INTRODUCTION
The pinworm Enterobius vermicularis persists as one of the most common helminths in humans [Reference Cook1], with an estimated 400 million individuals infected worldwide [Reference Lukes, Horák and Scholz2]. Infections with E. vermicularis are usually benign and asymptomatic [Reference Gale3–Reference Bøås5] with symptoms typically limited to perianal pruritus [Reference Burkhart and Burkhart6]. In 1947, 40–60% of European children were positive for pinworms [Reference Stoll7], but the reported prevalence has declined markedly in Western societies during the last decades [Reference Palmas8–Reference Kyronseppa11]. Reduced exposure to E. vermicularis is one of the suggested factors in the hygiene hypothesis [Reference Gale3]. This hypothesis proposes that a reduced exposure to common microorganisms and helminths has led to decreased efficiency of immunoregulatory mechanisms, and thus may be an underlying cause of the increase in asthma and other chronic inflammatory disorders seen in developed countries [Reference Rook12]. Interestingly, immunomodulatory effects of E. vermicularis and other helminths have been reported [Reference Gale3, Reference Yazdanbakhsh, van den Biggelaar and Maizels13, Reference Schäfer14].
A few studies have focused on investigating the possible link between E. vermicularis and allergic and atopic conditions, but with conflicting results. One study has shown that previous infections of E. vermicularis were associated with lower frequency of eczema and allergic sensitization [Reference Schäfer14]. Other studies have shown that a history of E. vermicularis infections has been associated with an increased risk of atopic dermatitis [Reference Wördemann15], allergic rhinoconjunctivitis [Reference Wördemann15] and current wheezing [Reference Bahceciler16], while another study found a negative association with current asthma and rhinitis [Reference Huang, Tsai and Yeh17]. A possible connection between current E. vermicularis infections and allergy has also been observed [Reference Herrström18].
In our recent study of Norwegian children [Reference Bøås5], 18·2% were positive for E. vermicularis, with a peak prevalence of 34·4% in children aged >5 years. These results are in line with earlier Swedish results [Reference Herrström4], and indicate that the decline in the prevalence of E. vermicularis is not as marked as reported by others [Reference Palmas8–Reference Kyronseppa11]. In light of our own recent report showing a very high prevalence of E. vermicularis in Norway, it was of interest to test if there are any associations between allergic disorders and E. vermicularis in Norwegian children.
MATERIALS AND METHODS
Subjects
The children studied participated in the ‘Environmental Triggers of Type 1 Diabetes’ (MIDIA) study, which is a longitudinal cohort study with the aim of identifying environmental factors of type 1 diabetes. Parents were invited to participate in the MIDIA study by a public health nurse during routine home visits at 1–2 weeks after birth. The children in the MIDIA study were screened for the human leukocyte antigen (HLA) genotype conferring the highest risk for type 1 diabetes (HLA-DQB1*02-DQA1*05-DRB1*03/DQB1*03:02-DQA1*03-DRB1*04:01) [Reference Stene19], found in 2·1% of the normal population [Reference Rasmussen20]. Children with the high-risk HLA genotype made up the DQ8/2 group, while children with other genotypes were assigned to the non-DQ8/2 group. At least one of the parents of children included in the MIDIA study had Norwegian or other European origin. Children born preterm or born with serious malformation or disease were excluded [Reference Stene19]. The recruitment was conducted on a nationwide scale between 2001 and 2007. For a more detailed description on the MIDIA study see Stene et al. [Reference Stene19].
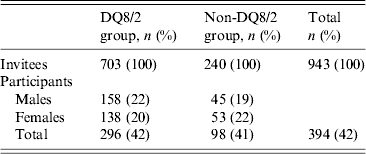
The parents of all children currently participating in the MIDIA cohort were invited to participate in this sub-study, with the aim of investigating possible associations between E. vermicularis and allergies, wheezing and atopic eczema. The numbers and characteristics of the invitees and the participants in this sub-study are given in Table 1. A flow diagram depicting the study participants of the MIDIA study and this sub-study is given in online Supplementary Figure S1. The children in the DQ8/2 group comprised 75% of the participants in the study, while the non-DQ8/2 genotype was carried by 25% of the participants. The compliance was similar by gender, and in the DQ8/2 and non-DQ8/2 groups. The children's age ranged from 2 to 11 years, with most being aged <6 years. The study was approved by The Regional Committee for Medical and Health Research Ethics South East A and the Norwegian Data Inspectorate, and written informed consent to participate in the MIDIA study was given by the parents.
Table 1. Characteristics of the invitees and the participants in the study
Data collection
The parents received sampling kits and instructions on how to collect scotch-tape anal samples from the children on three consecutive days. A sample was considered positive if one or more eggs were found on any of the three consecutive slides. The sample collection and slide examination has been described in greater detail elsewhere [Reference Bøås5]. Samples were collected from the whole of Norway in 2010 between January and August. The participants were also asked to complete a questionnaire, answering ‘yes/no’ or ‘don't know/remember’ about earlier treated episodes of E. vermicularis infection, present cases of eczema (atopic or other types), asthma, bronchiolitis and allergies (food, pollen or other allergies) or intolerances. A follow-up question inquired whether these conditions were confirmed by a physician, and whether the child received any medication for the condition. For unspecific conditions such as food allergy the questionnaires also contained a field where the condition or type of allergy could be described. Information given on other types of eczema and the descriptions was used for excluding cases that were not real cases of atopic eczema and allergy, blinded to information concerning pinworm infection status. Asthma and bronchiolitis proved difficult to differentiate based on the information given by the participants and these two illnesses were combined into one variable called wheezing. Questionnaires collected in the MIDIA study (see www.fhi.no/midia), were also used, giving information on age, gender and timing of the first symptoms of eczema, asthma/bronchiolitis or allergies. All data on eczema, asthma and allergies, and whether the reported condition was confirmed by a physician, were self-reported answers in the questionnaires. The questionnaires (in Norwegian) are available from http://www.fhi.no/studier/midia/informasjonsmateriell-og-skjemaer. The questionnaire was designed by H. Bøås, and revised by K. S. Rønningen. The questions were based on a questionnaire previously used by Herrström et al. [Reference Herrström18], and adapted to a similar structure as used in other MIDIA questionnaires in order that information would be compatible for comparison purposes. Efforts were made to exclude misclassification of conditions, based on the parents' description of the reported condition, and whether or not the condition was reported to have been confirmed by a physician. All questionnaires were controlled manually, and any unclear or ambiguous answers were recorded as missing.
Statistical analysis
The data were analysed by logistic regression. Pinworm infection status was used as a dependent variable and atopic eczema, wheezing, allergy, food allergy, pollen allergy and other allergies as independent variables. Generalized estimation equation was used to account for potential intra-family correlation, as 11·5% of the children participating were siblings. We adjusted for age, number of siblings in the household and HLA genotype, which were found to be possible factors affecting E. vermicularis infections in our earlier study [Reference Bøås5], by introducing these variables as independent variables in the logistic regression model. All statistical analyses were performed using Stata v. 11 (StataCorp, USA).
RESULTS
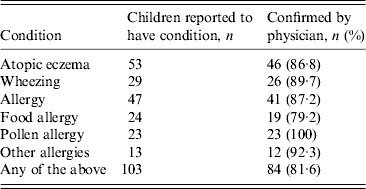
A total of 23·1% (84/364) of children who provided scotch-tape samples for E. vermicularis were reported to have a present case of any atopic eczema, allergy or wheezing, confirmed by a physician. Of these children, 21·4% (18/84) tested positive for pinworms. Of the children without atopic eczema, allergy or wheezing, 16·4% (46/280) tested positive for pinworms. Between 79% and 100% of the reported conditions were confirmed by a physician (Table 2).
Table 2. Percentage of atopic conditions confirmed by a physician
No significant results were found between current E. vermicularis infections and having any of atopic eczema, allergy or wheezing, confirmed by a physician (results not shown). To investigate if the association with pinworms and the different diseases and symptoms could have opposite effects and so mask the effect of each other, this main group was stratified by atopic eczema, wheezing and allergy. After analysing these variables against E. vermicularis positivity, only current allergy had a borderline significant association (P = 0·024) (Table 3). The proportion of positive samples was also higher in children with wheezing, but the difference was not significant (P = 0·069). Including children that did not have the reported condition confirmed by a physician resulted in essentially the same conclusion (Supplementary Table S1).
Table 3. Distribution and logistic regression of the main variables in the dataset

OR, Odds ratio; CI, confidence interval.
* Adjusted for age, number of siblings and HLA genotype (DQ8/2 vs. non-DQ8/2).
Since allergy is a very heterogeneous group of conditions, which could potentially conceal effects of specific allergies, this variable was divided into subgroups. However, due to the limited number of participants, it was only meaningful to group allergies into food, pollen or other allergies. Of the allergy variables both food and pollen allergies proved to be significant (Table 4, Supplementary Table S2). Only food allergy remained significant after adjusting for possible confounders (Table 4). However, when including children with a reported atopic eczema, allergy or wheezing without a physician's confirmation (Supplementary Table S2), the association was no longer significant.
Table 4. Distribution and logistic regression of the allergy variables in the dataset

OR, Odds ratio; CI, confidence interval.
* Adjusted for age, number of siblings and HLA genotype (DQ8/2 vs. non-DQ8/2).
Only 51 of the 398 children reported to have been treated for earlier pinworm infections. Of the children without a present case of atopic eczema, allergy or wheezing, 13·4% (38/283) reported earlier pinworm treatments, compared to 9·6% (10/104) of the children with atopic eczema, allergy or wheezing. Care was taken to ensure that the onset of atopic eczema, wheezing or allergy was reported as occurring after the first reported E. vermicularis treatment. No significant difference in reported current eczema, wheezing or allergy in these children compared to children without previous treatments for E. vermicularis was found (results not shown).
DISCUSSION
The purpose of our study was to investigate if any associations between E. vermicularis infections and allergies, wheezing and atopic eczema could be found in Norwegian children, as earlier studies had suggested the existence of a link between E. vermicularis infections and the development of these conditions [Reference Wördemann15, Reference Bahceciler16, Reference Herrström18]. The stratification of the HLA genotype using subjects from the MIDIA cohort in the study is thought to be of minor importance. Nonetheless, the HLA genotype is among the variables adjusted for in the analysis to make sure this did not introduce any unforeseen bias. The main strength of this study is that scotch-tape samples were collected on three consecutive days to address the present status of E. vermicularis infection while the reliance upon questionnaire data provided by the parents to assess atopic conditions is a limitation. There is a chance of misclassification of conditions like bronchiolitis as asthma or food intolerance as food allergy, due to the confusion of these conditions by the parents, or including other types of eczema than atopic eczema. To address this problem efforts were made to exclude cases of other types of eczema and intolerance (e.g. lactose intolerance), based on the parents’ description of the reported condition, and whether or not it was confirmed as made by a physician. Nevertheless, the association with food allergy must be interpreted with caution, as the total number of children reported to have a food-related allergy was quite low.
The percentage of children reporting wheezing is in line with earlier reports of the prevalence for asthma in Norway [Reference Lindbæk21, Reference Carlsen22], and the parents of all but two of the children with wheezing also reported use of anti-asthmatic drugs (data not shown). The parents' reported use of these medications has earlier been shown to be useful as a proxy for the presence of current asthma [Reference Furu23], and excluding children where the use of asthma medication was not reported, did not change the results significantly (results not shown). In this study we were unable to identify any significant association between E. vermicularis and wheezing or atopic eczema. This is in agreement with other studies including a meta-analysis from 2006 [Reference Leonardi-Bee24], which did not find any association between current E. vermicularis infections and asthma [Reference Wördemann15, Reference Bahceciler16, Reference Leonardi-Bee24] or atopy [Reference Wördemann15, Reference Bahceciler16].
Previously an association between current allergy and present E. vermicularis has been reported from Sweden. In the Swedish study the occurrence of E. vermicularis was similar in all children with any type of positive skin-prick test [Reference Herrström18]. This finding fits with our uncorrected results indicating that children with food and pollen allergies seemed to have significantly more E. vermicularis infections than children without these conditions. It is possible that the earlier reported associations from Sweden could stem from age or other confounding factors, as no such adjustments were performed in the Swedish study [Reference Herrström18]. There could also be real differences between countries, although the differences between Norway and Sweden are expected to be minor. After correcting for possible confounders in this study, only the association between food allergy and E. vermicularis remained significant. While there were more E. vermicularis-positive samples in children reported to have pollen allergy than in children without pollen allergy, this association did not reach statistical significance and should be studied in a larger study. The data do not allow for an explanation of the mechanisms or immunological pathways involved in the association between food allergy and E. vermicularis. It is possible that the association reflects other confounders not adjusted for in the study, e.g. socioeconomic status. Another possibility is that certain types of food allergies contribute to a more favourable environment in the gut for the pinworm to complete its life cycle, or symptoms of pinworm infections could possibly be mistaken for allergic symptoms. These results are in contrast to reports on Cuban and Turkish children [Reference Wördemann15, Reference Bahceciler16] that found no associations between allergies and E. vermicularis. However, these studies failed to differentiate between different types of allergies, and so would be more comparable to our main allergy variable, which gives similar results after correcting for known confounders.
We were unable to detect any association between earlier treatments of E. vermicularis and atopic eczema, asthma/bronchiolitis and allergies (results not shown). However, earlier infections with E. vermicularis are clearly heavily underreported as only 51 children were reported to ever have been infected and/or treated for this pinworm. Considering that 72/395 children tested positive for E. vermicularis [Reference Bøås5], and given the high prevalence, it is highly unlikely that the number of previous infections could be this low. It could be argued that only the most severe infections are detected, and that these are more likely to trigger an immune reaction, but even if this is the case, more severe infections do not appear to have any strong effects on development of these disorders. As the number of children is quite low we cannot exclude the possibility that small effects exists. However, the evidence for an effect of a history of E. vermicularis infections is weak; some studies found an association between E. vermicularis and asthma/wheezing [Reference Bahceciler16, Reference Huang, Tsai and Yeh17], allergy and atopy [Reference Wördemann15, Reference Huang, Tsai and Yeh17] and eczema/atopic dermatitis [Reference Schäfer14, Reference Wördemann15], but the results are often contradictory with both positive and negative effects being reported in different studies on the same condition. Moreover, other studies have failed to identify any associations or been inconclusive: asthma/wheezing [Reference Wördemann15, Reference Bager25], allergy and atopy [Reference Bahceciler16] and eczema/atopic dermatitis [Reference Huang, Tsai and Yeh17]. Of all the studies of E. vermicularis only one tried to establish a proper temporal sequence where worm infections occurred before the onset of eczema [Reference Schäfer14]. However, as in our study, the study relied upon the parents' reports of previous infections and timing of the infection [Reference Schäfer14]. Since most E. vermicularis infections go undetected [Reference Bøås5], the established temporal sequence seems highly uncertain.
Although the severity of the infection probably influences the likelihood of detection, it is more likely that the low number of earlier infections is due to E. vermicularis infections generally being asymptomatic [Reference Herrström4, Reference Bøås5]. With the real number of previous E. vermicularis infections in all likelihood being much higher than that reported by parents or detected from the history of previous treatments, a retrospective approach is unfortunately not well suited for investigating future effects on asthma, wheezing, allergies and eczema. Considering that E. vermicularis is still quite prevalent in Norway [Reference Bøås5] it is unlikely that the lack of this helminth is associated with the development of asthma, eczema and allergies. It should however be borne in mind that this study lacks longitudinal data on E. vermicularis infections, and the children's age at the time of first infection might be an important factor for development of a possible beneficial or adversary effect. Thus, it is difficult to assert an effect of E. vermicularis infections to the development of allergies, eczema and wheezing, although our study shows that strong effects are unlikely. Rather than collecting information on past infections, future studies should focus on a long-term screening approach to investigate these suggested effects of E. vermicularis infections. The association between current E. vermicularis infections and food allergy is interesting, and should be studied in a larger group.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268813003154.
ACKNOWLEDGEMENTS
The authors express their gratitude to the participants, and the MIDIA project at the Norwegian Institute of Public Health. Special thanks go to Lene Gustavsen for help with data collection, sample kits and questionnaires, and to Lars Christian Stene for useful help and discussion on the statistical analyses.
This work was supported by The Research Council of Norway (H.B., grant no.185610/V40), (K.S.R., grant no. 166515/V50). The study sponsor had no say in the study design; collection, analysis or interpretation of data; the writing of the paper or the decision to submit the paper for publication.
DECLARATION OF INTEREST
None.






