Introduction
The use of next-generation sequencing and other molecular detection methods has revealed the presence of complex communities of microbes within individual arthropod vectors of medical and veterinary importance. If vectors are infected with multiple potentially pathogenic microbes, then their vertebrate hosts might become exposed to >1 pathogen from a single bite from that vector, altering transmission dynamics. Such a possibility can alter traditional approaches to both medicine and disease ecology. Determining the ecological causes and health consequences of exposures to a specific pathogen may require explicit information on the presence of other co-occurring pathogens (Telfer et al., Reference Telfer, Lambin, Birtles, Beldomenico, Burthe, Paterson and Begon2010). Indeed, the expectation that vectors contain ‘pathobiomes’ suggests a new paradigm to replace the ‘one pathogen-one disease’ vision (Vayssier-Taussat et al., Reference Vayssier-Taussat, Kaximirova, Hubalek, Hornok, Farkas, Cosson, Bonnet, Vourch, Gasqui, Mihalca, Plantard, Silaghi, Cutler and Rizzoli2015). As transmission dynamics of any given vector-borne pathogen might be affected by the linked dynamics of other pathogens co-occurring with the focal pathogen within hosts and vectors (Diuk-Wasser et al., Reference Diuk-Wasser, Vannier and Krause2015).
Blacklegged ticks (Ixodes scapularis) are vectors of multiple zoonotic pathogens throughout North America. The causative agents of Lyme disease (Borrelia burgdorferi), human babesiosis (Babesia microti) and granulocytic anaplasmosis (Anaplasma phagocytophilum) in eastern and central North America are all transmitted predominantly by this tick vector (Eisen and Eisen, Reference Eisen and Eisen2018). All 3 of these diseases are increasing in prevalence and pose a mounting threat to public health in North America, as well as in Europe, and parts of Asia where they are vectored by related tick species (Rochlin and Toledo, Reference Rochlin and Toledo2020). Diagnosis and treatment of infected individuals can be complicated by the simultaneous presence of >1 of these pathogens, which can alter the presentation of symptoms, increase their severity, and change the recommended therapeutic response. The presence of coinfections of individual patients by more than 1 of these pathogens is increasingly being recognized as a public health challenge (Diuk-Wasser et al., Reference Diuk-Wasser, Vannier and Krause2015).
Coinfections in human patients can be caused by successive bites from different ticks, each delivering only 1 species of pathogen, or by the bite of 1 coinfected tick. Widespread detection of coinfection in individual nymph-stage blacklegged ticks, which is the stage most strongly linked to human cases of tick-borne infections (Ostfeld et al., Reference Ostfeld, Price, Hornbostel, Benjamin and Keesing2006; Pepin et al., Reference Pepin, Eisen, Mead, Piesman, Fish, Hoen, Barbour, Hamer and Diuk-Wasser2012), suggests that the latter happens frequently. Coinfection of blacklegged ticks by B. burgdorferi and B. microti is the coinfection most commonly detected, although other pairwise combinations and coinfection with all 3 of these pathogens also occur (Lehane et al., Reference Lehane, Maes, Graham, Jones, Delorey and Eisen2021). Consequently, coinfected ticks pose a particularly strong threat to public health and a challenge to disease ecologists.
Of these 3 zoonotic pathogens, prevalence of B. burgdorferi in both ticks and human patients is generally the highest and most geographically widespread, although all of them are spreading geographically and increasing disease incidence (Eisen and Eisen, Reference Eisen and Eisen2018). Patterns of co-occurrence of B. burgdorferi and B. microti in both reservoir hosts (Dunn et al., Reference Dunn, Krause, Davis, Vannier, Fitzpatrick, Rollend, Belperron, States, Stacey, Bockenstedt, Fish and Diuk-Wasser2014; Tufts et al., Reference Tufts, Adams and Diuk-Wasser2023) and blacklegged ticks (Little and Molaei, Reference Little and Molaei2020; Pokutnaya et al., Reference Pokutnaya, Molaei, Weinberger, Vossbrinck and Diaz2020; Zembsch et al., Reference Zembsch, Lee, Bron, Bartholomay and Paskewitz2021) suggest positive interactions between the 2 pathogens (i.e. facilitation), although interactions appear complex. For instance, host-to-tick transmission of B. microti increased when the host was also infected with B. burgdorferi (Dunn et al., Reference Dunn, Krause, Davis, Vannier, Fitzpatrick, Rollend, Belperron, States, Stacey, Bockenstedt, Fish and Diuk-Wasser2014). Delivery to white-footed mice (Peromyscus leucopus) of an oral vaccine that induces immunity to B. burgdorferi (but not to B. microti) (Richer et al., Reference Richer, Brisson, Melo, Ostfeld, Zeidner and Gomes-Solecki2014) reduced the odds of tick coinfection (B. burgdorferi and B. microti) by a factor of 7.5 despite having no direct effect on infection prevalence with the latter (Vannier et al., Reference Vannier, Richer, Dinh, Brisson, Ostfeld and Gomes-Solecki2023). Hersh et al. (Reference Hersh, Tibbetts, Ostfeld, Straus and Keesing2012) and Keesing et al. (Reference Keesing, Hersh, Tibbetts, McHenry, Duerr, Brunner, Killilea, LoGiudice, Schmidt and Ostfeld2012) demonstrated that 3 small mammal species, the white-footed mouse, the eastern chipmunk Tamias striatus and the short-tailed shrew Blarina brevicauda, were the most competent reservoir hosts for B. microti and A. phagocytophilum. These same 3 small mammals are also the most competent reservoir hosts for B. burgdorferi (LoGiudice et al., Reference LoGiudice, Ostfeld, Schmidt and Keesing2003, Reference LoGiudice, Duerr, Newhouse, Schmidt, Killilea and Ostfeld2008; Keesing et al., Reference Keesing, Brunner, Duerr, Killilea, LoGiudice, Schmidt, Vuong and Ostfeld2009). Hersh et al. (Reference Hersh, Ostfeld, McHenry, Tibbetts, Brunner, Killilea, LoGiudice, Schmidt and Keesing2014) determined that coinfection of nymphal blacklegged ticks with B. burgdorferi and B. microti was significantly more frequent than expected from a null model that assumed independent transmission by each pathogen, suggesting that these 2 pathogens were co-transmitted from hosts to ticks. Supporting that hypothesis, Hersh et al. (Reference Hersh, Ostfeld, McHenry, Tibbetts, Brunner, Killilea, LoGiudice, Schmidt and Keesing2014) found that the great majority of coinfected ticks had fed from 1 of the 3 species of small mammal hosts.
The Tick Project (Keesing et al., Reference Keesing, Mowry, Bremer, Duerr, Evans, Fischhoff, Hinckley, Hook, Keating, Pendleton, Pfister, Teator and Ostfeld2022) was designed to assess the efficacy of 2 tick-killing interventions in reducing abundance of blacklegged ticks, encounters with ticks and cases of tick-borne disease. One of the acaricidal interventions was a fungal biocontrol agent (Met52), consisting of spores of the entomopathogenic fungus Metarhizium brunneum, sprayed on low vegetation where host-seeking ticks are likely to occur. The other was a host-targeted acaricide deployed in devices (tick control system [TCS] bait boxes) that allowed small mammals to self-apply the acaricide fipronil, but excluded other hosts, thus targeting ticks that occur on mice, chipmunks and shrews. Placebo controls for both these interventions were also deployed. We predicted that nymphal blacklegged ticks in areas treated with placebo controls would show coinfection rates of B. burgdorferi and B. microti that were significantly more frequent than expected assuming independent host-to-tick transmission, reflecting results of Hersh et al. (Reference Hersh, Ostfeld, McHenry, Tibbetts, Brunner, Killilea, LoGiudice, Schmidt and Keesing2014). We further predicted that the use of acaricide targeted at ticks on small mammals would selectively reduce survival of the larval blacklegged ticks most likely to acquire infection with both pathogens. Survival of larval ticks feeding on other, larger hosts, such as raccoons (Procyon lotor), opossums (Didelphis virginiana) and white-tailed deer (Odocoileus virginianus), would not be affected by this treatment, and these non-small-mammal hosts do not contribute to coinfection (Hersh et al., Reference Hersh, Ostfeld, McHenry, Tibbetts, Brunner, Killilea, LoGiudice, Schmidt and Keesing2014). Therefore, we expected that the TCS bait boxes would eliminate the bias towards coinfection at the subsequent nymphal stage. If these predictions were supported, we expected that the use of acaricides targeted at small-mammal hosts could, in addition to reducing overall tick abundance, have the added benefit of reducing the probability of human exposure to multiple pathogens given a single tick bite.
Materials and methods
We collected data for this research as part of The Tick Project, a multi-year study to test the effects of environmental interventions on tick abundance and infection, as well as tick-borne diseases of humans and outdoor pets, in 24 residential neighbourhoods of Dutchess County, New York, USA (Keesing et al., Reference Keesing, Mowry, Bremer, Duerr, Evans, Fischhoff, Hinckley, Hook, Keating, Pendleton, Pfister, Teator and Ostfeld2022; Ostfeld et al., Reference Ostfeld, Mowry, Bremer, Duerr, Evans and Fischhoff2023a, Reference Ostfeld, Adish, Mowry, Bremer, Duerr, Evans, Fischhoff, Keating, Pendleton, Pfister, Teator and Keesing2023b), an area of very high endemicity for multiple tick-borne diseases (Keesing et al., Reference Keesing, Mowry, Bremer, Duerr, Evans, Fischhoff, Hinckley, Hook, Keating, Pendleton, Pfister, Teator and Ostfeld2022). In The Tick Project, we tested the effects of 2 commercially available products, Met52 and TCS bait boxes. Met52 contains spores derived from a naturally occurring fungus, M. brunneum, and is applied to habitats such as lawns and gardens with a high-pressure sprayer. Application was at 175–200 psi (pounds per square inch) and conformed to product labelling. This product is intended to kill all life stages of ticks while they are seeking hosts. The second product, the TCS bait box, consists of a small plastic and metal box (19.05 cm by 13.97 cm by 6.35 cm) that contains a small amount of bait. The bait attracts small mammals like rodents and shrews (Ostfeld, Reference Ostfeld2012), which are dabbed with an acaricide, fipronil, while they are inside the box, and then exit the box unharmed. The acaricide kills ticks for several weeks after application, with the goal of reducing transmission of tick-borne pathogens. Details of these treatments and other aspects of the study design are provided in Keesing et al. (Reference Keesing, Mowry, Bremer, Duerr, Evans, Fischhoff, Hinckley, Hook, Keating, Pendleton, Pfister, Teator and Ostfeld2022).
Each of the 24 neighbourhoods in The Tick Project was randomly assigned to receive 1 of 4 possible combinations of the 2 products – Met52 and TCS bait boxes – or their equivalent placebo controls. Placebo controls consisted of the product without its active ingredient. The placebo for Met52 was a high-pressure spray of water without the fungus. The placebo for TCS bait boxes was the box with the bait but without the acaricide. Thus, there were 4 treatment combinations – with active Met52 and active TCS bait boxes, with active Met52 and placebo bait boxes, with placebo Met52 and active TCS bait boxes and with placebos of both treatments. The 24 neighbourhoods were randomly grouped into 6 replicates of each of the 4 treatment combinations. The study was thus randomized, replicated and placebo-controlled. It was also double-masked (i.e. double-blinded) as neither the participants in the neighbourhoods nor anyone collecting data for the project knew the treatment assignments of any of the neighbourhoods.
Residents were invited to participate in the project if they lived in 1 of the 24 neighbourhoods, each of which consisted of ~100 homes and had a history of high incidence of tick-borne illnesses (Keesing et al., Reference Keesing, Mowry, Bremer, Duerr, Evans, Fischhoff, Hinckley, Hook, Keating, Pendleton, Pfister, Teator and Ostfeld2022). After thorough canvasing to determine interest and eligibility (e.g. willingness to forgo other acaricidal treatments for the duration of the study), we enrolled 24–44% (mean 34%) of households in each neighbourhood.
We collected questing nymphal ticks on the properties of 20 randomly selected households twice per year in each neighbourhood in May–July of 2017, 2018, 2019 and 2021, at the peak of nymphal activity for blacklegged ticks. (We were not able to collect ticks in 2020 because of the COVID-19 pandemic.) Ticks were collected by flag-sampling in 3 common habitat types in residential areas of Dutchess County, lawns, gardens and forests. Flag-sampling was standardized among all researchers by total effort (flagging time) and effort per habitat type (proportional to habitat area). All collected nymphal ticks from all 3 habitats were pooled by neighbourhood and stored alive in humidified vials until being flash-frozen and stored (see below).
We tested collected nymphal blacklegged ticks for the presence of 3 tick-borne pathogens, B. burgdorferi (causative agent of Lyme disease), A. phagocytophilum (anaplasmosis) and B. microti (babesiosis). Details of this procedure are provided in Ostfeld et al. (Reference Ostfeld, Adish, Mowry, Bremer, Duerr, Evans, Fischhoff, Keating, Pendleton, Pfister, Teator and Keesing2023b). Briefly, we surface-sterilized ticks with 10% bleach within 2–3 weeks of collection, and then rinsed them with deionized water, after which they were stored individually in 2 mL Eppendorf tubes at −80°C. After lysing via bead-beating and homogenizing the ticks, we extracted DNA using the DNeasy 96 Blood & Tissue kit (Qiagen, Maryland, USA). We used multiplex polymerase chain reaction(PCR) to detect A. phagocytophilum and B. burgdorferi, as described in Ostfeld et al. (Reference Ostfeld, Adish, Mowry, Bremer, Duerr, Evans, Fischhoff, Keating, Pendleton, Pfister, Teator and Keesing2023b). Briefly, A. phagocytophilum was detected by targeting the msp2 gene using primers ApMSP2f, ApMSP2r and ApMSP2p (Courtney et al., Reference Courtney, Kostelnik, Zeidner and Massung2004; Keesing et al., Reference Keesing, Hersh, Tibbetts, McHenry, Duerr, Brunner, Killilea, LoGiudice, Schmidt and Ostfeld2012). Borrelia burgdorferi was detected by targeting the 23S ribosomal RNA (rRNA) gene of B. burgdorferi using primers Bb23Sf, Bb23Sr and Bb23Sp. Babesia microti was detected by targeting the 18S rRNA gene using primers smbaJF and smbaKR following a melting curve analysis (Ostfeld et al., Reference Ostfeld, Adish, Mowry, Bremer, Duerr, Evans, Fischhoff, Keating, Pendleton, Pfister, Teator and Keesing2023b).
We ran all reactions on a LightCycler 480 II (Roche, Switzerland) thermal cycler following manufacturer recommendations, and used DNA extract from tick larvae and PCR-grade water (Roche) as negative controls (Ostfeld et al., Reference Ostfeld, Adish, Mowry, Bremer, Duerr, Evans, Fischhoff, Keating, Pendleton, Pfister, Teator and Keesing2023b). Positive controls were taken from previous positive control samples (Hersh et al., Reference Hersh, Tibbetts, Ostfeld, Straus and Keesing2012; Keesing et al., Reference Keesing, Hersh, Tibbetts, McHenry, Duerr, Brunner, Killilea, LoGiudice, Schmidt and Ostfeld2012). We tested 3 replicates of each nymphal tick sample, and assigned infection status based on the details provided in Ostfeld et al. (Reference Ostfeld, Adish, Mowry, Bremer, Duerr, Evans, Fischhoff, Keating, Pendleton, Pfister, Teator and Keesing2023b). For analysis, we considered nymphal ticks collected in 2018–2021 for which we were able to determine infection status for all 3 pathogens. The nymphal ticks collected these years represent the cohorts that could have been affected by the acaricidal interventions, which began in 2017.
We determined whether nymphal ticks in each treatment were coinfected more or less than expected by chance using a permutation test developed by Hersh et al. (Reference Hersh, Ostfeld, McHenry, Tibbetts, Brunner, Killilea, LoGiudice, Schmidt and Keesing2014). First, we combined infection status for the nymphal ticks from each of the 4 treatment combinations, so that we had 4 sets of data. For each treatment combination, we randomly resampled nymphal tick infection status for each pathogen, independently and without replacement, 100 000 times. This allowed us to determine the expected frequencies of each pathogen alone and in combination with other pathogens, which we then compared to the observed frequencies of single and multiple infections from that treatment combination. To do this, we determined the proportion of samples in which the difference between the observed prevalence of each infection type and the permutation mean was as or more extreme than the difference between the permutation mean and each permuted sample. The statistical significance of this result was determined as P = (number of samples in which [permutation mean – observed data] ⩾ [permutation mean – permutation data point] + 1)/(number of permutations + 1) (Chihara and Hesterberg, Reference Chihara and Hesterberg2011; Hersh et al., Reference Hersh, Ostfeld, McHenry, Tibbetts, Brunner, Killilea, LoGiudice, Schmidt and Keesing2014). We quantified the effect size by determining the ratio of the observed level of co-infection to the permutation mean.
To determine whether there were effects of treatment on observed levels of coinfection, we first excluded neighbourhood–year combinations in which we did not collect at least 10 ticks, of which there were 7 occurrences out of 72 total. We used generalized linear mixed models (glmm), with an interaction between the 2 treatments (Baitbox × Met52) and year (2018, 2019, 2021) as fixed effects, and neighbourhood as a random effect. For these analyses, we had 6 replicates (i.e. neighbourhoods) for each of the 4 treatments, and we used the number of infected ticks in a neighbourhood each year as the dependent variable, with an offset for the total number of ticks collected from that neighbourhood. Models were fitted with a negative binomial distribution. We tested that data met assumptions of tests using package DHARMa (Hartig, Reference Hartig2022), and determined statistical significance using the function Anova from package car (Fox and Weisberg, Reference Fox and Weisberg2019). Data were analysed using version 4.0.1 of R.
Results
We collected and tested 5231 questing nymphal blacklegged (I. scapularis) ticks for the presence of all 3 pathogens. Borrelia burgdorferi had the highest prevalence, infecting an overall mean of 21% (±0.02% s.e.m.) of ticks in the control neighbourhoods (i.e. those neighbourhoods treated only with placebo interventions). In contrast, 13% (±0.03%) of nymphs in control neighbourhoods were infected with A. phagocytophilum, and 9% (±0.01%) with B. microti.
Compared to single infections, coinfections were relatively rare, with a mean of 4% (±0.01%) of ticks in control neighbourhoods infected with both B. burgdorferi and B. microti, the most common coinfection. An overall mean of 2% (±0.01%) of nymphal ticks were infected with both B. burgdorferi and A. phagocytophilum, and 1% (±0.004%) with A. phagocytophilum and B. microti. Ticks infected with all 3 pathogens were uncommon, accounting for <1% of ticks in control neighbourhoods.
Based on our permutation analysis, ticks in control neighbourhoods were significantly less likely to be infected with only B. burgdorferi or only B. microti than expected from the assumption of independent transmission of each pathogen. For example, if infection were random, 6% (95% CI 5.30–6.64) of ticks on control plots would be infected with just B. microti, but we found that only 4% were, which is 2/3 of the expected value (Table S1; Fig. 1; Additional file 1: Fig. S1).
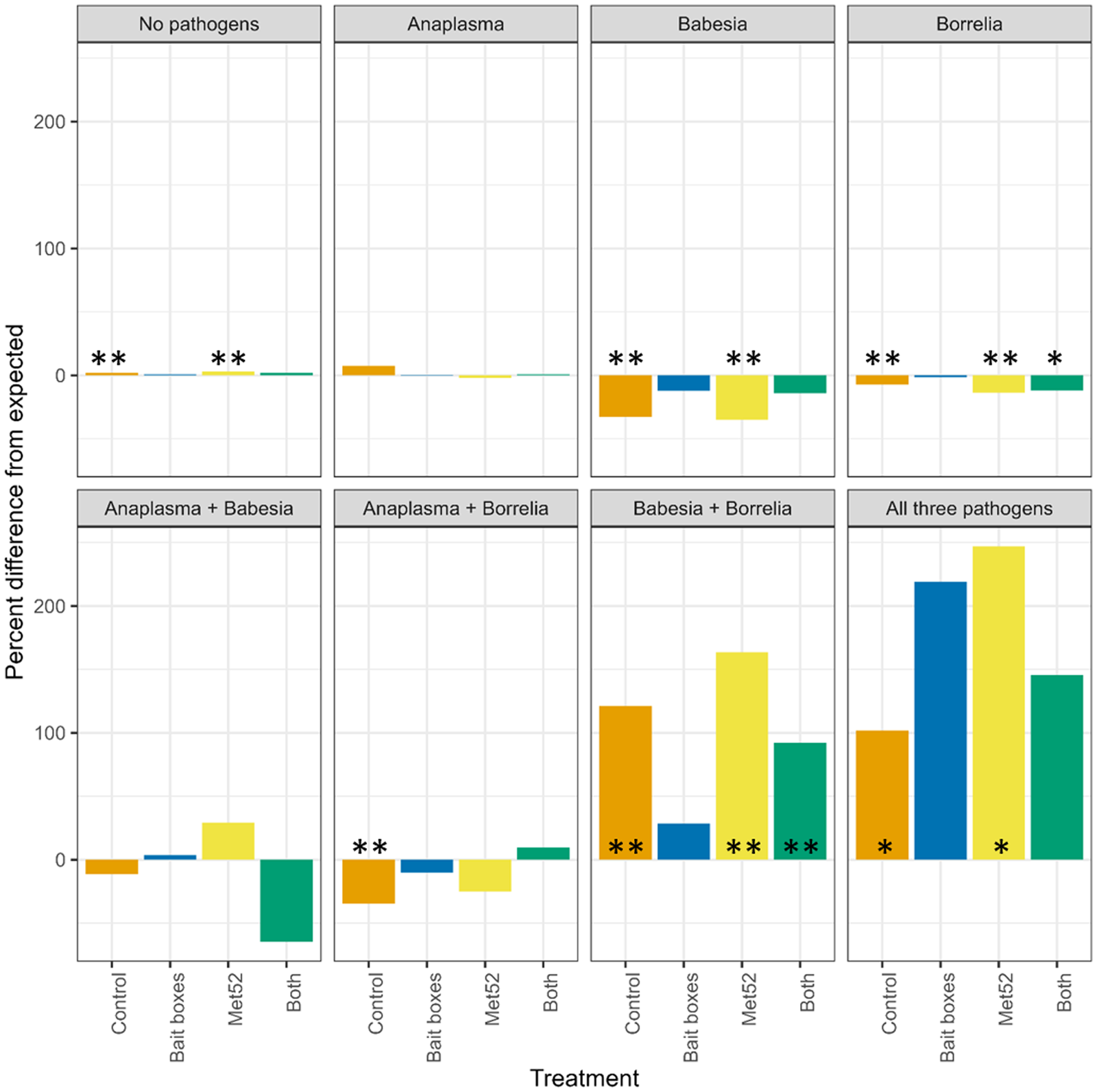
Figure 1. Per cent difference in observed prevalence of questing nymphal blacklegged ticks from values expected if prevalence of a particular pathogen in ticks were random with respect to that of other pathogens. Zero values indicate that observed prevalences were equal to expected. Control neighbourhoods were untreated, ‘both’ indicates neighbourhoods treated with bait boxes and Met52 spray, see details in Methods. Asterisks indicate statistically significant differences, * indicates P < 0.05, and ** indicates P < 0.01. See also Table S1 and Fig. S1.
The permutation analysis also revealed that some coinfections in control neighbourhoods were more common than expected from the assumption of independent transmission of each pathogen. In particular, ticks infected with B. burgdorferi were significantly more likely than expected to also be infected with A. phagocytophilum (P = 0.010), B. microti (P < <0.01) or both (P < 0.05) (Table S1; Fig. 1).
Neighbourhoods treated with active Met52 fungal spray showed patterns of infection and coinfection similar to those seen in control neighbourhoods (Table S1; Fig. 1). However, in sharp contrast to the patterns observed in control neighbourhoods, none of the observed levels of multiple infections in neighbourhoods treated with active bait boxes were significantly different from those expected by chance (Table S1; Fig. 1). In neighbourhoods treated with both bait boxes and Met52, B. burgdorferi was significantly less common than expected as a single infection, and significantly more common as a coinfection with B. microti.
Using the 6 neighbourhoods receiving each treatment as replicates, we asked whether treatment significantly affected the proportion of ticks coinfected with each combination of pathogens. In the neighbourhoods treated with bait boxes, we observed a statistically significant reduction in the proportion of ticks coinfected with both B. burgdorferi and B. microti, as compared to neighbourhoods treated with placebo controls (P = 0.04; Fig. 2B). There was also a significant interaction between bait boxes and Met52 spray (P = 0.03; Fig. 2B). None of the other combinations of coinfection showed a significant effect of treatment. In contrast, 3 of the 4 possible coinfections showed statistically significant effects of year (Fig. 2; Additional file 2: Fig. S2), with the prevalence of coinfections generally declining over time in all treatments, including the controls.
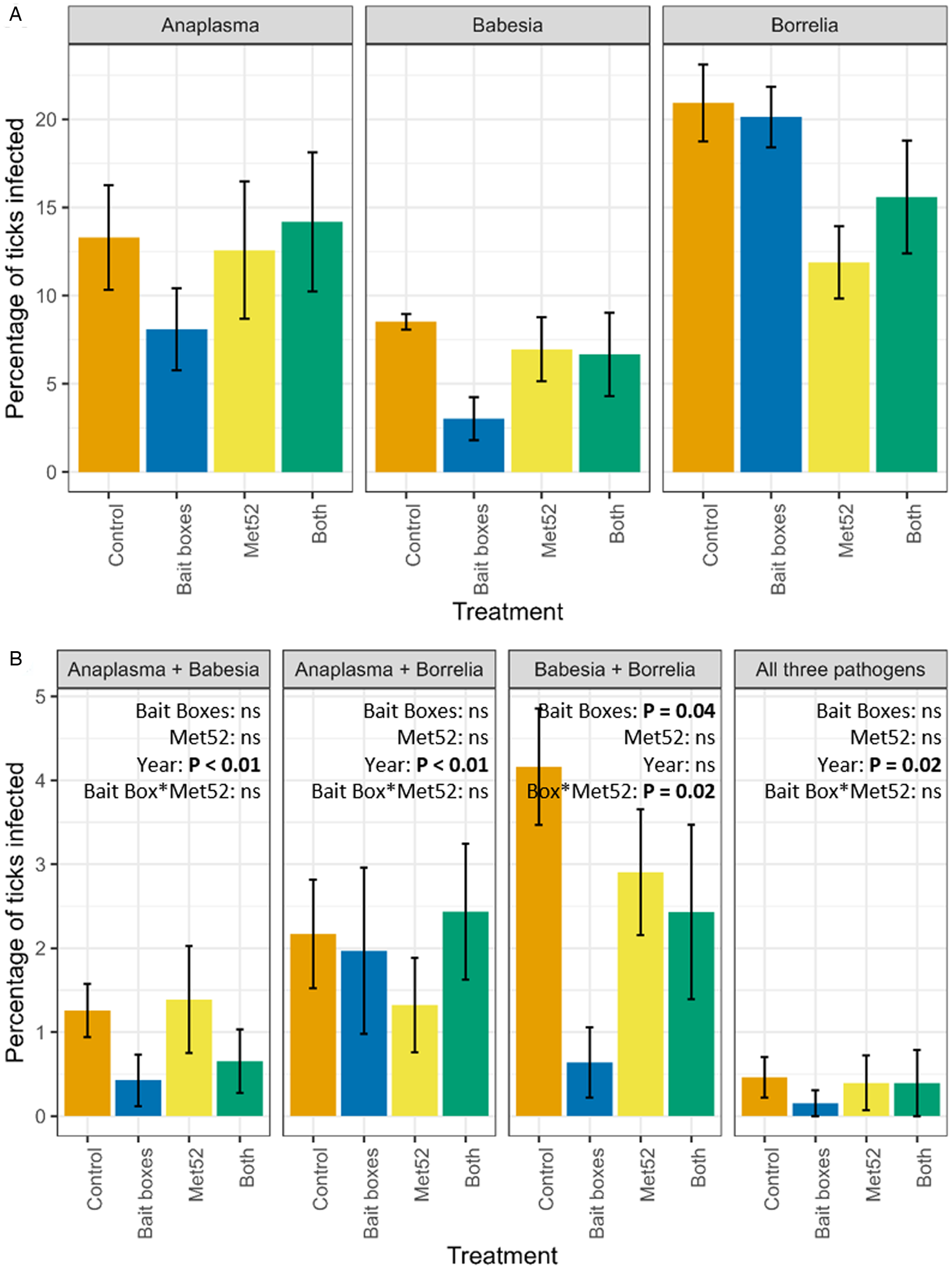
Figure 2. Mean (±standard error of the mean) percentage of questing nymphal blacklegged ticks infected with (A) individual pathogens, and (B) multiple pathogens in neighbourhoods in each of the 4 treatments of the Tick Project. Data on individual pathogens include ticks that were coinfected, and data on double infections include ticks that were triply infected. For example, the percentage of ticks infected with Anaplasma phagocytophilum in (A) includes ticks that were also infected with other pathogens, as in (B). Control neighbourhoods were untreated, ‘both’ indicates neighbourhoods treated with bait boxes and Met52 spray, see details in Methods. Effects of treatments on individual pathogens were previously reported in Ostfeld et al. (Reference Ostfeld, Mowry, Bremer, Duerr, Evans and Fischhoff2023a, Reference Ostfeld, Adish, Mowry, Bremer, Duerr, Evans, Fischhoff, Keating, Pendleton, Pfister, Teator and Keesing2023b) and are included here for reference. Note that y-axis values vary.
Discussion
We tested 5231 questing nymphal I. scapularis ticks collected over 4 years in Dutchess County, NY, to estimate the prevalence of single infections and 2- and 3-way coinfections with the most frequently encountered tick-borne, zoonotic pathogens in eastern North America. Assessments of ticks collected from control neighbourhoods, in which no acaricidal treatments were deployed, confirmed that ticks coinfected with B. burgdorferi and either B. microti or A. phagocytophilum occurred more frequently than expected assuming independent transmission (Hersh et al., Reference Hersh, Ostfeld, McHenry, Tibbetts, Brunner, Killilea, LoGiudice, Schmidt and Keesing2014). In parallel, ticks infected with only 1 of these pathogens occurred less frequently than expected under independent transmission, in the control neighbourhoods. Prior research revealed that blacklegged ticks acquire coinfections of tick-borne pathogens predominantly by feeding as larvae on white-footed mice, eastern chipmunks or short-tailed shrews (Hersh et al., Reference Hersh, Ostfeld, McHenry, Tibbetts, Brunner, Killilea, LoGiudice, Schmidt and Keesing2014), because these hosts are themselves often infected with multiple pathogens and are more efficient than other vertebrate hosts at transmitting infections to ticks. This observation led to the hypothesis that the selective killing of ticks that feed as larvae on this guild of small-mammal hosts would reduce coinfection prevalence within host-seeking ticks (because the remaining nymphs would have fed in higher numbers on other hosts), in addition to reducing tick abundance (which is the intended effect).
Supporting the hypothesis that selectively killing ticks that feed on small mammals would disrupt transmission biased towards multiple pathogens, we found that the questing nymphs collected from neighbourhoods in which TCS bait boxes were deployed showed coinfection patterns that were indistinguishable from expectations based on independent transmission. Prevalence of single infections with each of the 3 pathogens under bait box treatments was not different from expectations arising from the assumption of independent transmission. In other words, the bias towards coinfection that occurs under unmanipulated conditions (Hersh et al., Reference Hersh, Ostfeld, McHenry, Tibbetts, Brunner, Killilea, LoGiudice, Schmidt and Keesing2014) was eliminated by the use of bait boxes. The selective killing of ticks feeding as larvae on small mammals (Dolan et al., Reference Dolan, Maupin, Schneider, Denatale, Hamon and Cole2004; Stafford et al., Reference Stafford, Williams and Molaei2017; Williams et al., Reference Williams, Little, Stafford, Molaei and Linske2018a, Reference Williams, Stafford, Molaei and Linske2018b) eliminated the observed bias towards coinfection (Hersh et al., Reference Hersh, Ostfeld, McHenry, Tibbetts, Brunner, Killilea, LoGiudice, Schmidt and Keesing2014; Little and Molaei, Reference Little and Molaei2020; Zembsch et al., Reference Zembsch, Lee, Bron, Bartholomay and Paskewitz2021; but see Little et al., Reference Little, Williams, Stafford, Linske and Molaei2020) and away from single infection that arises because mice, chipmunks and shrews are the most competent reservoirs for all 3 of the zoonotic pathogens under study (Levi et al., Reference Levi, Keesing, Holt, Barfield and Ostfeld2016). We surmise that the questing nymphal ticks we collected from the neighbourhoods with active bait boxes had fed as larvae largely on other, non-small-mammal members of the host community, which would not result in a bias towards co-occurrence of multiple pathogens in individual nymphal ticks. Indeed, in neighbourhoods treated with Met52, which affects host-seeking ticks unselectively, the bias towards coinfection observed in the controls was maintained, as expected. Not surprisingly, ticks collected from neighbourhoods with both bait boxes and Met52 showed intermediate patterns of single- and coinfection.
Our prior analyses of the effects of both Met52 and TCS bait boxes on prevalence of tick infection with zoonotic pathogens focused exclusively on single, rather than multiple, infections (Ostfeld et al., Reference Ostfeld, Adish, Mowry, Bremer, Duerr, Evans, Fischhoff, Keating, Pendleton, Pfister, Teator and Keesing2023b). Those analyses revealed no significant effects of TCS bait boxes on infection prevalence of nymphal ticks with any of the 3 single pathogens but a significantly reduced infection prevalence with B. burgdorferi (but not of B. microti or A. phagocytophilum) in neighbourhoods with active Met52. These results caused us to expect that coinfections of B. burgdorferi and either of the other 2 pathogens would be reduced in the Met52-treated neighbourhoods, all else equal, but this hypothesis was not supported.
The use of bait boxes significantly reduced coinfection of B. burgdorferi and B. microti but not of B. burgdorferi and A. phagocytophilum. A reduced effect of bait boxes on the latter coinfection might arise because small mammals are only modestly more competent reservoirs for A. phagocytophilum as compared to other vertebrate hosts (Keesing et al., Reference Keesing, Hersh, Tibbetts, McHenry, Duerr, Brunner, Killilea, LoGiudice, Schmidt and Ostfeld2012). For B. microti, the difference in reservoir competence between small mammals and other hosts is more distinct (Hersh et al., Reference Hersh, Tibbetts, Ostfeld, Straus and Keesing2012). Thus, targeting ticks that fed as larvae on small mammals might be expected to have a weaker effect on coinfections involving A. phagocytophilum. The potential roles of indirect, host-mediated interactions between B. burgdorferi and the other 2 pathogens (Tufts et al., Reference Tufts, Adams and Diuk-Wasser2023; Vannier et al., Reference Vannier, Richer, Dinh, Brisson, Ostfeld and Gomes-Solecki2023), and of vertical transmission of B. microti (Tufts and Diuk-Wasser, Reference Tufts and Diuk-Wasser2018), are not clear but are worthy of further study.
Reports of human patients concurrently infected with B. burgdorferi and B. microti have accumulated since the 1990s, with evidence for potentially increased severity and duration of symptoms (Krause et al., Reference Krause, Telford, Spielman, Sikand, Ryan, Christianson, Burke, Brassard, Pollack, Peck and Persing1996, Reference Krause, McKay, Thompson, Sikand, Lentz and Lepore2002; Caulfield and Pritt, Reference Caulfield and Pritt2015), but see Wormser et al. (Reference Wormser, McKenna, Scavarda, Cooper, El Khoury, Nowakowski, Sudhindra, Ladenheim, Wang, Karmen, Demarest, Dupuis and Wong2019). Coinfections in human patients can arise from multiple tick bites, each resulting in single infections, or from bites from coinfected ticks, with the relative frequency of these 2 modes of transmission unknown, to our knowledge. Means of preventing such coinfections could protect public health by avoiding difficulties involved with diagnosing and treating coinfections within individuals. Although acaricides are expected to aid in preventing exposures by reducing tick abundance (Dolan et al., Reference Dolan, Maupin, Schneider, Denatale, Hamon and Cole2004; Schulze et al., Reference Schulze, Jordan, Schulze, Healy, Jahn and Piesman2007, Reference Schulze, Jordan, Williams and Dolan2017; Williams et al., Reference Williams, Little, Stafford, Molaei and Linske2018a, Reference Williams, Stafford, Molaei and Linske2018b; Jordan and Schulze, Reference Jordan and Schulze2019; Little et al., Reference Little, Williams, Stafford, Linske and Molaei2020; but see Hinckley et al., Reference Hinckley, Meek, Ray, Niesobecki, Connally and Feldman2016; Hinckley et al., Reference Hinckley, Niesobecki, Connally, Hook, Biggerstaff and Horiuchi2021; Keesing et al., Reference Keesing, Mowry, Bremer, Duerr, Evans, Fischhoff, Hinckley, Hook, Keating, Pendleton, Pfister, Teator and Ostfeld2022), an under-recognized pathway for acaricides directed at small-mammal hosts might consist of reducing the bias towards coinfections in host-seeking nymphal ticks.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182024000349
Data availability
Data will be posted on the Cary Institute's Figshare site upon acceptance of the final manuscript.
Acknowledgements
We thank our partners: the Cary Institute of Ecosystem Studies, Bard College, the US Centers for Disease Control, the New York State Department of Health and the Dutchess County Department of Community and Behavioral Health. The Cary Institute of Ecosystem Studies provided extensive logistical support. We benefited enormously from the expertise and dedication of the team at Arkus, Inc., especially Amy Bucciffero. Associates at Salesforce.org, Pardot, SMSMagic, FormAssembly and DocuSign provided software and support at reduced fees. Our colleagues at Pestech Pest Solutions provided the professional applications of our treatments, under the committed leadership of Rob Robinson and Noah Startup. This project would not have been possible without the staff of the Tick Project: Rose Adelizzi, Shefali Azad, Daniella Azulai, Kate Badour, Callie Barth-Dwyer, Connor Baush, Mohammad Harris Bayan, Anna Butler, Reilly Carlson, Samantha Cassata, Adrian D'Souza, Dylan Dahan, Stephanie Dea, Deanna DePietro, Joshua DiPaola, Lizzy Elliott, Michelle Ferrell, Amanda Gabryszak, Annie Geiger, Jared Jaeger, Abigail Johnson, Amanda Jones, Alyssa Kamrowski, Ashley Kolton, Troy Koser, Bethany Krug, Jacob Kulyniak, Beckett Lansbury, Rachel Livengood, Morgan Long, Daniel Medeiros, Sara McBride, Mica McCarty-Glen, Waynette McCracken, Sarah McGregor, Timothy McSweeny, Vince Meyer, Alison Molnar, Victoria Palfini, Nadine Pershyn, Nick Petterelli, Nicole Pierro, Luke Porter, Sophia Raithel, Madeline Rivard, Jared Russ, Elizabeth Rush, Samantha Sambado, Makayla Says, Megan Schierer, Brandi Strand, Katie Sweeney, Charlene Gray Tarsa, Abraham Turner, Nick Urbin, Tyler Walters, Imogene Welles, Agatha Winiarski and Alex Wolf. The project benefited from the professional support of Josh Ginsberg, Holly Talbot, Lori Quillen, David Fischer, Amanda Johnson, Heather Malcom, Fred Merritt, Catherine Forbes, Alison Hinckley, Sarah Hook, Ben Beard, Lars Eisen, Paul Mead, Jill Auerbach, Sue Serino, Didi Barrett and Sarah Dunphy-Lelii. Special thanks to Denise Schmidt for support of all aspects of the molecular analyses. This research was made possible by the generous participation of thousands of residents of Dutchess County, New York, and we extend our great appreciation to all of them.
Author contributions
R. S. O. and Keesing designed the study. A. S. E. provided input on study protocols. S. A., W. B., S. D., J. P. and M. T. collected data. Keating, A. P. and S. M. coordinated treatments and data. Keesing and S. M. analysed the data. R. S. O. and Keesing wrote the first draft of the manuscript and all authors edited it.
Financial support
We are grateful for the support we received from the Steven and Alexandra Cohen Foundation, the Centers for Disease Control, New York State, the Dutchess County Water and Wastewater Authority, the Ian Mactaggart Trust, the John Drulle, MD Memorial Lyme Fund, Inc., the Pershing Square Foundation, The Walbridge Fund, Nina Brown deClercq, Susan and Jim Goodfellow, Elyse Harney, Eric Roberts, Pamela and Scott Ulm, and other private donors.
Competing interests
None.
Ethical standards
Ethical standards were approved by the Cary Institute's Institutional Animal Care and Use Committee under protocol #02-16.