were obtained through linking data from the NTR with those from the Pathologisch Anatomisch Landelijk Geautomatiseerd Archief [Pathological Anatomy National Automatic Archive of the Netherlands] (PALGA) database and biobank. Data on PTE were obtained in a separate questionnaire administered to the mother when the twins were three years of age.
The Australian and Dutch samples were analyzed independently, with the Dutch sample providing a replicate sample for comparison with the Australian sample. Summary statistics for gestational age, birth weight, birth length, and PTE were calculated using one randomly selected twin from each pair. Comparisons between groups were made using ANOVA (Proc GLM, SAS 9.2) followed by Tukey's post-hoc tests, with statistical significance set at p < .05.
Data from MZ twins were analyzed subsequently to explore the association between chorion type and intra-pair BWD, and to explore the association between BWD and discordance in PTE. BWD between co-twins were expressed as percentages, and ordinalized as follows: BWD <10%; 10% ≤BWD ≤20%; BWD >20%. Discordance in PTE was recorded when there was greater than 14 days difference in the timing of emergence between co-twins, which is in accordance with Race et al. (Reference Race, Townsend and Hughes2005) and Mihailidis et al. (Reference Mihailidis, Woodroffe, Hughes, Bockmann and Townsend2009). Frequency data were analyzed using Fisher's exact test due to small sample sizes.
Results
For both the Australian and Dutch samples, data for DC (fused) and DC (separate) twins were combined, as analyses of variances and mean values using F and t tests respectively found no significant differences between the two groups.
Associations were observed between MC placentation, gestational age, birth length, and birth weight. In the Australian sample, the average gestation length of MC twins was 35 weeks relative to the normal singleton gestation length of 37 weeks, and significantly shorter than the average gestation length of DC twins (p < .05). MC twins were also found to have a significantly lower mean birth length (p < .05) compared with DC twins. Analysis of chorion type in relation to mean PTE found no significant association between the two variables for either chronological (p > .05) or post-conception age (p > .05).
In the Dutch sample, the average gestation length of MC twins was 36.6 weeks. There was no significant difference in gestation length for Dutch MC twins when compared with DC twins (p > .05). There was also no statistical difference found for birth length or birth weight for Dutch MC twins compared to Dutch DC twins. Analysis of chorion type in relation to mean PTE found no significant association between the two variables for chronological age (p > .05). Post-conception age was not tested in the Dutch sample. Table 1 illustrates the distribution of the Australian MZ twins according to chorion type and BWD. MZMC co-twins were found to exhibit intra-pair birth weight differences significantly more frequently (85%) than MZDC co-twins (15%), particularly when BWD greater than 20% were evaluated. The distribution of the Dutch MZ twins according to chorion type and BWD can be seen in Table 2. Dutch MZMC co-twins were also found to exhibit intra-pair birth weight differences significantly more frequently (82%) than MZDC co-twins (18%), particularly when BWD greater than 20% were evaluated.
TABLE 1 Frequencies of Percentage Birth Weight Discordance and Chorionicity in Australian MZ Twins
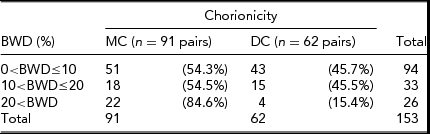
Table probability, p < .05 (χ 2 test); BWD = birth weight discordance.
TABLE 2 Frequencies of Percentage Birth Weight Discordance and Chorionicity in Dutch MZ Twins
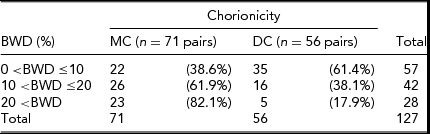
Table probability, p < .05 (χ 2 test); BWD = birth weight discordance.
Since monochorionicity was found to be associated with increased frequency of intra-pair birth weight differences, investigation of whether differences in birth weight were also associated with differences in PTE was undertaken. Results from the analysis of males for BWD >10% are presented in Table 3 (Australian sample) and Table 4 (Dutch sample). When BWD was evaluated at >10% difference in birth weight between co-twins, 10 out of 15 (67%) Australian MZMC male co-twins who showed intra-pair birth weight differences also exhibited differences in PTE (p < .05). However, when BWD was set at >10% difference in birth weight in Dutch co-twins, no relationship was found between intra-pair birth weight differences and differences in PTE (p > .05).
TABLE 3 Frequencies of Birth Weight Discordance (>10%) and Primary Tooth Emergence for Australian MZMC Male Twins

Table probability, p < .05 (χ 2 test); BWD = birth weight discordance; PTE = primary tooth emergence.
TABLE 4 Frequencies of Birth Weight Discordance (>10%) and Primary Tooth Emergence for Dutch MZMC Male Twins

Table probability, p > .05 (χ 2 test); BWD = birth weight discordance; PTE = primary tooth emergence.
Furthermore, when BWD was set at >20% difference in birth weight between co-twins, 7 out of 8 (87.5%) Australian MZMC male co-twins who were discordant for their birth weight also exhibited differences in their PTE. No significant relationship between BWD and discordance in PTE was found between Dutch MZMC male co-twins (p > .05) when BWD was set at >20%.
Results from the analysis of females for BWD >10% are presented in Table 5 (Australian sample) and Table 6 (Dutch sample). No relationship was found between BWD and discordance in PTE for either the Australian or Dutch MZMC female co-twins (p > .05).
TABLE 5 Frequencies of Birth Weight Discordance (>10%) and Primary Tooth Emergence for Australian MZMC Female Twins

Table probability, p > .05 (χ 2 test); BWD = birth weight discordance; PTE = primary tooth emergence.
TABLE 6 Frequencies of Birth Weight Discordance (>10%) and Primary Tooth Emergence for Dutch MZMC Female Twins

Table probability, p > .05 (χ 2 test); BWD = birth weight discordance; PTE = primary tooth emergence.
Similarly, when BWD was evaluated at 20% difference in birth weight, no significant relationship between BWD and discordance in PTE was found between MZMC female co-twins in either cohort (p = 1.00) and (p = .4) for Australian and Dutch samples respectively.
Discussion
The present study supports the view that the timing of emergence of the first primary tooth is strongly influenced by genetic factors and is relatively protected from environmental disturbances. In the Australian sample, monochorionicity was found to be significantly associated with reduced gestational age, low birth weight, and low birth length. In the Dutch sample, however, no significant association was found between monochorionicity and either low birth weight or low birth length. Chorion type was not found to have a significant influence on the mean PTE in either the Australian or Dutch sample.
In both the Australian and Dutch samples, a significant association between chorion type and BWD was found, with MZMC co-twins exhibiting greater frequency of BWD compared with MZDC co-twins. This finding is in accordance with other published studies that have hypothesized that the increased frequency of BWD present in MC twin pregnancies is most likely due to hemodynamic imbalance. This may arise from vascular anastomoses between the twin fetuses, or as a result of greater capture of chorionic vessels of one co-twin over the other (Benirschke, Reference Benirschke1995; Lewi et al., Reference Lewi, Gucciardo, Huber, Jani, Mieghem, Doné and Deprest2008; Nikkels et al., Reference Nikkels, Hack and Gemert2008; Salafia & Maas, Reference Salafia and Maas2005).
A significant finding from the present study was that discordance in PTE occurred most frequently between Australian MZMC male co-twins who were discordant for birth weight. This finding, however, was not supported in the Dutch sample. Several studies in the literature have suggested females have a greater buffering capacity to environmental disturbances than males (Garn et al., Reference Garn, Lewis and Kerewsky1967; Johnson et al., Reference Johnson, Carothers and Deary2009). Although the exact mechanism for this effect is unclear, the extra X chromosome present in females is postulated to play an important role. The female XX chromosome pattern is thought to be more resistant to external influences than the male XY chromosome pattern (Garn et al., Reference Garn, Lewis and Kerewsky1967; Harris, Reference Harris2007; Sobhi et al., Reference Sobhi, Mihailidis, Rogers, Hughes and Townsend2007). As a result, females tend to buffer prenatal environmental factors more readily than males and thus proceed on a more consistent developmental course both pre-and postnatally (Garn et al., Reference Garn, Lewis and Kerewsky1965; Harris, Reference Harris2007; Sobhi et al., Reference Sobhi, Mihailidis, Rogers, Hughes and Townsend2007). It is postulated that, in this study, the poorer buffering capacity of MZMC male co-twins may have contributed to a greater frequency of intra-pair differences in PTE.
The observations from this study provide some support for the proposition that it is not only the ‘nature and severity of the stress’, but also the ‘inability of the individual to buffer against stress’ that causes disturbances in development (Kieser & Groeneveld, Reference Kieser and Groeneveld1988, p. 1204). In the Australian sample of twins, a combination of monochorionicity, BWD (>10% or >20%), and male sex were necessary to effect a high level of discordance in the timing of emergence of the first primary tooth between MZ co-twins. Without all three of these factors, PTE was relatively consistent between MZ co-twins. This reinforces the notion that PTE is a regulated event; one that is well protected from environmental disturbances. The Dutch sample of twins remained well buffered and there was no effect seen on PTE between MZ co-twins.
Studies that use different twin models are valuable as they allow researchers to gain insight into the various influences that genetic, epigenetic, and environmental factors have on dental traits (Townsend et al., Reference Townsend, Richards, Hughes, Pinkerton and Schwerdt2005; Reference Townsend, Hughes and Richards2006; Reference Townsend, Hughes and Richards2009; Reference Townsend, Bockmann, Hughes and Brook2012a; Reference Townsend, Bockmann, Hughes, Mihailidis, Seow, Brook, Townsend, Kanazawa and Takayama2012b). A distinct advantage of the MZ co-twin design is that genetic and common environmental factors are controlled for, which allows for determination of whether specific unique environmental factors, such as chorion type and BWD, influence a defined variable or trait (Townsend et al., Reference Townsend, Hughes, Luciano, Bockmann and Brook2009). However, a weakness of the MZ co-twin design is that it does not control for the influence that epigenetic factors may have on phenotypic variation. There are two proposed mechanisms by which epigenetic factors may influence human dental development. First, differences in DNA methylation or histone acetylation may lead to alterations in gene expression despite an unchanged nucleotide sequence. Second, differences in the cellular responses to the nucleotide sequence and subsequent genes expressed may lead to altered behavior of cells at the localized tissue level (Machin, Reference Machin2009; Machin & Keith, Reference Machin and Keith1999; Townsend et al., Reference Townsend, Richards, Hughes, Pinkerton and Schwerdt2005; Reference Townsend, Hughes, Luciano, Bockmann and Brook2009; Wong et al., Reference Wong, Gottesman and Petronis2005). Previous studies have found that epigenetic factors are likely to contribute to variation in the expression of tooth emergence times, tooth size, supernumerary teeth, and agenesis amongst MZ twin pairs (Townsend et al., Reference Townsend, Richards, Hughes, Pinkerton and Schwerdt2005; Reference Townsend, Hughes, Luciano, Bockmann and Brook2009).
In the present study, data for PTE were obtained from parental reports. Although parental reports present challenges for researchers in terms of their reliability and accuracy, they offer advantages over clinical examinations for large cohort studies (Bockmann et al., Reference Bockmann, Hughes and Townsend2010). Increased power of resolution (days as opposed to months) for tooth emergence times is achieved and the study sample is easier to manage logistically (Bockmann et al., Reference Bockmann, Hughes and Townsend2010). In the Australian sample, the validity of parental recordings was tested on a randomly selected 10% of the study sample. Clinical examinations were conducted on this subset of twins and clinical recordings of emerged teeth in the arch were compared with parental recordings (Hughes et al., Reference Hughes, Bockmann, Seow, Gotjamanos, Gully, Richards and Townsend2007). Data for PTE in the Dutch sample were acquired by questionnaire when the twins were three years old and this must be recognized as a limitation due to issues of recall bias and power of resolution.
Parental reports were also utilized for chorion type determination in the Australian sample. According to Race et al. (Reference Race, Townsend and Hughes2005), although parental reports attempt to differentiate between the different chorion types, errors can arise when attempting to differentiate between MC placentas and DC-fused placentas (Race et al., Reference Race, Townsend and Hughes2005). A significant proportion (approximately 50%) of DC twins have fused placentas, an important fact that is commonly misunderstood by the general community (Derom et al., Reference Derom, Derom, Loos, Jacobs and Vlietinck2003; Loos et al., Reference Loos, Derom, Derom and Vlietinck2001; Race et al., Reference Race, Townsend and Hughes2005). In the Dutch sample, accurate information on chorion type was obtained through access to the PALGA database and biobank and this represents a strong advantage of this sample. The Australian sample would benefit from a more accurate method of chorion type determination, which could now be achieved through investigation of ultrasound reports from hospital records. This could potentially be done in the future to improve the accuracy of results obtained in this study.
Conclusion
Findings from this study support the notion that primary tooth emergence in humans is a regulated event; one that is reasonably resistant to environmental disturbances. In both samples, chorion type was not found to significantly affect the mean timing of primary tooth emergence; however, there were mixed findings in relation to evidence of an association between BWD and discordance in the timing of primary tooth emergence in MZMC male co-twins. Although not supported by the Dutch cohort, the fact that discrepancies in the timing of emergence occurred between birth weight discordant Australian MZMC male co-twins implies that genetic factors may not be solely responsible for primary tooth emergence. This is an area for further investigation.
Acknowledgments
We wish to thank the twins and their families for their ongoing participation in the twin investigations carried out by the Craniofacial Biology Research Group, School of Dentistry, The University of Adelaide. We would also like to acknowledge the Financial Markets Foundation for Children, Australian Dental Research Foundation (ADRF) and the National Health and Medical Research Council (NHMRC) for their financial contributions to this study. This research was facilitated through access to the Australian Twin Registry, a national resource supported by an Enabling Grant (ID 628911) from the National Health & Medical Research Council and access to the Netherlands Twin Register (NTR) and the Pathologisch Anatomisch Landelijk Geautomatiseerd Archief [Pathological Anatomy National Automatic Archive of the Netherlands] (PALGA) database and biobank.








