Introduction
Childhood undernutrition remains a global public health concern because it is an underlying cause of 3⋅1 million child deaths annually(Reference Black, Victora and Walker1). Stunting also contributes to the global burden of childhood diseases; 80 % of which is affecting children in developing countries(Reference Ezzati, Lopez and Rodgers2). In low- and middle-income countries, as many as 165 million children are estimated to be stunted(Reference Black, Victora and Walker1). In the Upper West Region of Ghana where the present study was conducted, the stunting rate as of 2014 was 22⋅2 %(3).
Globally, about 32 % of the world population does not have access to adequate sanitation, about 9 % lacks access to safe drinking water, and this could contribute to infections especially at the household level (United Nations, 2016). In the Jirapa Municipal of Ghana where the present study was carried out, about 81⋅0 % of the households do not have toilet facilities in their homes and therefore resort to open defaecation(3).
As depicted in the UNICEF conceptual framework, the causes of undernutrition are multifactorial and vary according to geographical locations, but poor feeding and sanitary practices contribute to this in developing countries(4,5) . Some studies indicate that inadequate infant and young child feeding (IYCF) practices, particularly low dietary diversity, are critical determinants of stunted child growth(Reference Mallard, Houghton and Filteau6–Reference Dewey and Adu-Afarwuah8). Furthermore, some studies have identified strong associations between poor water, sanitation and hygiene (WASH) and stunting(Reference Budge, Parker and Hutchings9–11).
Poor WASH conditions coupled with inappropriate infant and young feeding practices are therefore expected to have an increased risk of child malnutrition. It is plausible that combining improved WASH and appropriate complementary feeding practices could more positively impact child growth, although there is little evidence available. We therefore investigated how the distribution of stunted child growth varies according to individual and joint composite indicators of poor WASH and inappropriate complementary feeding practices.
Materials and methods
Study setting
The present study was carried out in Jirapa District which is mainly rural and located in the north western corridor of the Upper West Region of Ghana. It lies roughly between latitudes 10⋅25° and 11⋅00° North and longitudes 20⋅25° and 20⋅40° West. It occupies 6⋅4 % of the regional landmass, representing a territorial size of 1188⋅6 square km.
The district is mainly agrarian and farming serves as the main source of economic activity as the majority (82⋅7 %) of the households have agriculture as their main occupation, while the few are engaged in trading and formal employment. Many households are food secured from October to March (i.e. the harvesting period). There is one rainy season from May to October. Crops cultivated are mainly maize, guinea corn, millet, beans, groundnuts and bambara beans. Most of the women are involved in peasant farming with a cross-section of them combining that with pito brewing for the sake of family upkeep.
The majority of the households depend on boreholes for water. However, for those who rely on the unimproved water, 94⋅1 % do not treat their drinking water. The rate of open defaecation among households is quite high as about 81⋅0 % of the households do not have toilet facilities in their homes (GDHS, 2014).
Sampling size estimation, study design and population
An analytical community-based cross-sectional design was carried out from 25 November 2019 to 22 December 2019.
The sample size was determined by using a single population proportion formula with the following assumptions: 23⋅0 % prevalence of the main outcome variable (i.e. prevalence of stunting) from a previous study in the area(Reference Saaka, Larbi and Mutaru12), 95 % confidence interval (CI) and 5 % margin of error. A sample size of 301 was estimated after considering 10 % of unexpected events (e.g. damaged/incomplete questionnaire) was factored in the sample size determination.
Children aged 6–23 months and their mothers/caregivers were recruited for the study using a multistage sampling procedure. A stratified sampling procedure was used to stratify the sub-districts where each of the seven sub-districts constituted a stratum. A simple random sampling procedure was used to select the communities within each stratum using the Emergency Nutrition Assessment (ENA) sampling software. A comprehensive list of all households that constituted the sample frame was compiled from selected communities/clusters, and the systematic sampling techniques were used to select the study households. In the selection of the study participants in each household for the interview, only one eligible mother–child pair was selected using a simple random sampling.
Classification of households
To assess the independent and joint effects of improved complementary feeding practices and WASH, we classified households in the following way: (1) households meeting the WASH criteria (i.e. safe disposal of child faeces, use improved drinking water source, availability of improved toilet facility and handwashing with soap); (2) household feeding children with appropriate complementary feeding practices and (3) households meeting WASH + appropriate complementary feeding practices.
Independent and dependent variables
Appropriateness of complementary feeding practice and WASH status were the key independent variables, while child growth was the dependent variable.
Data collection methods
The data were collected from the mothers/caregivers using a structured questionnaire which was administered through face-to-face interviews at the household level. The data included socio-demographic and economic characteristics of the participants, young child feeding practices, child's age, gender, mothers’ educational level, child illness in the past 2 weeks, birth spacing, utilisation of prenatal care, WASH practices, and mother and child anthropometric measures.
Measurement of the dependent variable: stunting (low length-for-age)
Stunted child growth was defined as low length-for-age z-scores (LAZ < minus 2 standard deviations of the median)(13). Other child growth indicators measured were weight-for-length Z-score (WLZ) and weight-for-age Z-score (WAZ). A brief description of the main independent and dependent variables is as follows.
Anthropometric measurements
Anthropometric measurements were taken by the researchers and trained field assistants.
The length and weight were taken using standardised procedures.The length of the children less than 24 months was measured with an infantometer in a recumbent position to the nearest 0⋅1 cm. The weight of children was taken with minimal clothing using a digital SECA 890 digital scale to the nearest 0⋅1 kg. Anthropometric measures were then converted to indicators of LAZ, WAZ and WLZ as per the World Health Organization guidelines(14).
Assessment of the appropriateness of complementary feeding
Complementary feeding practice was assessed using a composite indicator comprising three of the WHO core IYCF indicators which were determined based on the 24 h dietary recall of mothers(15): timely introduction of solid, semi-solid or soft foods at 6 months, minimum meal frequency (MMF) and minimum dietary diversity (MDD). MDD was defined as the proportion of children aged 6–23 months of age who received foods from five or more food groups. The eight foods aregrains, roots and tubers; legumes and nuts; dairy products; flesh foods; eggs; vitamin-A rich fruits and vegetables; other fruits, and vegetables; and breastmilk(16).
MMF is defined as the proportion of breastfed and non-breastfed children aged 6–23 months, who receive solid, semi-solid or soft foods (but also including milk feed for non-breastfed children) the least number of times or more. The least number of times or more (i.e. two times for breastfeeding infants within aged 6–8 months, three times for breastfeeding children within 9–23 months and four times for non-breastfeeding children aged 6–23 months, within 24 h). Meals may include extraordinarily rich nutritious snacks and must be devoid of trivial quantities, and the frequency should be based on the caregiver report.
Minimum acceptable diet (MAD) was defined by the WHO as the proportion of children aged 6–23 months who received both MDD and MMF during the previous 24 h(15).
Appropriate complementary feeding practice was thus defined in the present study to comprise timely introduction of complementary foods at 6 months and meeting MAD(Reference Macías and Glasauer17–Reference Aggarwal, Verma and Faridi19). A child was thus classified as having received appropriate complementary feeding if he/she met all the following three criteria: complementary feeding started at the sixth month of birth, meeting MDD and MMF was adequate for the age of child.
A score of 1 was assigned for meeting each of the criteria and zero for not. The summative score for the appropriate complementary feeding practice score comprised scores for MMF + MDD + timely introduction of complementary foods. Complementary feeding practice was treated as a categorical variable and classified as appropriate if the mother fulfilled the three practices as recommended. Complementary feeding was inappropriate if any of the three criteria was not met.
Assessment of household water, sanitation and hygiene practices
WASH practices assessed included access to and use of safe drinking water, availability of sanitation facilities (e.g. latrines) and hygiene practices (e.g. handwashing with soap at critical times). Respondents were asked the method used to dispose of the youngest child's stool; the responses were categorised as ‘Safe’ or ‘Unsafe’. Disposal of faeces was classified as ‘Safe’ if the child is helped to use a toilet or latrine or faecal matter is disposed into a toilet or latrine. All other methods were considered ‘Unsafe’.
Based on the criteria set by the Joint Monitoring Programme (JMP) for Water and Sanitation(20,21) , improved water sources including protected wells, boreholes, piped water to houses or rainwater and unimproved water sources were defined as drinking water from an unprotected spring or well or surface water.
Improved sanitation facilities are those designed to hygienically separate excreta from human contact and these include wet sanitation technology (flush and pour-flush toilets connected to sewers, septic tanks or pit latrines) and dry sanitation technology (ventilated improved pit latrines, pit latrines with slabs or composting toilets). Unimproved sanitation facilities were pit latrines without a slab, bucket latrines, bush or no facilities(21).
Assessment of household wealth index
A household wealth index, which is a composite measure of a household's cumulative living standard, was used as a proxy indicator for the socio-economic status (SES) of households. Principal component analysis was used to determine the household wealth index from information collected on housing quality (floor, walls and roof materials), source of drinking water, type of toilet facility, the presence of electricity, type of cooking fuel and ownership of modern household durable goods and livestock (e.g. bicycle, television, radio, motorcycle, sewing machine, telephone, cars, refrigerator, DVD/CD player, bed, computer and mobile phone)(Reference Vyas and Kumaranayake22–Reference Howe, Hargreaves and Huttly25).
These facilities or durable goods are often regarded as modern goods that have been shown to reflect household wealth. A household of zero index score, for example, means that the household has not a single modern good. The scores were thus added up to give the proxy household wealth index.
Statistical data analysis
Statistical Package for the Social Sciences (SPSS) Version 21 (SSPS Inc. Chicago, IL, USA) software was used for data entry, cleaning and analysis. The nutritional indicators of the child were computed using the Anthro Plus which converted anthropometric measures into z-scores. The various z-scores were transferred to the SPSS software for further analysis.
Missing data and wrong entries were checked by generating frequency tables after the data entry. Univariate and multivariable logistic regression analyses were performed to identify determinants of stunting. Only variables that showed significant association (P < 0⋅05) with each dependent variable in the univariate analysis were selected and adjusted for in the multivariable binary logistic regression analysis (forward LR). Multicollinearity among the predictor variables was checked before their inclusion in the final regression model. Multicollinearity among independent variables was assessed by using the variance inflation factor (VIF)(Reference Robinson and Schumacker26,Reference Freund, Littell and Creighton27) , which assesses the increment in regression coefficients if the independent variables are correlated. VIF > 5 is an indication that multicollinearity may be present, while VIF > 10 is certainly multicollinearity among the variables. We did not have any VIF exceeding 5, indicating no collinearity.
Crude and adjusted odds ratios (AORs) and their corresponding 95 % CIs were used to measure the strength of the association between stunting and its predictors. The Nagelkerke R 2 value provides an indication of the amount of variation in the dependent variable explained by the model.
The potential confounding factors of stunting that were measured and tested were: age of the child, educational status of the mother/caregiver, maternal height, number of children under 2 years in the household, household wealth index, knowledge of complementary feeding practices and antennal clinic attendance.
Ethical consideration
The School of Allied Health Sciences, University for Development Studies, Ghana approved the study protocol. After providing the needed information and explanation, informed written consent was obtained from the participants. In situations, where the participants were illiterates, verbal informed consent was sought. Participants were provided copies of the signed forms for their records.
Results
Socio-economic and demographic characteristics
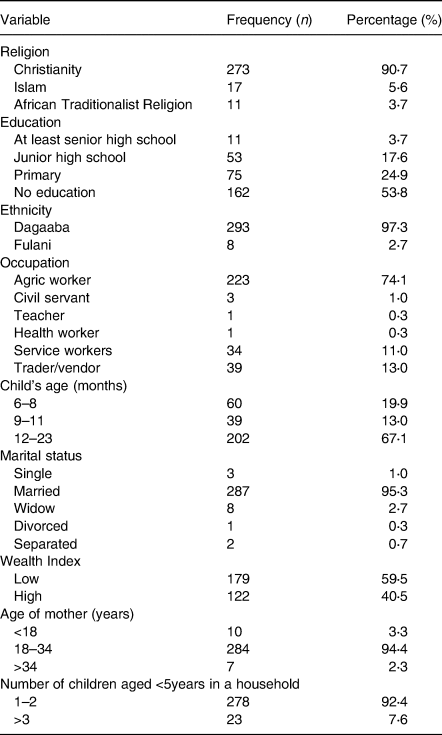
Table 1 summarises the main characteristics of the study participants. The majority (53⋅8 %) of the mothers had no formal education at all. A vast proportion (95⋅3 %) of the respondents were married. The main ethnic group was Dagaabas (97⋅3 %), and 90⋅7 % of them were Christians. Most mothers/caretakers (74⋅1 %) were engaged in peasant farming/agricultural work, and only 40⋅5 % of the households were classified as having a high household wealth index.
Table 1. Sample characteristics (N = 301)
Nutritional status, WASH practices and dietary intake of children aged 6–23 months
In the study population, 13⋅3, 11⋅3 and 18⋅3 % were stunted, wasted and underweight, respectively. The proportion of children aged 6–23 months who met the MDD (≥5 food groups) was 23⋅9 % and 47⋅5 % had adequate meal frequency. Only 17⋅6 % of the children aged 6–23 months met the MAD. The overall appropriate complementary prevalence was therefore 15⋅9 %. The households with unimproved WASH practices were the majority (59⋅2 %). Households with access to improved water sources were 92⋅7 %. The practice of open defaecation among households was high (71⋅1 %) (Table 2).
Table 2. Nutritional status, IYCF and WASH practices
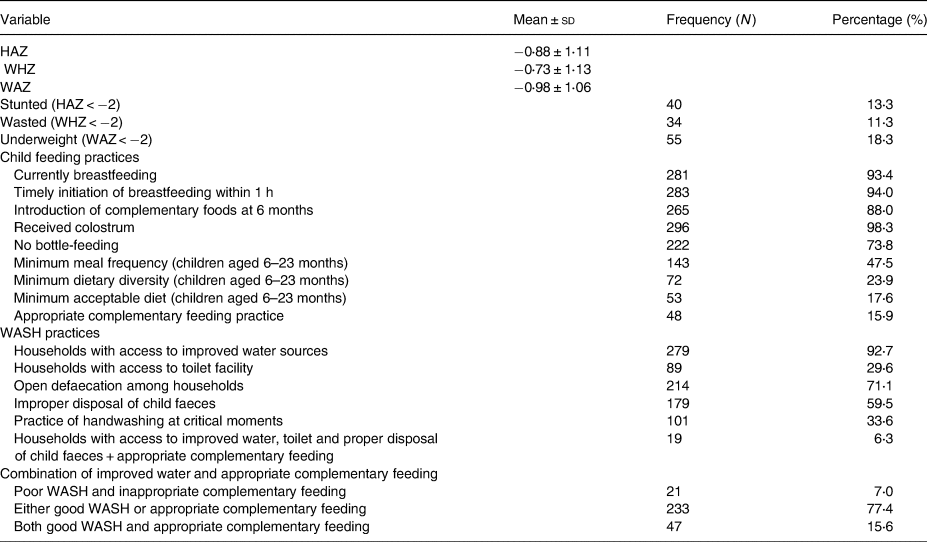
HAZ, height-for-age z-score; WHZ, weight-for-height z-score; WAZ, weight-for-age z-score; WASH, water, sanitation and hygiene.
Distribution of stunted child growth according to individual and combined composite indicators of poor WASH and inappropriate complementary feeding practices
The results indicate that except for unimproved drinking water sources, poor sanitation and hygiene, which comprised the use of unimproved household toilet facilities, washing hands without soap and improper disposal of child faeces were not associated with the risks of stunting among children aged 6–23 months. Children from households with unimproved drinking water sources had greater odds of having stunted child [crude odds ratio (COR) = 3⋅48; 95 % CI: 1⋅32, 9⋅16]. Inappropriate complementary feeding practice was positively and significantly associated with stunted growth. Children receiving both unimproved water and inappropriate complementary feeding had a greater odds of becoming stunted compared to children receiving either only inappropriate complementary feeding or unimproved water (Table 3).
Table 3. Relationship between stunted child growth and selected independent variables (univariate logistic regression analysis)
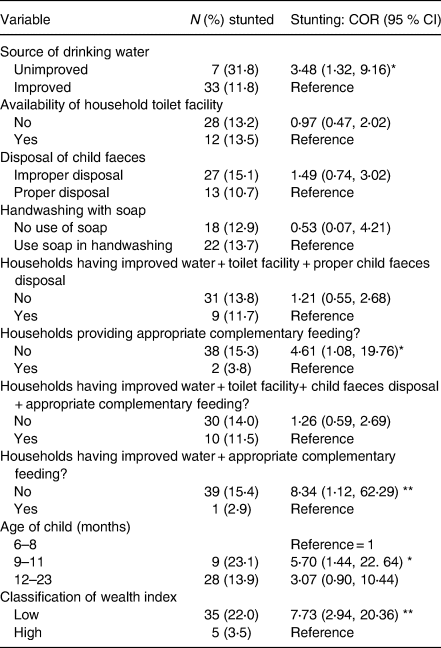
COR (95 % CI), crude/unadjusted odds ratio at 95 % confidence level.
* Significant at P < 0⋅05; **significant at P < 0⋅01.
Predictors of chronic malnutrition among children aged 6–23 months
In a multivariable logistic regression model, the combined effect of unimproved drinking water and inappropriate complementary feeding practices on stunting was significantly positive after adjusting for the age of the child and household wealth index. Children receiving both unimproved water and inappropriate complementary feeding had a higher and significant odds of becoming stunted (AOR = 33. 92; 95 % CI 3⋅04, 37⋅17; P = 0⋅004) compared to households having both improved water sources and appropriate complementary feeding practices (Table 4).
Table 4. Predictors of stunted growth (multivariable logistic regression analysis)
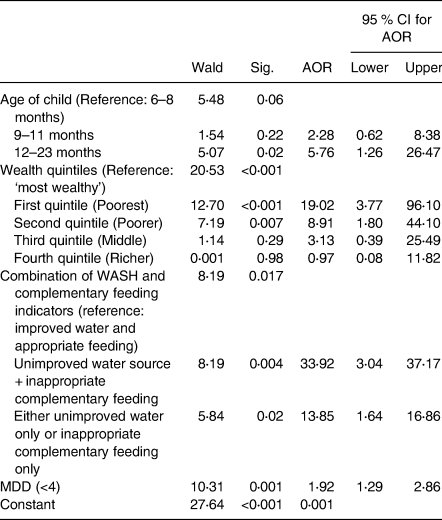
AOR, adjusted odds ratio; MDD, minimum dietary diversity.
Children were 5⋅8 times more likely to be stunted if they were older (12–23 months) compared to younger children aged 6–8 months, odds increasing with age (AOR = 5⋅76; 95 % CI 1⋅26–26⋅47). Compared with children from the richest households, children from the poorest wealth quintile were 19⋅0 times more likely to be stunted (AOR = 19⋅02; 95 % CI 3⋅77, 96⋅10), and the poorer wealth quintile (AOR = 8⋅91; 95 % CI 1⋅80, 44⋅10) was also positively associated with stunted growth. Low MDD is associated positively with the prevalence of stunting among children aged 6–36 months.
The set of three variables explained 32⋅4 % of the variation in stunting (Nagelkerke R 2 = 0⋅324).
Discussion
There had been no studies undertaken in Ghana to understand whether there is a significant joint effect of poor WASH and feeding practices on child nutrition status in Ghana. This is the first paper that has investigated in Ghana how stunted child growth relates to individual and joint composite indicators of poor WASH and inappropriate complementary feeding practices.
The results indicate that except for unimproved drinking water sources, poor sanitation and hygiene, which comprised the use of unimproved household toilet facilities, washing hands without soap and improper disposal of child faeces were not associated with the risks of stunting among children aged 6–23 months.
The joint protective effect of households having improved water sources and appropriate complementary feeding on stunting was reduced in the binary logistic multivariable regression analysis that adjusted for other explanatory variables including the age of child and household wealth index.
Nutritional status, WASH practices and dietary intake of children aged 6–23 months
The prevalence of stunting in the study sample was 13⋅3 % which is higher than the national average that was reported in the 2014 Ghana Demographic and Health Survey(3). The finding is, however, consistent with the findings of other studies in Northern Ghana(Reference Glover-Amengor, Agbemafle and Hagan28,Reference Saaka, Wemakor and Abizari29) .
In the present study, suboptimal feeding practice was highly prevalent. Except for MMF, the other IYCF core indicators (MDD, MAD and appropriate complementary feeding) were abysmally low. The world today is confronted with poor dietary quality, as foods often served to children aged 6–23 months are characterised by too little variety, inappropriate in terms of consistency of food, lack of essential micronutrients, no/too little essential fatty acids and may be too less dense in calories especially among non-breastfeeding infants(Reference Abeshu, Lelisa and Geleta30). Similar poor complementary feeding practices have severally been reported in many places including Northern Ghana and Southern Ethiopia(3,Reference Saaka, Larbi and Mutaru12,Reference Mekonnen, Workie and Yimer31–Reference Kassa, Meshesha and Haji33) .
Evidence from the present study revealed that WASH practices were poor. Low ownership of household’s toilets and as such the practice of open defecation among many households was widespread. It was observed that most respondents were engaged in bad hygienic and poor sanitation practices, a finding that has been similarly reported in the 2010 Population and Housing Census and the Ghana Demography and Health Survey(3,34) . However, the study found that most households had access to boreholes and small water township projects provided by politicians as part of campaign promises.
Association between child nutritional status and poor household WASH practices
In the present study, poor sanitation and hygiene practices including non-availability of household toilet facilities and improper disposal of child faeces were not associated with the risk of stunting among children aged 6–23 months. However, unimproved drinking water sources were associated with chronic malnutrition. This finding is consistent with a study in Ethiopia where children in households with the unimproved source of drinking water had higher odds of stunting(Reference Wondimu35). In the Ethiopian study, the bivariate analysis showed that household access to the unimproved source of drinking water and sanitation increased the likelihood of malnutrition but adjustment of child, maternal and household characteristics attenuated this association(Reference Wondimu35). This means the contribution of poor WASH to undernutrition was not strong in the presence of other predictors. The meta-analysis of some studies similarly reported that unimproved sources of drinking water among households were significantly associated with stunting among children(Reference Akombi, Agho and Hall36–Reference Kwami, Godfrey and Gavilan39).
There is currently conflicting evidence on the effect of WASH on linear growth, as there are discordant results from studies conducted in so many countries. While some studies suggest that malnutrition is significantly linked to poor WASH practice(Reference Prüss-Üstün, Bos and Gore40–Reference Pickering, Djebbari and Lopez47), others have reported that there is no significant association between WASH and linear growth(Reference Bowen, Agboatwalla and Luby48–Reference Muhoozi, Atukunda and Diep50).
Some recent randomised controlled trials (RCTs) in Bangladesh, Zimbabwe and Kenya concluded that the integration of water, sanitation and handwashing with nutrition did not have any benefit on improved child's growth(Reference Luby, Ra hman and Arnold51–Reference Humphrey, Mbuya and Ntozini54). In India, another study showed that improved sanitation facilities decreased open defaecation but did not improve children's growth(Reference Patil, Arnold and Salvatore55) and meta-analysis including 4627 children identified a borderline statistically significant effect of WASH interventions on height-for-age z-score (HAZ)(Reference Dangour, Watson and Cumming56).
It has been reported that repeated exposure to diarrhoeal and parasitic infections causes environmental enteric dysfunction (EED), an inflammatory condition of the gut of children, which is characterised by increased permeability, inflammatory cell infiltration and modest malabsorption(Reference Kelly, Menzies and Crane57–Reference Brown, Cairncross and Ensink59). Poor or unimproved WASH contributes to EED, which is reported to be strongly associated with linear growth faltering(Reference Wolf, Prüss-Ustün and Cumming60–Reference Owino, Ahmed and Freemark62).
Diarrhoeal parasitic infections and EED have been suggested to be key mediating pathways linking poor WASH to developmental deficits(Reference Lin, Arnold and Afreen43,Reference Bhutta, Ahmed and Black63,Reference Checkley, Gilman and Black64) . This is based on the fact that diarrhoea and intestinal worms cause nutrient loss and diversion of nutrients from growth to the immune system to fight the infection(Reference Petri, Miller and Binder65–Reference Checkley, Buckley and Gilman68) and EED increases the small intestine's permeability and reduces nutrient absorption(Reference Korpe and Petri69–Reference Lunn71).
Humphrey has indeed argued that the primary causal pathway from poor sanitation and hygiene to undernutrition is tropical enteropathy, and not diarrhoea(Reference Humphrey70), and he therefore proposes that the provision of toilets and promotion of handwashing after faecal contact could reduce or prevent tropical enteropathy and its adverse effects on growth. It should be noted that in the present study, we compared the distribution of stunting in households that were categorised as having proper and improper disposal of child faeces mechanisms, but we found no significant difference between them. Similarly, a study in Ethiopia found that sanitation facility, i.e. faeces disposal, was not associated with stunting(Reference Kwami, Godfrey and Gavilan39).
We also did not find differentials in stunting with regard to hand washing practice, although there is also the possibility that households with poor sanitation facilities may nullify the effect of handwashing with clean water. This suggests that households with good handwashing practices may offset the consequences of poor-quality sanitation facilities.
In some other studies, a strong significant association was found between WASH conditions and wasting and stunting(Reference Rah, Cronin and Badgaiyan44,Reference Manzoni, Laillou and Samnang72,Reference Dodos, Mattern and Lapegue73) . Furthermore, both RCTs and non-randomised studies conducted elsewhere, including Indonesia and Mali, showed that sanitation interventions reduced stunting or improved HAZ(Reference Pickering, Djebbari and Lopez47,Reference Cameron, Shah and Olivia74) . A literature review of WASH interventions on nutrition status found WASH to be a slightly significant factor in reducing the risk of stunting and could increase the average HAZ by 0⋅08 standard deviation(Reference Dangour, Watson and Cumming75). In yet another study, improved sanitation and a reduction in open defaecation by 20 % was found to increase the average HAZ by 0⋅1 standard deviation(Reference Spears76). Another meta-analysis has found that WASH interventions were significantly associated with increased pooled mean HAZ and that the effect of WASH on linear growth differed markedly according to the age and type of intervention, either single or combined(Reference Gizaw and Worku77).
Effect of joint poor WASH and inappropriate complementary feeding practices on child growth
One would expect that a combination of poor WASH and inappropriate complementary feeding practices, which are the underlying causes of undernutrition, would amplify the effect on the overall prevalence of stunting. However, in the present study, there was no strong evidence of the combined and interaction between them on stunting. Only either unimproved water sources or inappropriate complementary feeding practices and the combination of both manifested in stunted growth. The finding of the present study is consistent with earlier published studies. For example, a community-based trial in Zimbabwe (SHINE trial) tested the impact of combined WASH and IYCF counselling on infant LAZ and reported that the IYCF intervention alone reduced the number of stunted children from 35 to 27 % and the number of children with anaemia from 13⋅9 to 10⋅5 %(Reference Humphrey, Mbuya and Ntozini54). The WASH intervention (alone or combined with IYCF) had no effect on either primary outcome(Reference Humphrey, Mbuya and Ntozini54,78) . There have been two similar trials in which a WASH intervention alone or in combination with an IYCF intervention had no effect on linear growth(Reference Luby, Ra hman and Arnold51,Reference Null, Stewart and Pickering53) .
It is interesting that a favourable combination of WASH-nutrition interventions/exposure did not appear to yield the desired outcome. It is possible that the effect of improved WASH conditions or interventions on child growth may be context-specific.
It is to be noted, however, that in the present study sample, a combination of improved water and appropriate complementary feeding only offered marginal protection against stunting when other explanatory variables were adjusted for in a multivariable regression analysis. However, a recent systematic review reported that WASH intervention alone improved HAZ of children aged <2 years and that combined WASH with nutrition showed a strong effect on HAZ and WAZ and a borderline effect on WHZ(Reference Bekele, Rawstorne and Rahman79). Additionally, a study from the Millennium Villages Project attributes a reduction in stunting to a combination of factors that cut across several sectors(Reference Remans, Pronyk and Fanzo80), all of this suggests integrated WASH with nutrition interventions may be effective in improving child growth outcomes in some contexts.
Predictors of chronic malnutrition
In the present study, it was found that unimproved water sources, inappropriate complementary feeding, child's age and household wealth index were consistently significant predictors of child stunting. Although a number of studies have reported a positive association between inappropriate feeding practices indicators such as infant child feeding index and chronic malnutrition(Reference Zhao, Gao and Li81), others reported no association at all(Reference Wondafrash, Huybregts and Lachat82).
The higher prevalence of chronic malnutrition among children aged 12–23 months compared to children aged 6–8 months in the present study is consistent with other studies done elsewhere including Uganda, Ethiopia, Oromia regional state and Northern Nigeria(Reference Endris, Asefa and Dube83–Reference Udoh and Amodu85). This linkage could be attributed to the fact that children at the age of 12–23 months will be eating family food with a reduction in the frequency of breastmilk intake but more exposed to environmental infections. Indeed, on many occasions, the foods fed to these children are less diversified and are not even fed the minimum required number of times in a day and that will contribute to poor growth in the children.
Households’ wealth index was negatively linked to child chronic malnutrition status. Rich households/families are better positioned and therefore more likely to be able to afford and provide a diversified of foods to their children more frequently. Seeking and accessing quality medical and health services becomes easier no matter the cost. This association between child malnutrition and wealth index has also been reported in other studies(Reference Endris, Asefa and Dube83).
Conclusions
The results of the present study indicate that except for unimproved drinking water sources, poor sanitation and hygiene, including non-availability of household toilet facilities, and improper disposal of child faeces were not associated with risk of stunting among children aged 6–23 months. Secondly, the combined effect of unimproved water and inappropriate complementary feeding on stunting was reduced when the age of the child and the SES of the household were adjusted for.
Limitations of the study
The present study was cross-sectional and that makes it difficult to demonstrate cause-and-effect relationships. Notwithstanding this, we have provided important insights into the contribution of poor WASH and inappropriate complementary feeding practices to stunting in children.
Acknowledgments
The authors wish to thank the research assistants Gordon, Daniel, Solomon for their commitment and dedication to data collection. Our sincerest thanks also go to the Ghana Health Service (the regional director, District Directors and sub-municipal In-charges) and the community leaders for permission to carry out the present study. Last but not the least, our appreciation goes to the principal of the St. Joseph Nursing Training College, Jirapa for encouragement and support during the study.
F.N.S. and M.S. conceived and designed the study. M.S. and R.N.D. performed data analyses, scientific content and interpretation of findings. F.N.S. and M.S. drafted the manuscript. All authors contributed to the data interpretation, critical revision of the manuscript, read and approved the final manuscript.
No funding was received for this study.
The quantitative data presented in the form of SPSS used to support the findings of the present study are included within the supplementary information file(s).
The authors declare that they have no competing interests.






