Introduction
Epiphytic plants constitute a significant proportion of the biodiversity in tropical forests (Gentry and Dodson Reference Gentry and Dodson1987, Kress Reference Kress1989). However, they are often understudied due to accessibility issues and lack of resources. The absence of basic ecological information, e.g. habitat requirements, poses challenges in conserving epiphytes, particularly in a time of rapid global change. There is a need to plan population translocations to counter current and anticipated future threats, as is done with some endangered plants worldwide (Liu et al. Reference Liu, Ren, Liu, Wen, Maunder and Gao2015, Liu et al. Reference Liu, Liu, Jin, Gao, Chen, Liu and Zhang2020, Maschinski and Haskins Reference Maschinski and Haskins2012). The success of such actions depends on understanding the habitat limitations for the species of concern. For example, a good understanding of host species requirements can be used to inform the conservation strategy of threatened epiphytic species (Benzing Reference Benzing1978, Callaway et al. Reference Callaway, Reinhart, Moore, Moore and Pennings2002, Segovia-Rivas et al. Reference Segovia-Rivas, Meave, González and Pérez-García2018, Yang et al. Reference Yang, Sun, Zhu, Downing, Song and Liu2017). Studies of this kind are rare in the tropics, especially on orchids, one of the most diverse plant families among tropical plants.
Throughout a species’ range, there exists a spectrum of habitats, defined by the species’ degree of dependence on various biotic and abiotic factors, including the nature and quality of species interactions (Cassini Reference Cassini2013, HilleRisLambers et al. Reference HilleRisLambers, Harsch, Ettinger, Ford and Theobald2013, Louthan et al. Reference Louthan, Doak and Angert2015, Parmesan Reference Parmesan2006). When species are threatened, conservation strategies depend on understanding those sustaining factors and also anticipating potentially rapid landscape changes (Katina et al. Reference Katinas, Crisci and Posadas2009, Seddon et al. Reference Seddon, Van Heezik and Berkoff2013). As one nears the edge of a species’ distribution, habitat quality may decline until the distribution “limit” for the species is reached (Sexton et al. Reference Sexton, McIntyre, Angert and Rice2009). Distribution edges can include large swaths of habitat types, elevations, and climate zones extending across latitudes.
Southern Florida lies at the northern edge of the distribution for many tropical plant species, orchids included, that have their core range in the Caribbean or tropical America (Nieto-Blázquez et al. Reference Nieto-Blázquez, Antonelli and Roncal2017, Santiago-Valentin & Olmstead Reference Santiago–Valentin and Olmstead2004, Trejo-Torres & Ackerman Reference Trejo-Torres and Ackerman2001). The subtropical climate of southern Florida may not be as ideal for tropical species, but the varied transitional climate may be tolerable, due to tropical seasonality, despite occasional frost events (Downing et al. Reference Downing, Borrero and Liu2016, Obeysekera et al. Reference Obeysekera, Browder, Hornung and Harwell1999). The northward movement of species from the Caribbean and tropical America to southern Florida has already been documented for species with strong dispersal capability naturally (Paulson Reference Paulson2001) or aided by human activities (Pemberton & Liu Reference Pemberton and Liu2007, Pemberton & Liu Reference Pemberton and Liu2008a,b, Skov & Wiley Reference Skov and Wiley2005), and it is likely that more species will follow suit. For endangered species that have limited dispersal abilities, or small populations at the limit of a distribution, range shift or expansion into nearby unoccupied yet desirable habitat may be difficult (Liu et al. Reference Liu, Feng, Chen, Wang, Xie, Deng, Wei, Liu, Zhang and Luo2012, Martin Reference Martin2001). Under such circumstances, a conservation strategy might be used to identify suitable habitats for assisted dispersal, which should enhance the probability of establishment and reproductive success (Münzbergová et al. Reference Münzbergová2004).
The dust-like wind-dispersed seeds of orchids may readily disperse to great distances, but can be site-limited due to their dependencies on mycorrhizae for germination and other periods of their life histories (McCormick & Jacquemyn Reference McCormick and Jacquemyn2014, Yang et al. Reference Yang, Sun, Zhu, Downing, Song and Liu2017). Determination of host tree diversity and substrate specificity for epiphytic orchids is a critical component to understanding their life histories and habitat suitability as a baseline for conservation planning (Adhikari et al. Reference Adhikari, Fischer and Fischer2012, Ilves et al. Reference Ilves, Metsare, Seliškar, García, Vassiliou, Pierce, Tatarenko, Tali and Kull2016, Laube & Zotz Reference Laube and Zotz2006, Migenis & Ackerman Reference Migenis and Ackerman1993, Mújica et al. Reference Mújica, Mably, Skarha, Corey, Richardson, Danaher, Gonzalez and Zettler2018, Tremblay et al. Reference Tremblay1997a, Xiqiang Reference Xiqiang2005). Comparison of host identities and associated plant communities between the core and edge distribution of an orchid species may offer insight into the limiting factors along with its distribution range. The goals of this study are to: (a) identify host trees of Trichocentrum undulatum (Sw.) Ackerman & M.W.Chase in the core range area, i.e. Cuba; (b) compare and contrast host plant community types in Cuba and in southern Florida, the species’ northern distribution edge; and (c) identify potential suitable but unoccupied habitats for T. undulatum in southern Florida where the species is highly threatened with extinction.
Study species
Trichocentrum undulatum is an epiphytic orchid with a distribution in the Greater and Lesser Antilles and southern Florida of the United States (Cetzal-Ix et al. Reference Cetzal-Ix, Carnevali and Romero-González2016). The orchid can be found throughout the entirety of the island country of Cuba and in historically large numbers in Jamaica, with these two islands being the core range in the Caribbean (Ackerman Reference Ackerman2014, Ackerman & Chase Reference Ackerman and Chase2001). Current conservation status of the species in Jamaica is unknown, but populations were reported in decline following high levels of wild harvest and habitat destruction (NEPA 2007). Only one population is currently known to persist in the USA, and it is limited to a thin coastal stretch on the southernmost border of peninsular Florida. Southern Florida is considered the northern latitudinal limit of the species. Throughout the entire distribution of this species, it is subject to anthropogenic threats such as habitat alteration, destruction, collection, and natural forces like hurricanes and specialized herbivory (Borrero et al. Reference Borrero, Alvarez, Prieto and Liu2018, Gann et al. Reference Gann, Hines, Saha and Bradley2009).
Study site
Populations of T. undulatum were studied across the species core range on the island of Cuba as well as the leading northern edge of the species distribution in southern Florida, USA. Cuba, the largest of the Caribbean islands, is home to over 312 orchid species and is thought to be the centre of radiation for many wind-dispersed plant species like orchids and bromeliads (Ackerman Reference Ackerman2014). Due to Cuba’s geological age, as well as its mountainous landscape, there are diverse habitats and microclimates from which these wind-dispersed species can spread (Borhidi & Muñiz Reference Borhidi and Muñiz1985, Nieto-Blázquez et al. Reference Nieto-Blázquez, Antonelli and Roncal2017). In contrast, the Everglades National Park (ENP) is lower in elevation than much of Cuba, ranging from 0 to 2.4-m above sea level. The ENP is the largest wetland preserve in the USA covering over 64,238 ha in Miami-Dade, Monroe, and Collier counties (https://www.nps.gov/ever/learn/news/parkstatistics.htm). Boasting a diverse sub-tropical region of its own, the ENP houses 39 native orchid species.
Methods
Field methods
Study sites were selected based on prior knowledge of the species distribution and legal accessibility. Over the 4-year (2015–2019) study period, we visited a total of 29 sites with T. undulatum populations in Cuba across seven provinces including Artemisa, Cienfuegos, Matanzas, Mayabeque, Pinar del Rio, Sancti Spiritus, and Santiago. In this study, we defined a population of T. undulatum as a collection of all individuals that occur at a site. We surveyed plant communities at eight sites using transects at four provinces in Cuba: Matanzas, Mayabeque, Pinar del Rio, and Sancti Spiritus. A single transect was also surveyed at the ENP site in the USA. The transects range 499 km in the distance from each other with a median of 216 km. The identity of each host tree species for every T. undulatum encountered was documented at all sites. Non-host species were also documented along the transects.
Population and plant community sampling via transects
At each site where plant community sampling was possible, a 1-km non-linear transect was set-up where T. undulatum occurrence was deemed representative of the site. Most of these transects were along informal forest trails, including the one at the ENP. Once we encountered T. undulatum, we would search all trees within a 5-m radius for additional individuals. This approach was taken to maximize the probability of locating T. undulatum individuals for the orchid’s population study (not presented here). A transect ended when it reached 1 km in length. Both host and non-host trees, shrubs, and lianas were identified as species for the entire transect length. Shrubs and lianas were included in the plant community study because they were occasional hosts of T. undulatum (pers. obs.). Diameter at breast height (DBH) of the host and the height at which the individual T. undulatum was found were recorded. In addition, abundance of host and non-host species were categorized into the following five categories within the transects: (1) very abundant, with 15 or more individuals, (2) moderately abundant, between 11 and 14 individuals, (3) somewhat abundant, between 6 and 10 individuals, (4) occasional presence, between 3 and 5 individuals, and (5) species with a rare presence, 1–2 individuals within the study area. While the transects were not a random sample as they maximized inclusion of host species, they nonetheless generated reasonable quantification of host and non-host species diversity and relative abundance where T. undulatum occurred. For the nine plant communities where a transect was sampled, South Florida included, habitat description was based on vegetation types as defined by Borhidi (Reference Borhidi1991).
Data analyses
To assess the thoroughness of our sampling effort, we plotted in Cuba two species accumulation curves: one for all recorded tree species and another for just the orchid host species (Figure 3 a&b). Only plants that were identified to species level were plotted. Differences in mean host tree DBH and height frequencies at which T. undulatum occurred were compared among sites using one-way ANOVA in SPSS 26 (SPSS, Chicago, Illinois, USA). Host preference was evaluated both qualitatively and quantitatively. Qualitatively, host preference was evaluated in three ways to provide a range between liberal and conservative evaluation scenarios. The most inclusive interpretation for host preference includes as a host every species that has been observed with the presence of a T. undulatum across all study sites. An intermediate interpretation is provided by creating species-wide abundance categories for every plant recorded along the Cuban transects, as follows: (1) 10 or fewer individuals, (2) between 11 and 30 individuals, and (3) 31 or more individuals. We then classified the species with the least abundant score and being a host as preferred hosts. The third approach, also the most conservative, was to calculate a host proportion using the abundance of host species at the transect sites divided by the overall abundance for every plant species. This preference interpretation criterion used a host proportion equal to or greater than 0.5 occurrences for each species.
Quantitatively, for species that are identified as preferred hosts in the strictest sense, the total number of trees encountered for each species and the number that were observed as hosts was compared to the total number of trees of all species and the total that were hosts, using Chi-square tests (Vergara-Torres et al. Reference Vergara-Torres, Pacheco-Álvarez and Flores-Palacios2010). The species must have been observed at a minimum of three times to be tested statistically. The significant p value was adjusted by Bonferroni method.
Results
Habitat and host species in Everglades National Park, Florida, USA
The ENP population site is known as a coastal transitional buttonwood woodland or hammock (TBH) with a calcite marl substrate and thin detritus layer at approximately 0.3 m elevation above sea level (25°10ʼ18" N, 80°54ʼ28" W) (Ross et al. Reference Ross, Meeder, Sah, Ruiz and Telesnicki2000, Rutchey et al. Reference Rutchey, Schall, Doren, Atkinson, Ross, Jones and Gann2006, Saha et al. Reference Saha, Lobo O’Reilly Sternberg and Miralles‐Wilhelm2009). Flooding at the site between May and October occurs between the open salt marsh and tropical hardwood hammock. Post-storm disturbances can cause an influx of sea water at the site (Saha et al. Reference Saha, Lobo O’Reilly Sternberg and Miralles‐Wilhelm2009, Saha et al. Reference Saha, Sadle, Van Der Heiden and Sternberg2015). The ENP site consists of a predominant canopy of buttonwood trees, Conocarpus erectus, with occasional occurrence of other woody plants such as Sideroxylon celastrinum and Randia aculeata, and an understory of herbaceous plants including Alternanthera flavescens, Chromolaena frustrata, and Dicliptera sexangularis (Saha et al. Reference Saha, Sadle, Van Der Heiden and Sternberg2015, Wendelberger Reference Wendelberger2016). The TBH habitat is considered a threatened habitat type and is shrinking due to increasing salinity and sea-level rise at the ENP (Saha et al. Reference Saha, Lobo O’Reilly Sternberg and Miralles‐Wilhelm2009). This vegetation type can also be found in shallow coastal regions of Cuba (Borhidi Reference Borhidi1991), but we have not yet located populations of T. undulatum in these habitats (personal observations).
A total of 277 individuals of T. undulatum were documented on the 1-km transect at the ENP population site and all were found growing on the dominant canopy tree species, C. erectus. The heights above the ground where T. undulatum was attached varied between 0.41 and 4 m (average = 1.36 ± 0.6 SD meter height for N=158). Host tree DBH ranged between 6 and 100 cm (average = 31 ± 18.5 SD cm, N=151) (Table 3).
Habitats in Cuba
The general plant communities identified for the Cuban transect sites were Semi-deciduous Mogote Complex (MC), Tropical Semi-deciduous Forest (TSF), Lowland Seasonal Rainforest (LSR), and Wet Montane Forest (WMF), all occupying an exposed limestone karst (Borhidi Reference Borhidi1991).
A Semi-deciduous Mogote Complex (MC) is a type of Tropical Karstic Forest with four subdivisions that are based on species richness, location on the island, canopy height, and precipitation. Two MC subdivisions visited within this study include the Spatheloio-Gaussian Forest and the Thrinacion-Punctulatae Forest. The latter is a species-poor forest found between the Habana and Mayabeque provinces at 200–600 m elevation, while the former is a species-rich deciduous forest found in the western mountains exhibiting high endemism (Borhidi Reference Borhidi1991). The Spatheloio-Gaussian Forest site (MC 1) visited was found near a popular hiker’s trail in Pinar del Rio (22°33ʼ39" N, 83°49ʼ58" W). The Thrinacion-Punctulatae Forest site (MC 2) transect was laid out near Ceiba Mocha, Mayabeque, on two small mogotes (limestone hills) surrounded by pasture (22°57ʼ25"N, 81°46ʼ05" W). The mogotes are steep and the rocky cliffs make it difficult for pastoral animals to climb and damage the vegetation.
The Tropical Semi-deciduous Forest (TSF) community is commonly found along coastal areas where seawater flooding is common. Sites on the coasts of Yaguajay, Sancti Spiritus (TSF 1) (22°16ʼ03" N, 79°12ʼ33" W) (Figure 4 a&b), and Cienega de Zapata, Matanzas (TSF 2, TSF 3) are a microphyllous community also known as Coccolobeto-Buresertum (22°15ʼ56" N, 81°07ʼ05" W and 22°13ʼ14" N, 81°08ʼ08" W respectively). At the Guanahacabibes National Park, Pinar del Rio site (TSF 4), the coastal forest is known for the microphyllous Bombacopsi-Catalpetum plant community (21°55ʼ24" N, 84°28ʼ33" W) (Borhidi Reference Borhidi1991).
LSRs were historically widespread in Cuba, but most are now agricultural zones. The LSR that we visited, Comunidad 23, Sancti Spiritus, is a predominantly shade-coffee region with high epiphyte richness (21°52ʼ06" N, 79°40ʼ54" W) (pers. comm., Aliesky Gil Carballo). Our LSR transect was in a riparian area with limestone substrate that had not been converted to coffee plantations. Canopy trees in the LSR forest type can reach 40 m in height (Borhidi Reference Borhidi1991).
WMF are characterized by elevation (above 800 meters), annual precipitation of 1,700–3,000 mm, and a 20–25 m high canopy layer. They are found in the mountains of central to eastern Cuba (Borhidi Reference Borhidi1991). One WMF was visited in the Sancti Spiritus Province along a river near the Banao Biology Research station in Jarico (21°51ʼ36" N, 79°34ʼ48" W).
Sampling efforts and observations of host and non-host species in Cuba
A total of 246 plant species were identified across the eight Cuban transects, with 74 of them observed as hosts for a total of 1,021 T. undulatum (Table 1). The two MC sites had the highest recorded number of woody plant species and host trees with a total of 46 host and 160 non-host species between them (Figure 2 a&b). The most common host species at MC1 were Adelia ricinella and Gymnanthes lucida at MC2 comprising 10% [N=245] and 6% [N=222] of the host species, respectively. Thirteen percent of the orchids at MC1 that were found growing epilithically (growing on the limestone substrate) with a majority of orchids (62%) at MC2 were found to be epilithic.
Table 1. List of all observed host species for T. undulatum, host plant family, and vegetation types found at the Cuban and Florida transect sites. The “*” denotes a preferred host based on a species low abundance at sites, yet with the presence of a T. undulatum epiphyte (36 species). The “ψ” symbol is used to distinguish the strict interpretation of a preferred host species based on whether a plant species had an observed T. undulatum at a minimum of 50% of the time that species was encountered (13 species). Site vegetation where the host species were found is abbreviated with the following acronyms: Mogote Complex (MC), Wet Montane Forest (WMF), Semi-deciduous Mogote Complex (SMC), Tropical Semi-deciduous Forest (TSF), Buttonwood Hammock (BH), and Lowland Seasonal Rainforest (LSR). Following the host species name in brackets [C] means that the species is native to Cuba, [FL] native to Florida - USA and [E] for exotic.
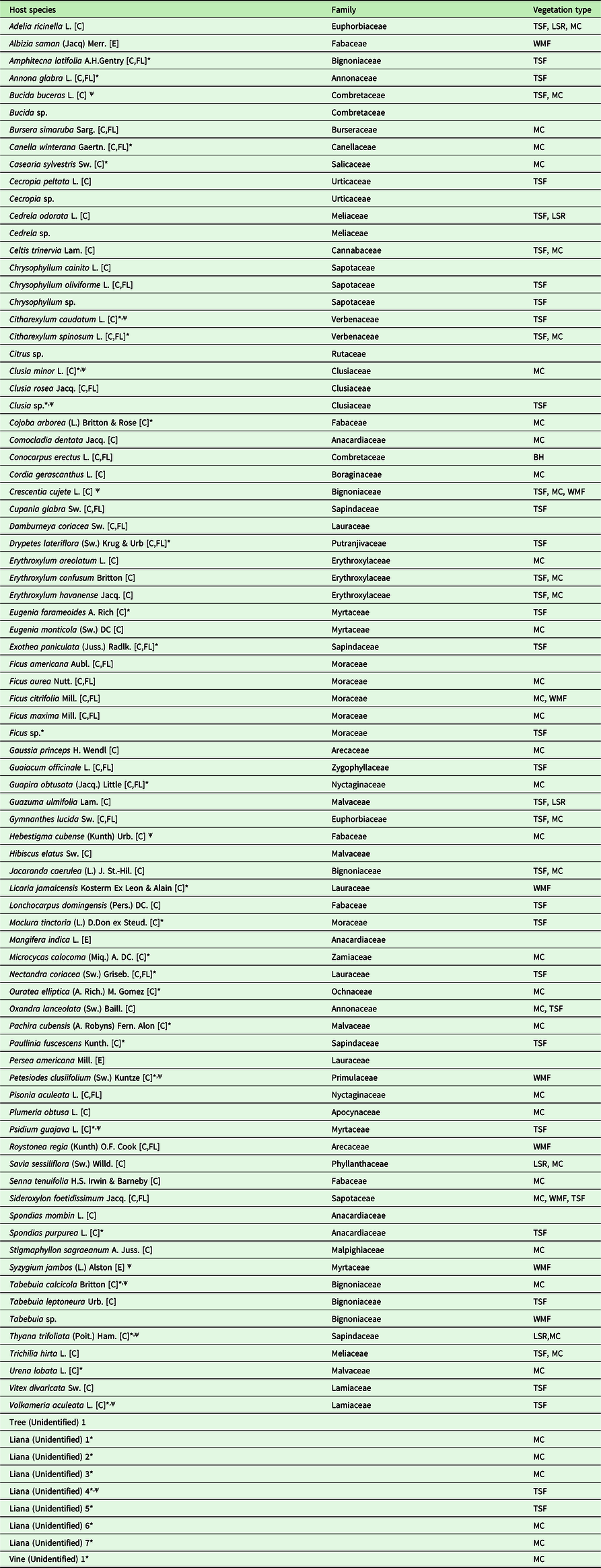
The TSF sites combined had a total 37 host species and 118 non-host species. The most dominant host species recorded at each site were Oxandra lanceolata (12 % at TSF 1) [N=71], Bucida buceras (55% at TSF 2 [N=281] and 30% at TSF 3 [N=23]), and Adelia ricinella (33% at TSF 4 [N=74]). The WMF and LSR sites had the least host richness with totals of nine host species [N=71] and six [N=34], respectively. Syzygium jambos made up the most common host species (55%) at the WMF site. The most common host recorded at the LSR site was Guazuma ulmifolia making up 33% of the host tree diversity. Few orchids were found growing epilithic at the WMF site (6%) and none at the LSR site.
We identified 92 host species at the 29 sites across seven provinces in Cuba, from a total of N=1,095 host tree observations (Table 2). Twenty-three of the 92 host species documented are native to both Cuba and southern Florida. Species accumulation curves show that sampling effort on the Cuban transects plateaus for both all species and host species encountered (Figure 3 a&b).
Table 2. List of all plant species (236 taxonomically confirmed species, 72 families) recorded at eight 1-km long survey sites across 4 provinces in Cuba. Included are whether or not the plant species was observed as a host, vegetation types that the species was observed in, and the average abundance of the species at the sites.

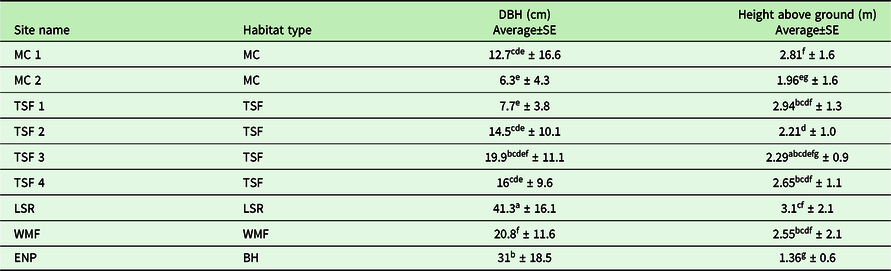
Table 3. The range and average (± SD) diameter at breast height (DBH) and height of the T. undulatum observed was recorded for the nine 1-km transect sites across habitat types.
Host tree DBH and orchid height on host tree
We recorded the heights of over 845 T. undulatum in the Cuban transects that ranged between 0.1 and 8 meters (Figure 1b). The lowest height that a T. undulatum was observed is for those orchids found on the ground (0 m). The MC 2 site had the lowest heights on a host which indicates where the T. undulatum germinated is 0.1 m for three host trees: Bursera simaruba, Erythroxylum sp., Erythroxylum havanense, and Gymnanthes lucida. The tallest that a T. undulatum was observed was on a Bucida buceras at 8 m at TSF 2 site. We measured 698 host tree DBH at the transects that ranged between 1 and 100 cm (Figure 1a). The largest DBH recorded was a Ficus sp. at 105 cm at TSF 3 site. The smallest host plants were recorded at MC 2 and measured 0.25 cm from a Stigmaphyllon sagraeanum and two B. simaruba plants at 0.6 and 0.65 cm. The mean DBH among and within the nine sites were statistically significant (F8,803 = 40.14, P = 0.0014) as well as the heights (F8,937 = 18.31, P = 0.0014). The most distinct sites with respect to DBH were the LSR, ENP, and WMF (Figure 2a) (Bonferonni post hoc; P = 0.00139) (Figure 1 a&b).
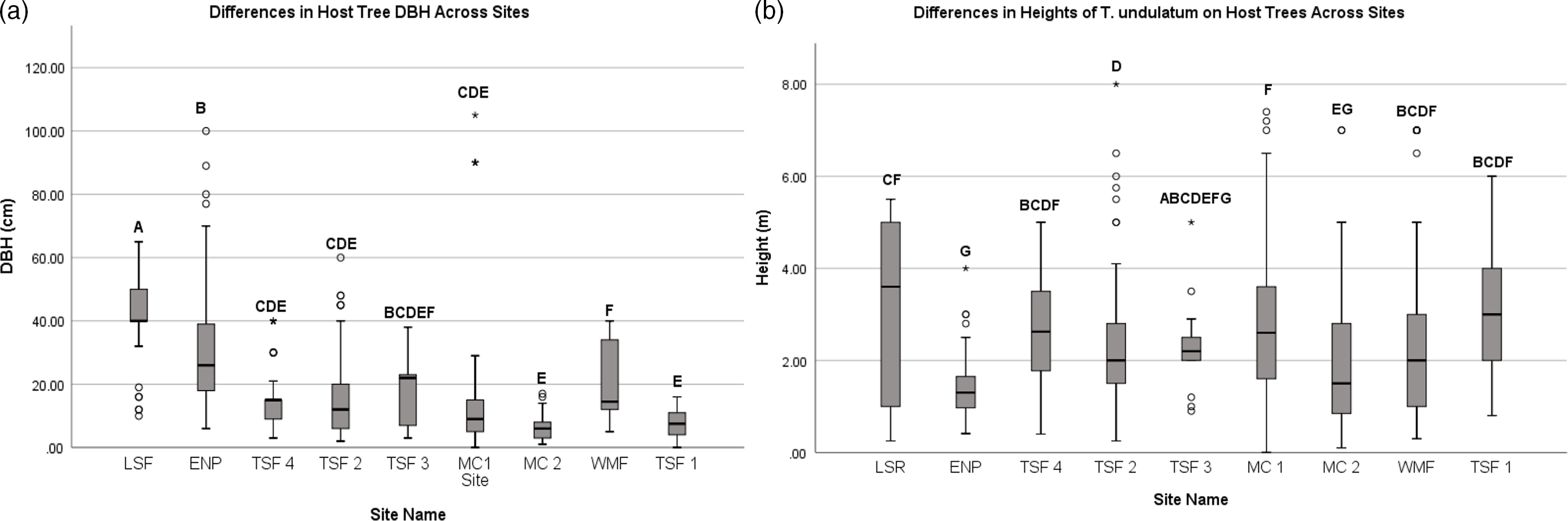
Figure 1 a&b. The range and average (± SD) diameter at breast height (DBH) and height of the T. undulatum observed was recorded for all nine 1-km transect sites across habitat types in Cuba and Florida, USA.
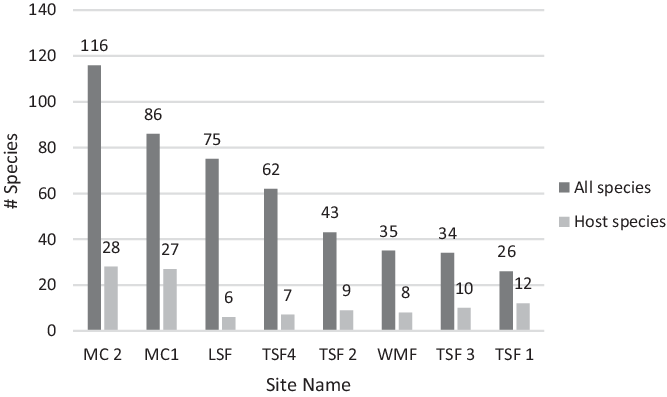
Figure 2 a&b. Species richness for the following a) total species found across eight transect sites in Cuba and b) T. undulatum host species found across eight transect sites in Cuba.
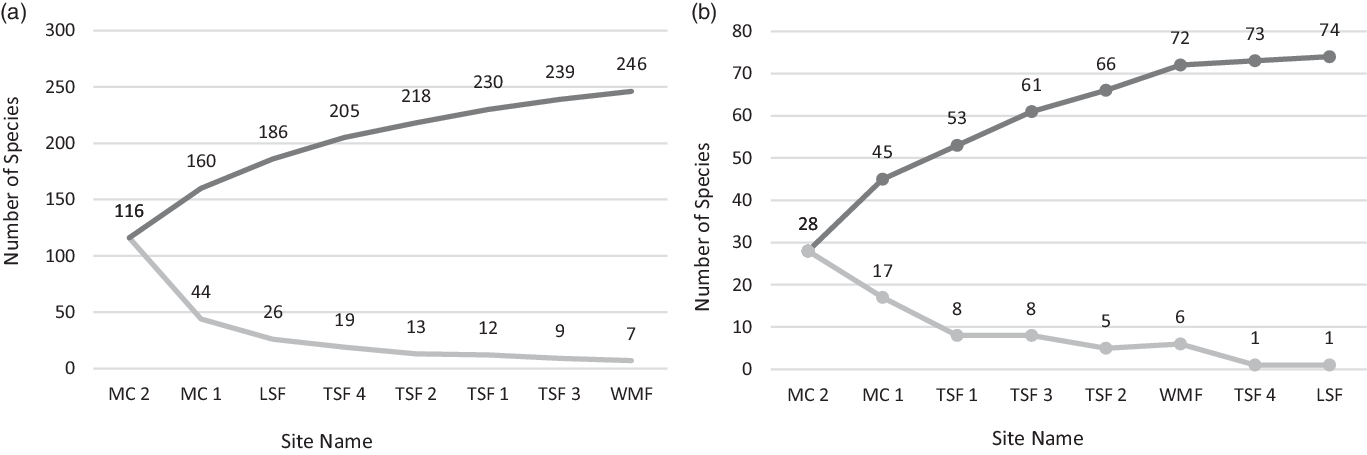
Figure 3 a&b. Species accumulation curves for the following a) total species found across 8 transect sites in Cuba and b) T. undulatum host species found across 8 transect sites in Cuba. The darker line represents the compounding total of new species and the lighter colored line represents the number of new species encountered at subsequent sites.

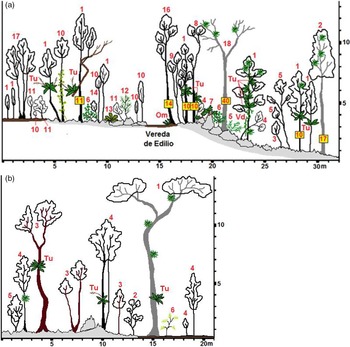
Figure 4 a&b. Two profiles were drawn from within transects in August of 2018 off the trail of La Vereda de Edilio, Sancti Spiritus, Cuba where demographic information was collected for Trichocentrum undulatum at a tropical semideciduous forest in Jobo Rosado protected area (N 22°29614 W -79°22910). Courtesy of MSc. Armando Falcón Méndez, Biologist, Specialist of Parque Nacional Caguanes, CSASS, CITMA. a) The woody species have numerical denominations while smaller herbaceous species are an acronym of the first letter of both genus and species: 1 - Oxandra lanceolata, 2 - Zanthoxylum caribaeum, 3 - Adelia ricinella, 4 - Picramnia pentandra, 5 - Olyra latifolia, 6 - Erythroxylum havanense, 7 - Philodendron lacerum, 8 - Cupania glabra, 9 - Casearia aculeata, 10 - Eugenia axillaris, 11 - Amyris balsamifera, 12 - Eugenia ligustrina, 13 - Anthurium cubense, 14 - Cordia gerascanthus, 15 - Trichilia hirta, 16 - Exothea paniculata, 17 - Gossypiospermum praecox, 18 - Cedrela odorata, 19 - Bignonia diversifolia, Tu - Trichocentrum undulatum , Om - Oeceoclades maculata, Tf - Tillandsia fasciculata, and Vd - Vanilla dilloniana. b) The woody species have numerical denominations while smaller herbaceous species are an acronym of the first letter of both genus and species: 1 - Cedrela odorata, 2 - Adelia ricinella, 3 - Sideroxylon foetidissimum, 4 - Oxandra lanceolata, 5 - Picramnia pentandra, 6 - Acacia tenuifolia, Tu - Trichocentrum undulatum , and Tf - Tillandsia fasciculata.
Host preferences
Our combined data from the eight transects show that T. undulatum was found growing on 74 species or 43% of the total species encountered (Table 1). Most species on the transects were found not to be hosts [N=171], although some were scored as very abundant (Table 2). The preferred host list generated using the intermediately conservative criteria includes a total of 36 species and the more exclusive preferred host list identifies 13 species (Table 1). Statistically, proportions of trees being hosts for all of the 13 species identified as preferred hosts using the strictest criterion were significantly higher than the overall proportion of trees being hosts with all species pooled. Specifically, for Bucida buceras, χ2 = 116.7, P < 0.001; for Citharexylum caudatum, χ2 = 126.6, P < 0.000; for Clusia minor, χ2 = 52.4, P < 0.001; for Crescentia cujete, χ2 = 276.4, P < 0.001; for Hebestigma cubense, χ2 = 227.2, P < 0.001; for Petesiodes clusiifolium (χ2 = 52.4, P < 0.001; for Syzygium jambos, χ2 = 503.8, P < 0.001; for Thyana trifoliata, χ2 = 135.2, P < 0.001; and for Volkameria aculeata, χ2 = 99.5, P < 0.001. The following were not subject to the chi-square tests because they violated the test assumptions, Clusia sp., Liana (unidentified 4), Psidium guajava. Tabebuia calcicola.
Discussion
Our study illustrates that T. undulatum has a large number of host species in its core distribution, and it showed preference on a few of them. This information can be used to inform conservation strategy of this threatened species at its northern most range, as we will discuss in detail below. Studies of this kind are rare in the tropics, especially on orchids, one of the most diverse plant families among tropical plants (Tremblay et al. Reference Tremblay, Zimmerman, Lebrón, Bayman, Sastre, Axelrod and Alers-García1998). There is often a lack of resources and time to study epiphytic species in their current ranges before stochastic events or other rapid environmental changes, which demand emergency rescue and translocation actions. In some cases, it is difficult to know where a fallen epiphyte came from and prior knowledge on host species would be helpful to such actions (Tremblay et al. Reference Tremblay, Zimmerman, Lebrón, Bayman, Sastre, Axelrod and Alers-García1998). Active restoration initiatives for anticipating threats to the population growth of endangered plants are often needed (Liu et al. Reference Liu, Ren, Liu, Wen, Maunder and Gao2015, Liu et al. Reference Liu, Liu, Jin, Gao, Chen, Liu and Zhang2020, Maschinski & Haskins Reference Maschinski and Haskins2012). Success of such actions varies depending on the species habitat limitations. Restoration initiatives may have a greater likelihood of success when out-planting occurs on trees of the right species and size, aided by a list of host trees with preference orders (Mujica et al. Reference Mújica, Raventós, González and Bonet2013, Segovia-Rivas et al. Reference Segovia-Rivas, Meave, González and Pérez-García2018, Tremblay et al. Reference Tremblay, Zimmerman, Lebrón, Bayman, Sastre, Axelrod and Alers-García1998, Yang et al. Reference Yang, Sun, Zhu, Downing, Song and Liu2017). Collecting baseline information for understudied species, like T. undulatum, can provide alternative solutions for conservation planners.
Habitats of T. undulatum as defined by hosts
The most diverse habitat in terms of host species was the Mogote Complex (MC) sites, which also have the highest percentages of epilithic plants. The T. undulatum plants at the MC sites are found at higher elevations and are possibly protected from both flooding and herbivory by large grazing herbivores (i.e. goats and cattle) (Aukema et al. Reference Aukema, Carlo and Collazo2007). We observed many plants growing on the Mogote rocky ground. Some orchid species are known to grow and recruit on rocky substrates (Kendon et al. Reference Kendon, Yokoya, Zettler, Jacob, McDiarmid, Bidartondo and Sarasan2020, Yokoya et al. Reference Yokoya, Zettler, Kendon, Bidartondo, Stice, Skarha, Corey, Knight and Sarasan2015), but we did not observe any protocorms or seedlings of T. undulatum growing on rocky surfaces or crevices during the course of our study as we had on host trees. Judging from the size of the plants on the ground, it is likely that they fell from the host trees nearby. In habitats that experience periodic floodings, such as coastal TSF, BH, or LSR sites, T. undulatum individuals are unlikely to survive while on the ground.
The three sites with the lowest host species richness were the ENP, WMF, and LSR sites. The LSR and WMF habitats are particularly impacted by human presence. The LSR habitats are considered the most common habitat type in Cuba and are seen as ideal for agricultural usage, which makes these sites severely impacted by human presence (Borhidi Reference Borhidi1991). Two of eight hosts documented at the WMF site are listed as invasive, including the most common host tree at the site, Syzygium jambos, which is found along rivers and waterways (CABI 2021). The ENP site is periodically flooded by salt water, dominated by Conocarpus erectus trees throughout, and the population runs between an open saltwater marsh with no canopy tree species as well as manmade canals skirted by dense Rhizophora mangle. It comes as no surprise that C. erectus is the only host within this population due to a lack of alternative woody species in the area with a relatively open canopy. Conocarpus erectus was not reported in any of the Cuban vegetation assays and therefore not reported as a host in any of the T. undulatum populations in Cuba.
Trichocentrum undulatum is likely not microsite limited when germinating since such a wide-scale usage of tree species and growing locations were observed in Cuba. However, in southern Florida, coastal hammocks near the sole existing population were explored in the search for more populations of T. undulatum but none have been found (pers. obs.). There may be pollination and seed limitations caused by T. undulatum’s deceptive pollination strategy and the lower pollinator availability in the southern Florida population (Ackerman et al. Reference Ackerman, Sabat and Zimmerman1996, Turnbull et al. Reference Turnbull, Crawley and Rees2000). The presence of specialized herbivores as well as high herbivory rates found in the southern Florida population exacerbates fruit set and limits seed production, so although the habitat is there, the seeds may not be (Borrero et al. Reference Borrero, Alvarez, Prieto and Liu2018, Higgins and Gann Reference Higgins and Gann2007). Varied mycorrhizal diversity between host trees and habitat types is also likely to be important, particularly because epiphytic orchids may depend on mycorrhizal fungi for water in harsh and dry conditions (Gowland et al. Reference Gowland, Van der Merwe, Linde, Clements and Nicotra2013, Kartzinel et al. Reference Kartzinel, Trapnell and Shefferson2013, Rock-Blake et al. Reference Rock-Blake, McCormick, Brooks, Jones and Whigham2017, Yoder et al. Reference Yoder, Zettler and Stewart2000). Bark rugosity, seasonal light penetration through canopy, as well as throughfall of nutrients adds yet more dimensions of complexity that may have an effect to some extent on host tree choices (Callaway et al. Reference Callaway, Reinhart, Moore, Moore and Pennings2002, Hirata et al. Reference Hirata, Kamijo and Saito2008, Sayago et al. Reference Sáyago, Lopezaraiza-Mikel, Quesada, Álvarez-Añorve, Cascante-Marín and Bastida2013, Zarate-Garcia et al. Reference Zarate-García, Noguera-Savelli, Andrade-Canto, Zavaleta-Mancera, Gauthier and Alatorre-Cobos2020).
Implications for management
This study is the first to evaluate the differences among habitats for populations of T. undulatum across its distribution. Our study is also a reflection of our best effort in understanding the orchid’s hosts and vegetation communities in Cuba while access to natural areas is limited. Due to the diversity of host tree species, substrates, elevations, and plant species richness across the Cuban sites, we are certain that the Cuban T. undulatum populations are not host-specific (Ackerman et al. Reference Ackerman, Trejo-Torres and Crespo-Chuy2007, Nieto-Blázquez et al. Reference Nieto-Blázquez, Antonelli and Roncal2017). The restricted southern Florida population occurred in mangroves with very limited tree diversity, and only one species, Conocarpus erectus, serves as a host tree for T. undulatum. It is not uncommon for specialist species to express stress characteristics on the leading edges of their distributions due to lower habitat quality (Franco et al. Reference Franco, Hill, Kitschke, Collingham, Roy, Fox, Huntley and Thomas2006). Yet there may be hope for T. undulatum since the wide range of hosts found in the core range overlaps with southern Florida (23 out of the 69 identified Cuban native host species are also native to Florida, USA). Our host list recommendations suggest that particular species be targeted in translocation and conservation projects in Florida, beginning with the species that are both native as well as noted as preferred, followed by intermediately preferred, and finally the more inclusive hosts. Unfortunately, there are no species in the most preferred host list that are native to mainland Florida, an indication that the habitats in South Florida are marginal.
The most similar habitat type in southern Florida to the sites observed in Cuba is the Tropical Hardwood Hammock or Rockland Hammock (G2/S2 Global/State Rank), in which 25 species have been recorded that are T. undulatum host trees in Cuba (Institute for Regional Conservation 2021). A listing of “exemplary” Rockland Hammocks that may be adequate planting sites for future projects focused on T. undulatum includes: Dagny Johnson Key Largo Hammock Botanical State Park, John Pennekamp Coral Reef State Park, and Lignumvitae Key Botanical State Park (all in Monroe County) as well as Matheson Hammock, Royal Palm Hammock, and Everglades National Park in Miami-Dade county (Florida Natural Areas Inventory 2010). Management of host tree species within orchid distribution is encouraged since available host trees can be a limiting factor for epiphytic orchid populations (Migenis & Ackerman Reference Migenis and Ackerman1993). Although sites in Cuba did have healthy individuals growing on the ground, recommendations for ground planting will not be made for future conservation work due to the flooding risk, ease of potential poaching, as well as increasing herbivory potential.
Acknowledgements
We would like to thank Dr. James D. Ackerman, Dr. Suzanne Koptur, Aliesky Gill Carballo, Paul Michael Nuñez, Jimi Sadle, Leyani Caballero Tihert, Armando Falcón Méndez and Luce Echeverria for their help at various stages of the research. This work was completed under scientific research permit #EVER-2015-SCI-0048 from Everglades National Park.
Financial Support
Fieldwork was supported by Florida International University’s (FIU) International Center for Tropical Botany; FIU Latin American and Caribbean Center’s Tinker Foundation; the Judith Evans Parker Travel Scholarship; and the Kelly Foundation’s Tropical Botany scholarship.
Competing Interests
The authors declare none.
Ethical Statement
None.