Introduction
During the past 20 years, there has been a dramatic increase in obesity in the United States. The incidence of obesity has reached epidemic dimensions, and there are no signs that it will decrease given the current trend. Recent statistics estimate that about one-third of U.S. adults (more than 35%) and approximately 17% (or 12.5 million) of children and adolescents in the age of 2–19 years are obese (Odgen CL 2012). Obesity is a condition that is characterised by excess body fat and is defined as a body mass index (BMI) ≥ 30 kg/m2. The likelihood of becoming obese does not depend on sex, age, or ethnicity, but children who are overweight have an increased likelihood of becoming obese adults (Hedley and others Reference Hedley, Ogden, Johnson, Carroll, Curtin and Flegal2004). The incidence of obesity is undoubtedly an important contributor to the increase in insulin resistance and the metabolic syndrome, as well as in type 2 diabetes (T2D) (Mokdad and others Reference Mokdad, Ford, Bowman, Dietz, Vinicor, Bales and Marks2003). Obesity therefore ranks as an independent, high-risk factor for T2D (Storz and others Reference Storz, Döppler, Wernig, Pfizenmaier and Müller1999).
The principal role of the hormone insulin is to mediate the redistribution of the GLUT-4 isoform from an intracellular vesicle pool into plasma membranes of insulin-responsive tissues, thus regulating the uptake of glucose. In the presence of insulin stimulation, the translocation of GLUT4 to the membrane surface is necessary for glucose transport into the cell (Abel and others Reference Abel, Kahn, Shepherd, Le Roith, Taylor and Olefsky2003). Elevated plasma glucose concentrations stimulate pancreatic ß-cell secretion of insulin (Viscarra and others Reference Viscarra, Vázquez–Medina, Crocker and Ortiz2011). Insulin binds to the insulin receptor (IR) on target tissues and stimulates the phosphorylation of insulin receptor substrate-1 (IRS-1). IRS-1 associates with phosphatidylinositol 3-kinase (PI3-kinase). After association of these two substrates, the phosphorylation of Akt2 takes place (Czech Reference Czech1995; Heller-Harrison and others Reference Heller–Harrison, Morin and Czech1995; Leney and Tavaré Reference Leney and Tavaré2009) causing the translocation of GLUT4 to the plasma membrane, increasing the uptake of glucose and decreasing plasma glucose levels (Czech Reference Czech1995; Leney and Tavaré Reference Leney and Tavaré2009).
GLUT4 has a central role in whole-body glucose homeostasis and defective GLUT4 trafficking might represent one of the earliest defects contributing to insulin resistance in humans (Stöckli and others Reference Stöckli, Fazakerley and James2011). Insulin resistance is characterised by an inability of cells to respond to insulin upon stimulation with glucose and presents as an important risk factor for the development of T2D (Bastard and others Reference Bastard, Maachi, Lagathu, Kim, Caron, Vidal, Capeau and Feve2006). GLUT4 is the glucose transporter most responsive to insulin and is found predominantly in muscle and adipose cells. Quantification of GLUT4 would then require invasive biopsies. A transformative study done by Maratou and others (Reference Maratou, Dimitriadis, Kollias, Boutati, Lambadiari, Mitrou and Raptis2007) demonstrated that there is GLUT4 in insulin stimulated white blood cells (WBC) collected from human subjects in response to insulin activation. In a subsequent study this research group further validated the use of insulin-stimulated GLUT4 expression in mononuclear cells as a reliable diagnostic tool by correlating these levels with HOMA-IR in diabetic patients (Maratou and others Reference Maratou, Hadjidakis, Kollias, Tsegka, Peppa, Alevizaki, Mitrou, Lambadiari, Boutati and Nikzas2009). Hence, this technique would seem to offer great potential as a relatively non-invasive diagnostic tool and creates unique possibilities for studying the molecular basis for T2D (Zorzano and others Reference Zorzano, Palacín and Gum2005).
Historically, dogs have played a critical role in our understanding and treatment of diabetes and scientists have used dogs as a biochemical research model for diabetes for over a century (Catchpole and others Reference Catchpole, Ristic, Fleeman and Davison2005). Though there are species-specific pathologies associated with diabetes, dogs develop insulin dependent and independent forms of diabetes, and gestational diabetes akin to humans (Bergman and others Reference Bergman, Kim, Catalano, Hsu, Chiu, Kabir, Hucking and Ader2006; Catchpole and others 2005; Johnson Reference Johnson2008). The prevalence of canine diabetes (classified into insulin deficiency diabetes) is significantly lower than human, which could be a result of better diagnostics or an increased incidence of risk factors like obesity, as seen in humans, or both, but an increasing trend has been observed (Catchpole and others 2005). Dogs are a proved model for biochemical research (Dunlap and others 2006; Greeley and others 2001; Milgram and others 2002), and can be an innovative model to link activity and nutrition to the physiological and immune effects seen in metabolic syndrome and related disorders. For the circumpolar north, racing sled dogs are excellent models for studying health effects related to exercise, nutrition and metabolic syndrome (Felsburg Reference Felsburg2002; Kararli Reference Kararli2006). Nutritional intervention and exercise has shown to improve insulin sensitivity and increase GLUT4 expression (Carey and Kingwell Reference Carey and Kingwell2009; Ruel and Couillard Reference Ruel and Couillard2007). The main purpose of this pilot study was to validate quantifiable amounts of GLUT4 in white blood cells of dogs using a simple commercially available ELISA and furthermore compare GLUT4 levels in young versus old sled dogs. Additionally, we examined the blood insulin response to a meal.
Materials and methods
Animals and diet
Sled dogs, raised in Salcha, Alaska (Latitude 65°N, 147°W) were used as test subjects. All procedures were reviewed and approved by the Institute of Animal Use and Care Committee at the University of Alaska Fairbanks (#02–14). The dogs that were used were typical racing sled dogs that were evenly distributed for sex. Both populations of dogs, healthy young racing sled dogs (n = 6) and healthy old retired sled dogs (n = 5), were from the Piledriver Kennel in Salcha, AK. The age distribution for young dogs ranged from 1 to 5 years (3.2 years ± 1.8) and for old dogs ranged from 7 to 13 years (10 years ± 2.1). All dogs were sexually intact. Housing arrangements consisted of 2-m chains on which the dogs were tethered for the duration of the study. Each dog had access to his or her own house. Dogs in both groups were fed the same diet (Purina Pro Plan Performance) and were allowed ad libidium access to water. Each dog was fed to maintain its ideal body condition score of 3 (Laflamme, Reference Laflamme1997).
Sample collection and preparation
All dogs were sampled pre meal (PRE) to measure fasting plasma insulin levels and GLUT4 and post meal (POST) for plasma insulin levels. Blood samples (4mL) were collected via cephalic venipuncture into prechilled EDTA-treated vacutainer sample tubes containing protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Tubes were stored upright at room temperature for no longer than 2 hours prior to centrifugation and centrifuged for 15 min at 3600 RPM at room temperature. For the insulin analysis, the plasma was transferred into freezer vials, flash frozen in liquid nitrogen and immediately stored at –80˚C. For the GLUT4 analysis, the buffy coat (mononuclear interphase layer containing white blood cells) was collected, washed in 1x phosphate buffer saline twice, stimulated with 100nM insulin for 20 minutes, sonicated, transferred into freezer vials, flash frozen in liquid nitrogen and stored at –80˚C.
Biochemical analysis
The concentrations of GLUT4 in white blood cells, were measured with a commercially available ELISA (Uscn Life Science Inc., United States). The Uscn Life Science GLUT4-ELISA kit is a sandwich enzyme immunoassay for the in vitro quantitative measurement of GLUT4 in canine tissue homogenates and other biological fluids. The micro filter plate in the kit has been pre-coated with a monoclonal antibody specific to GLUT4. GLUT4 concentrations of the samples were then determined by further extrapolation from a standard curve developed from known concentrations of GLUT4. After appropriate sample and standard dilution, the procedure supplied with the assay was followed. GLUT4 concentrations were determined by comparing the optical density (read spectrophotometric with a microplate reader at 450nm) of each sample to the standard curve. The plasma concentrations of insulin (Porcine/Canine; ALPCO, Salem NH) were measured with a commercially available ELISA. The ALPCO insulin ELISA is also a sandwich type immunoassay. Monoclonal antibodies specific for insulin are immobilised to the 96-well microplate as the solid phase. Sample concentration was determined by extrapolation from a standard curve. Again, the procedure supplied with the assay was followed. Optical density was measured with a microplate reader at 450nm and reference wavelength at 620 nm.
Statistics
The curves were constructed using Prism5 Software (GraphPad Software, La Jolla, CA). All samples were analysed in duplicates and run in a single ELISA kit. PRE and POST meal insulin means ± SE were compared between young and old sled dogs using a paired t-test and considered significantly different at P < 0.05. GLUT4 means ± SE were compared between young and old dogs using an unpaired Student's t-test and considered significantly different at P < 0.05. Outliers were determined using Grubbs’ test (significance levels α = 0.05).
Results and discussion
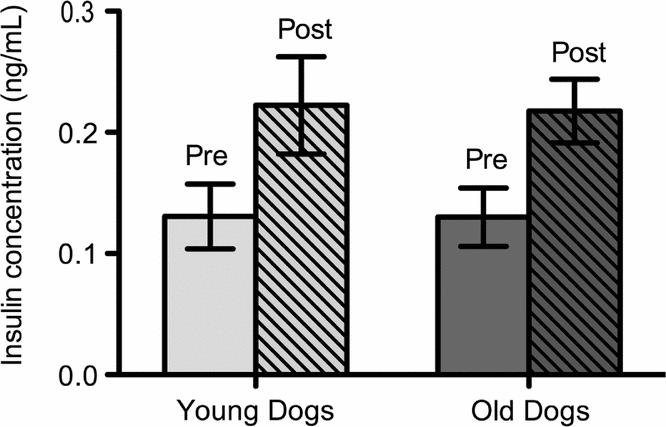
Insulin concentrations increased post meal. There was a 59% increase in mean plasma insulin pre and post meal for both dog populations. Pre and post meal insulin was increased significantly in young and old dogs (Fig. 1). Age had no effect on insulin levels.

Fig. 1. Mean insulin levels of young (n = 6) and old (n = 5) sled dogs. Insulin levels for young dogs were: pre meal 0.13 (±0.03 ng/mL, n = 6); post meal 0.22 (±0.04 ng/mL, n = 6); p < 0.05. Insulin Levels for old dogs were: pre meal 0.13 (±0.02 ng/mL, n = 5); post meal 0.22 (±0.03 ng/mL, n = 5); p < 0.05. Insulin level means pre and post meal are significantly different at the 95% confident level in old and in young dogs (p < 0.05). There was no age-induced difference in insulin levels in sled dogs (p > 0.05).
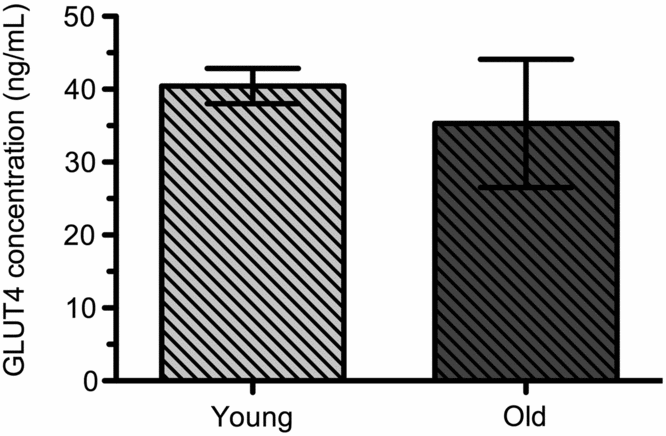
GLUT4 levels in white blood cells. GLUT4 was quantified in white blood cells of sled dogs. The GLUT4 concentration of young dogs was 40.4 (± 2.4 ng/mL). GLUT4 concentration of old dogs was 35.3 (±8.8 ng/mL). Age had no significant effect in the concentration of GLUT4 between the populations of old and young dogs (Fig 2). There was one outlier in each population of dogs so the number was reduced to n = 5 (young dogs) and n = 4 (old dogs).

Fig. 2. GLUT4 concentrations in WBC of young (n = 5) and old (n = 4) sled dogs. GLUT4 in WBC of Young Dogs was 40.4 (± 2.4 ng/mL, n = 5) and Old Dogs 35.3 (±8.8 ng/mL, n = 4). Means are not significantly different at the 95% confidence level between Young and Old Dogs (p > 0.05).
Currently, the cellular mechanisms underlying insulin resistance and T2D are often studied in vitro using myocytes and adipocytes, or in vivo with invasive muscle or adipose biopsies. Our data support the results of Maratou (Maratou and others 2007) and indicates that there is significant and quantifiable activity of GLUT4 in WBC of sled dogs in response to insulin, a finding that opens up many opportunities for understanding the molecular mechanisms associated with insulin resistance and of particular interest, a minimally invasive diagnostic tool. A major hindrance to proper diagnosis and treatment of insulin resistance lies in the fact that there is currently no direct method for determining insulin resistance that has a widespread clinical application. Insulin resistance is typically diagnosed with a combination of comorbidities and mathematical formulation based on glucose-insulin ratios, such as HOMA-IR (Ascaso and others 2003; Olatunbosun and Dagogo-Jack 2011). This approach severely compromises the reliability of diagnosis, especially during the early onset of insulin resistance, when treatment and lifestyle changes would likely be most effective.
This study focused on sled dogs, incredible athletes, which provide a homogenous population for studying environmental impacts such as nutrition and exercise on blood parameters (Dunlap and others 2006; Reynolds and others 1997, 1999). Even the older sled dogs in this study are relatively fit compared with other canine models in our study group (Dunlap and others 2006). While older dogs are no longer competitive, they still remain physically fit because they are routinely used to teach younger animals. Because of the uniformity and rigorous exercise regime, sled dogs provide a unique model for studying insulin signaling in response to exercise and maybe even age, but may not be the perfect model for obesity and diabetes. The results of this study indicate that sled dogs exhibit a typical insulin spike after a meal, which further indicates that sled dogs provide a reliable model for normal insulin response.
Perhaps the most important finding of this study was a non-significant, but apparent trend in GLUT4 in WBC with aging. This has also been observed in other species in muscle (Kern and others 1992). This trend may become significant if expression on the cell surface, rather than overall concentration is considered. The main objective of this project was to see whether significant and quantifiable amounts of GLUT4 could be detected in WBC of dogs. Now that we have established this, the next step is to assess expression of GLUT4 and correlate GLUT4 levels with HOMA-IR. We are now developing and refining this technique to allow for a fast, reliable, and simple method for quantifying GLUT4 expression on the cell surface. While our findings are quite preliminary, our results are very promising. Sled dogs are proving to be a fantastic model for insulin signaling because exercise and conditioning has a well-established effect on GLUT4 levels (Ebeling and others 1993). Furthermore, the energy needs and expenditure of a sled dog is 3–8 times that of the most elite human athlete (Hinchcliff and others 1997). Our future studies will benefit from comparisons with the established conditioning response in muscle to further validate this technique.
Acknowledgements
We are grateful for the assistance and support of Pile Driver Kennels and Jing Zheng for helping with the sample collection. This project was partly funded by the Department of Chemistry and Biochemistry, UAF and Nestle-Purina, St. Louis, MO. This publication was made possible by grants from the National Center for the Research Resources (5P20RR016466) and the National Institute of General Medical Sciences (8P20GM103395–12), from the National Institutes of Health. Its contents are the sole responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.