According to the definition of the FAO and the WHO, probiotics are ‘live bacteria that offer a health benefit to the host when administered in adequate amounts’(1). Measuring beneficial effects in healthy populations is, however, challenging. The WHO has defined health as ‘…a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity’(2). However, what we are able to measure and interpret as ‘health’ is a continuum from obvious dysfunction (disease) to optimal function (health) with a wide range of ‘normal’ values in between. Many currently available biomarkers are linked to disease or progression of disease, and are not appropriate for evaluating changes within the normal range in healthy populations(Reference van Ommen, Keijer and Heil3). Useful markers for evaluating effects in healthy subjects do exist but it can be difficult to interpret if a change is beneficial. This is due to the fact that there is a huge resilience in the human system and changes in these markers within the normal range tell most about the robustness of homeostasis. Additionally, the large inter- and intra-subject variation of many markers results in a very broad ‘healthy range’, and analytical variability contributes further to the difficulty of evaluating modest changes within the normal range. Therefore, it has been suggested to define health as the ability of the human body to adapt to changing environmental challenges such as stress or pathogens(Reference van Ommen, Keijer and Heil3). This may be a more workable definition when assessing health in ‘healthy’ individuals, for example by challenging homeostasis and evaluating the body's ability to mount an appropriate response or adaptation to the challenge.
The immune system possesses a great degree of redundancy such that an excess functional capacity of some component may compensate for a reduced functional capacity of another component. Furthermore, the system may have a certain amount of excess capacity(Reference Albers, Antoine and Bourdet-Sicard4). Therefore, assessing the status of immune markers might not provide much information about the ability of the immune system to deal with a challenge. This has been supported by a study in athletes which demonstrated that subjects with antibody levels in the lowest 10th percentile relative to clinical norms were still able to mount clinically appropriate antibody responses when immunised with a pneumococcal vaccine(Reference Gleeson, Pyne and McDonald5). A more useful measure is to assess the functional capacity of the immune system to deal with common pathogens. Clinical endpoints such as morbidity from common infections are ultimately most relevant but reflect the balance between immune defence and natural pathogen exposure, the latter being uncontrolled and unpredictable. To control antigenic exposure, it is possible to investigate the integrated in vivo immune response to an experimental challenge such as attenuated or killed pathogens. This provides valuable information on the ability of the immune system to respond to a ‘model infection’ in which the dose of pathogens as well as the modality and the timing of exposure are standardised, and is currently believed to be the best way to investigate the effect of nutritional interventions on immune function(Reference Albers, Antoine and Bourdet-Sicard4, Reference Cummings, Antoine and Azpiroz6). Evidence from in vitro, animal and ex vivo studies has suggested that probiotics may have immune-modulating properties(Reference Kekkonen, Lummela and Karjalainen7–Reference Lomax and Calder12). The possible ability of different probiotic strains to improve immune function in human subjects has been shown in studies employing vaccine challenges(Reference Boge, Remigy and Vaudaine13–Reference French and Penny16), as well as in studies with clinical endpoints such as common infections(Reference Hatakka, Savilahti and Ponka17–Reference de Vrese, Winkler and Rautenberg23). Because health benefits of probiotics are strain-dependent, the functional effects demonstrated for one probiotic strain cannot necessarily be extrapolated to other strains(1, Reference Rijkers, Bengmark and Enck24). As human data on the immune-modulating effect of Bifidobacterium animalis ssp. lactis (BB-12®) and Lactobacillus paracasei ssp. paracasei (L. casei 431®) are very sparse, it was considered important to further investigate the effects of these two probiotic strains in a controlled, adequately powered trial using the challenge of influenza vaccination.
The primary objective was to determine the effect of each of these two probiotic strains on the vaccine-specific antibody responses. As secondary objectives, adaptive and innate immune responses as well as influenza-like illness and safety measures were assessed.
Methods
Study design
The study was a randomised, double-blind, placebo-controlled, four-arm, parallel-group study in healthy adult volunteers. Study products were consumed daily for 6 weeks, 2 weeks before and 4 weeks after a challenge with a seasonal influenza vaccination. Immune responses were assessed at baseline, before starting supplementation with study products, and 4 weeks after vaccination. Information on influenza-like illness and infections was collected during the 6-week supplementation period. Finally, the subjects were contacted by phone 10 weeks after the end of supplementation for safety assessment.
Ethics and subjects
The study was performed in accordance with the principles in the Declaration of Helsinki and Good Clinical Practice. The ethical committee of the Luigi Sacco Hospital in Milan, Italy approved the study on 19 February 2009 (72/09/101/08/AP). The study is registered in the International Standard Randomised Controlled Trial Number Register (ISRCTN64739181). All individuals were informed about the study orally and in writing, and gave their written informed consent to participate. Subjects were recruited via posters in university and hospital buildings and flyers in diverse locations such as gyms and metro stations. The study took place at the Department of Infective Diseases at the Luigi Sacco Hospital in Milan between February and August 2009.
Potential subjects were screened for eligibility and randomised into four supplementation groups. Eligible subjects were healthy males and females aged 20–60 years old. Exclusion criteria were presence of acute/terminal disease, intolerance to milk protein or lactose, daily consumption of probiotic products 1 month before the start of the study, frequent gastrointestinal disorders, gastrointestinal surgery, and antibiotic treatment or treatment with any other drug known to affect the immune response during the trial. Furthermore, subjects were excluded if they had received any vaccination during the 15 d before the baseline visit, had already received the influenza vaccination for the season 2008–9, were participating in another research study, or had already suffered from influenza between September 2008 and the beginning of the study.
Subjects were instructed to refrain from eating fermented dairy products in addition to products containing probiotics from screening until the end of the study. The use of antibiotic treatment or any other drug treatment that, according to the investigators' discretion, could have an effect on the immune response was also not allowed during the study.
Interventions
The two probiotic strains were given in different forms, and to have a proper control for each strain, two placebo groups were included in the study. The four study groups consumed either a capsule containing the probiotic strain BB-12® (DSM15954), a placebo capsule, an acidified dairy drink (110 ml) containing the probiotic strain L. casei 431® (ATCC55544), or a placebo acidified dairy drink (110 ml). All study products were provided by Chr. Hansen A/S, Hørsholm, Denmark. The probiotic products contained a minimum of 1 × 109 colony-forming units/dose, and subjects consumed one drink or took one capsule once daily for 6 weeks. Placebo products were similar to the corresponding active product in appearance, smell and taste. The identity of the specific product (active or placebo) was blinded to subjects, investigators and support staff. Each product was labelled with a randomisation number and the randomisation list was kept confidential during the study.
Subjects received an intramuscular injection with 0·5 ml of the influenza vaccine specific for the viruses involved in the 2008–9 epidemic (Fluad®; Novartis Vaccines and Diagnostics, Siena, Italy) 2 weeks after starting supplementation. The strains that were present in the vaccine were A/Brisbane/59/2007 (H1N1)-like strain (A/Brisbane/59/2007, IVR-148), A/Brisbane/10/2007/(H3N2)-like strain (A/Uruguay/716/2007, NYMCX-175C) and B/Florida/4/2006-like strain (B/Florida/4/2006).
Endpoints
Primary efficacy variables were vaccine-specific plasma IgG and subclasses IgG1 and IgG3, and vaccine-specific salivary IgA, IgG and IgM.
Secondary variables were adaptive and innate immune responses assessed by total plasma IgA, IgM, IgG and subclasses IgG1 and IgG3; total salivary IgG, IgA and IgM; plasma concentrations of interferon-γ, IL-2 and IL-10; natural killer cell activity; CD4+T-lymphocytes and phagocytosis. All immune parameters were evaluated at baseline and 4 weeks after vaccination.
Influenza-like illness was rated by subjects in a diary, while infection status was evaluated at each study visit by a physician.
As safety variables, measurement of tetanus-specific IgG in plasma was included before and after the vaccination, and vital signs were measured at each study visit. Information on adverse events (AE) was collected from screening to the end of the study.
Laboratory methods
Blood samples were collected in EDTA tubes after an overnight fast at baseline and after 6 weeks of supplementation (day 42). After plasma collection, peripheral blood mononuclear cells were separated from the buffy coat on lymphocyte separation medium (Organon Teknica Corporation, Durham, NC, USA), washed twice in PBS (Organon Teknica) and centrifuged at 1500 rpm for 10 min. Working on ice, 1 ml of a freezing solution (80 % fetal bovine serum-supplemented Roswell Park Memorial Institute (RPMI) medium+20 % dimethyl sulfoxide) was added to the peripheral blood mononuclear cell pellet and the cells were resuspended. Finally, the cell suspension was transferred to 2 ml cryovials and frozen at − 80°C until use at a density of 10–15 × 106 viable peripheral blood mononuclear cells per vial (as determined by trypan blue exclusion). Saliva was collected by subjects spitting into a test-tube every 60 s for 5 min. All samples were immediately frozen at − 80°C until analysis. Samples were analysed in duplicate using commercially available kits or previously published methods as described below.
Vaccine-specific antibodies were analysed using Influenza A IgG/IgA/IgM ELISA test kits (IBL-America, Inc., Minneapolis, MN, USA). Vaccine-specific IgG1 and IgG3 were analysed using the IgG kit, with the modification that the specific antibodies were detected by a secondary horseradish peroxidase-conjugated antibody specific for human IgG1 or IgG3 (Alpha Diagnostic International, Inc., San Antonio, TX, USA).
Total antibodies in plasma were analysed using human IgG/IgA/IgM/IgG1/IgG3 ELISA Kits (Groundwork Biotechnology Diagnosticate, San Diego, CA, USA), salivary IgA using Human Secretory IgA SIgA ELISA Kit (USCNLIFE, Wuhan, China), total salivary IgG and IgM using the Quantitative Human IgG/IgM ELISA Kit (ZeptoMetrix Corporation, Buffalo, NY, USA), tetanus-specific IgG in plasma using Tetanus Antibody ELISA Kit (Wuhan Institute of Biologic Product, Wuhan, China) and circulating cytokines using Quantikine Human IL-2/IL-10/interferon-γ immunoassay (R&D Systems, Inc., Minneapolis, MN, USA).
Phagocytosis of Candida albicans blastospores by polymorphonuclear cells was performed as described previously by Saresella et al. (Reference Saresella, Roda and Speciale25). Briefly, CM2 strain blastospores were grown in Sabouraud broth and 2 % dextrose (Difco Laboratories, Detroit, MI, USA) at 37°C for 18–24 h, washed, resuspended, counted and checked for viability by trypan blue exclusion. C. albicans blastospores were then ethanol-fixed and labelled with fluorescein isothiocyanate (Sigma-Aldrich, St Louis, MO, USA). Human polymorphonuclear cells obtained from heparinised whole blood after lysis of erythrocytes by hypotonic shock were identified by labelling with the phycoerythrin-conjugated monoclonal antibody My-7 (My-7-RD1; Coulter Electronics, Miami Lakes, FL, USA); this antibody is directed against CD13 and selectively labels peripheral blood monocytes and polymorphonuclear granulocytes. The green and red fluorescence biparametric graph of the double-labelled blastospores was used to evaluate the percentage of killed fluorescein isothiocyanate-labelled C. albicans blastospores using an optimal phagocyte:blastospore cell ratio of 1:5. Analyses were performed using a Coulter EPICS XL Flow Cytometry (Coulter Electronics); multiparametric data were collected on 10 000 events and analysed using Coulter System II software (Coulter Electronics).
Natural killer cell activity was determined using K562 target cells at an effector:target ratio of 25:1 in a final volume of 200 μl and analysed by flow cytometry, and CD4+T-cells were analysed with flow cytometric analysis of normal human peripheral blood mononuclear cells using a monoclonal antibody for human CD4 (Phycoerythrin anti-human CD4; eBioscience, San Diego, CA, USA).
Subject diaries
Each week during the 6-week supplementation period, subjects recorded influenza symptoms in a diary containing a list of predefined symptoms. After 1 and 4 weeks of supplementation, subjects made a self-evaluation of influenza-like illness based on the presence of fever and at least one influenza symptom in the diary during the preceding week. Subjects also recorded any missing doses of the study product and AE experienced in the diary.
Sample size and randomisation
The sample size was determined based on group differences and standard deviations for vaccine-specific IgA in the study by Olivares et al. (Reference Olivares, Diaz-Ropero and Sierra14). To demonstrate a difference of similar magnitude with a power of 80 % and a significance level of 0·05, a sample size of at least forty-seven subjects per group would be required. To account for potential dropouts, it was planned to include fifty-five subjects in each group.
A Statistical Analysis Systems computer program was used to generate block randomisation assignment of the four supplementation groups stratified by age and sex and with a block size of six. Age was stratified as 20–39 years and 40–60 years. The randomisation list was generated by a statistician not involved in the study conduct. The clinical centre performed assignment of a randomisation number for each subject upon inclusion in the study chronologically within each of the age–sex strata.
Statistical analysis
Baseline characteristics were analysed with ANOVA or non-parametric tests. The main statistical analysis was the difference in change from baseline (day 42 − baseline) between the groups. Furthermore, difference between the groups at baseline and at day 42 and in mean fold increase (MFI) defined as (day 42 − day 0)/day 0 was analysed. Univariate ANOVA was used to identify study effects in models containing terms of intervention, sex, age and baseline. For the primary efficacy variables, non-parametric analyses (Mann–Whitney test) were performed as a sensitivity test, and all results of the non-parametric analyses were in line with the results from the parametric analyses.
Additionally, the number of individuals in each group with a substantial increase in vaccine-specific antibodies was calculated and the difference between the groups analysed with Fisher's exact test. Based on the literature, an increase in specific antibodies of at least 2-fold from baseline to day 42 was considered substantial, and defined as difference (day 42 − baseline) ≥ 2 × baseline(Reference Stepanova, Naykhin and Kolmskog26, Reference Kurstak27).
All randomised subjects with available data from day 42 were included in the intention-to-treat analyses (n 211). As a sensitivity analysis, the analyses on the primary variables were also performed on the per-protocol population (subjects with no major protocol deviations, n 196). As BMI was significantly different between the groups, a post hoc analysis was performed, with BMI as an additional covariate in the ANOVA models. None of these alternative analyses changed any of the conclusions.
The analyses of the primary endpoints, changes from baseline in the vaccine-specific antibody responses, were adjusted for multiple testing by the Holm–Bonferroni method(Reference Holm28). All statistical analyses were performed according to a written statistical analysis plan using the Statistical Analysis Systems package version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Comparisons were made for the BB-12® capsule v. placebo capsule and the L. casei 431® drink v. placebo drink.
Results
Subject disposition and compliance with study product
A total of 221 subjects were eligible to participate in the study and were randomised to one of the four intervention groups. Of these subjects, ten dropped out during the study, nine of these before receiving the influenza vaccine. The motivation was in all cases individual's own request to leave the study and none was due to AE (Fig. 1).

Fig. 1 Disposition of the subjects.
All subjects were Caucasian. The overall mean age was 33·2 (sd 13·1) years and ranged from 19 to 60 years. A total of 118 (56 %) females and 93 (44 %) males completed the study. Baseline characteristics for each study group are shown in Table 1.
Table 1 Demographic and baseline characteristics of the subjects (intention-to-treat population)
(Mean values, standard deviations, number of subjects and percentages)
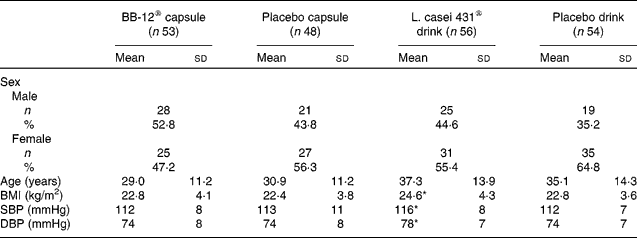
SBP, systolic blood pressure; DBP, diastolic blood pressure.
* Mean values were significantly different from those of the corresponding placebo drink group (P < 0·05; Kruskal–Wallis test).
Assessment of compliance was based on subjects' recordings of missing doses. The compliance with study product was high in all four groups: 99·1 % in the BB-12® capsule group; 99·6 % in the placebo capsule group; 98·2 % in the L. casei 431® drink group; 98·5 % in the placebo drink group.
Vaccine-specific antibody responses
The primary outcome parameters, changes from baseline in vaccine-specific IgG and subclasses IgG1 and IgG3, were significantly greater in each probiotic group compared with the corresponding placebo group (Fig. 2). Furthermore, a significantly greater MFI was shown in the BB-12® group v. placebo for vaccine-specific IgG (P = 0·016) and in both BB-12® and L. casei 431® groups v. the corresponding placebo group for vaccine-specific IgG1 and IgG3 (P < 0·001 for all comparisons).
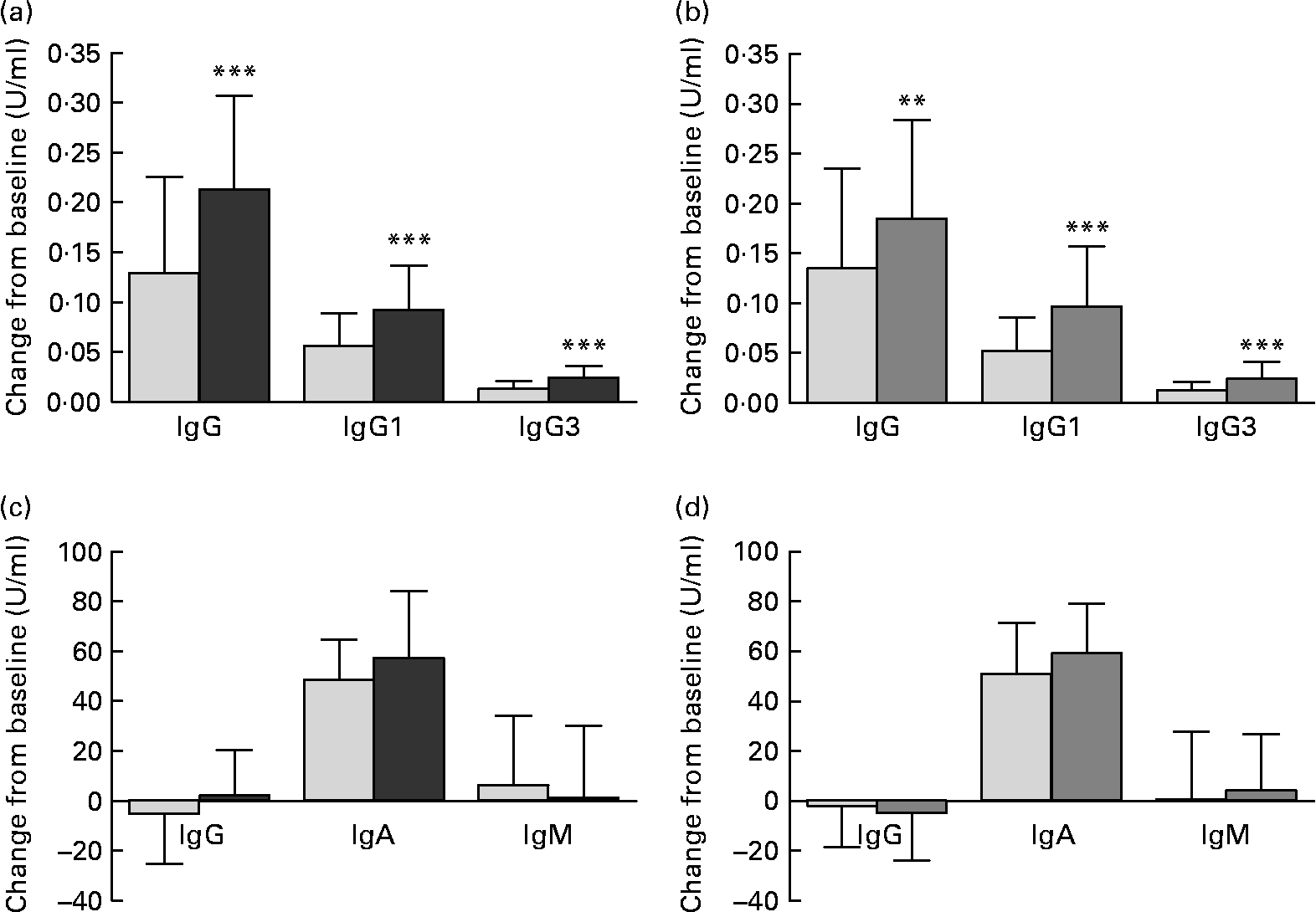
Fig. 2 Change from baseline (U/ml) in (a, b) vaccine-specific plasma IgG, IgG1 and IgG3, and (c, d) vaccine-specific salivary IgG, IgA and IgM for (a, c) the Bifidobacterium animalis ssp. lactis (BB-12®, ![]() ) capsule group and the corresponding placebo (
) capsule group and the corresponding placebo (![]() ) group and (b, d) the Lactobacillus paracasei ssp. paracasei (L. casei 431®,
) group and (b, d) the Lactobacillus paracasei ssp. paracasei (L. casei 431®, ![]() ) group and the corresponding placebo (
) group and the corresponding placebo (![]() ) group consuming the study product for 2 weeks before and 4 weeks after an influenza vaccination. Values are means for the intention-to-treat population, with standard deviations represented by vertical bars. ** Mean values were significantly different from those of the placebo group (ANOVA with age, sex and baseline value as covariates; P = 0·01). *** Mean values were significantly different from those of the placebo group (ANOVA with age, sex and baseline value as covariates; P < 0·001).
) group consuming the study product for 2 weeks before and 4 weeks after an influenza vaccination. Values are means for the intention-to-treat population, with standard deviations represented by vertical bars. ** Mean values were significantly different from those of the placebo group (ANOVA with age, sex and baseline value as covariates; P = 0·01). *** Mean values were significantly different from those of the placebo group (ANOVA with age, sex and baseline value as covariates; P < 0·001).
Importantly, the number of subjects in whom a substantial increase in plasma vaccine-specific antibodies was observed was significantly greater in each probiotic group than in the placebo groups (Table 2).
Table 2 Number of individuals (intention-to-treat population) with a substantial increase in vaccine-specific plasma IgG, IgG1 and IgG3 4 weeks after an influenza vaccination for the Bifidobacterium animalis ssp. lactis (BB-12®) capsule group and the corresponding placebo group and the Lactobacillus paracasei ssp. paracasei (L. casei 431®) group and the corresponding placebo group†
(Absolute number of subjects and percentages)
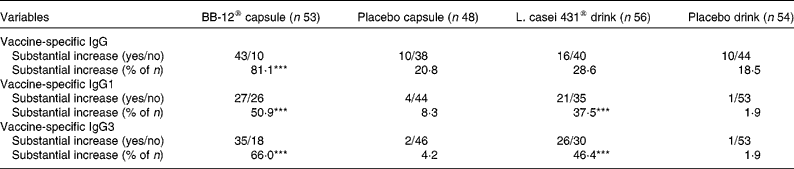
*** Values were significantly different from those of the corresponding placebo group (P < 0·001; Fisher's exact test).
† Study products were consumed for 2 weeks before and 4 weeks after an influenza vaccination. A 2-fold or more increase in specific antibodies was defined as a substantial increase (increase (day 42 − baseline) ≥ 2 × baseline).
For vaccine-specific salivary IgA, a significantly greater MFI was observed in both BB-12® (P = 0·017) and L. casei 431® groups (P = 0·035) v. their corresponding placebo group, while no significant changes from baseline were found (Fig. 2). No differences were found for salivary vaccine-specific IgG or IgM.
Total antibody responses
Significantly greater changes from baseline and MFI in total plasma IgG, IgG1 and IgG3 were observed in both L. casei 431® and BB-12® groups v. the relevant placebo group (P < 0·001 for all comparisons). No relevant differences were seen in total plasma IgA and IgM.
For total salivary IgG, both L. casei 431® and BB-12® groups showed significantly greater changes from baseline (P < 0·001) and higher MFI (P = 0·006 and P = 0·001, respectively) v. their corresponding placebo group. Furthermore, a significantly greater change from baseline and a greater MFI was observed in salivary IgA in the BB-12® group v. the corresponding placebo group (P = 0·046 and P = 0·005, respectively).
Cytokines and cellular immune responses
No significant differences were detected in the plasma concentration of interferon-γ, IL-2 and IL-10, or in natural killer cell activity, number of CD4+T-lymphocytes and phagocytosis (data not shown).
Influenza-like illness and infections
The incidence of influenza-like illness was very low in all groups. Influenza-like illness was reported for one subject (2 %) in the L. casei 431® group at week 1, and for three subjects (6 %) in the placebo drink group and two subjects (4 %) in the placebo capsule group at week 4. No infections were diagnosed during the study in any of the intervention groups.
Safety
Tetanus-specific IgG were not modified in any of the intervention groups from baseline to day 42, and no difference between the groups was observed, demonstrating that supplementation of L. casei 431® and BB-12® is not associated with non-specific stimulation of the immune system. Vital signs raised no safety concerns. In forty-nine subjects, ninety-eight AE were assessed as related to the study products; the pattern and incidence of AE were similar between the groups. The most prevalent of the related AE were high fever (26 % of events), rhinitis (13 % of events) and severe malaise (12 % of events). No AE led to discontinuation and no serious AE occurred during the study.
Discussion
These data suggest that dietary supplementation with BB-12® or L. casei 431® leads to an increased adaptive immune response to vaccination. This is in line with results on other probiotic strains tested in healthy subjects. In a recent study in healthy adults investigating the effect of Lactobacillus fermentum strain VRI 003 (PCC®) on the response to an influenza vaccine, a significantly enhanced haemagglutination-inhibition titre to the H1N1 antigen has been shown(Reference French and Penny16). Additionally, a lower percentage of non-seroconverters (defined as subjects with an increase in haemagglutination-inhibition titre of less than 4-fold post-vaccination) was found in the probiotic group compared with placebo. In a similar study in healthy adults, supplementation with a capsule containing L. fermentum CECT5716 resulted in increased concentrations of influenza-specific IgA and total IgM compared with placebo(Reference Olivares, Diaz-Ropero and Sierra14). Finally, a study investigating the effect of either of the strains L. casei 431® or Lactobacillus rhamnosus, LGG® on a booster polio vaccine has shown that the poliovirus-specific antibody response was increased in the probiotic groups when compared with placebo(Reference de Vrese, Rautenberg and Laue15). Similar results were also obtained in elderly subjects (mean age 85 years) consuming a fermented dairy drink (‘Actimel’) containing the probiotic strain Lactobacillus casei DN-114 001 in addition to common yogurt cultures(Reference Boge, Remigy and Vaudaine13). Results showed that influenza-specific antibody titres towards the B-strain of the vaccine were increased and seroconversion towards the B-strain of the vaccine was more frequent in the group consuming the fermented dairy drink compared with the control group consuming an acidified milk-based placebo drink.
The present study was performed in adults aged 20–60 years, while the study by Boge et al. (Reference Boge, Remigy and Vaudaine13) cited above enrolled subjects older than 70 years. The similarity of the findings suggests that certain probiotic strains are able to improve immune responses to a challenge in adults across a wide age range. Similar effects are expected in children, although further studies are needed to confirm this. It has, however, been demonstrated that supplementation with probiotics in children can reduce incidence and duration of upper-respiratory-tract infections which may be due to an effect on the immune system(Reference Hojsak, Snovak and Abdovic21).
In a healthy adult population aged < 65 years, it is expected that there is an optimal response to vaccination, with 70–90 % of vaccinated subjects being protected. Against this background, it could be difficult to demonstrate an increased protection with probiotic supplementation. However, in the present study, the specific antibody response to vaccination in the placebo groups was surprisingly low, and clear differences in response to the vaccination were shown between the probiotic and placebo groups. As we did not use the standard measures of vaccine efficacy in the present study, it could be that the low response observed in the placebo group is enough to confer protection as measured by the standard methods used in vaccinology.
Commonly, haemagglutination-inhibition titres are used to assess vaccine immunogenicity, with a haemagglutination-inhibition titre of ≥ 40 generally considered to be associated with at least a 50 % reduction in the risk of infection within a population. We chose, however, to use ELISA assays to measure the specific antibody responses because (1) these assays allow specific Ig classes to be measured and (2) the main purpose of the study was to evaluate the immune-modulating effect of the probiotics in a model system, and not specifically to assess whether it would confer a greater protection of the vaccine. However, although part of the antibodies detected by the ELISA assays may not be protective, the difference observed in specific antibody responses in the present study will most probably translate into a clinical benefit such as reduced incidence or duration of infection. A future study demonstrating an effect on protective antibody titres after vaccination could confirm that the effects on specific antibody levels seen in the present study will also result in a clinical benefit.
In the present study, supplementation with the probiotic strains BB-12® or L. casei 431® enhanced both mucosal and systemic antibody responses to the vaccine compared with a matching placebo. This is an important finding because protection against infection with pathogens that penetrate through the mucosa, such as the influenza virus, requires responses from both the mucosal and the systemic part of the adaptive immune system(Reference Kutzler, Kraynyak and Nagle29). Secretory IgA is currently the best way to measure the mucosal immune response, which is especially relevant in relation to an airborne virus infection such as influenza. An association between low levels of total secretory IgA and clinical endpoints (increased susceptibility to upper-respiratory-tract infections) has been demonstrated in athletes(Reference Gleeson30, Reference Neville, Gleeson and Folland31).
There was no clear effect on clinical parameters in the present study. However, as the study was conducted outside the main influenza season, resulting in a very low incidence of influenza-like illness in all study groups, there were not enough data to evaluate these parameters. Another study has demonstrated a lower incidence of influenza-like illness after 5 months in addition to an increased response to an influenza vaccination in the probiotic group compared with placebo(Reference Olivares, Diaz-Ropero and Sierra14). Other studies have either shown a reduced incidence of infections(Reference Leyer, Li and Mubasher20, Reference Hojsak, Snovak and Abdovic21, Reference Berggren, Lazou Ahren and Larsson32, Reference Merenstein, Murphy and Fokar33) or a reduced duration and/or severity of infections(Reference Hatakka, Savilahti and Ponka17, Reference Guillemard, Tondu and Lacoin18, Reference Cox, Pyne and Saunders22, Reference de Vrese, Winkler and Rautenberg23) after consumption of probiotics, while some studies have failed to show an effect on the incidence of infections due to an unexpectedly low infection rate during the season when the study was conducted(Reference Guillemard, Tondu and Lacoin18, Reference Merenstein, Smith and Scriven34).
The present study was designed to assess the response to the controlled exposure to vaccination which overcomes the uncontrolled conditions in studies of clinical endpoints. Exposing the immune system to a fixed quantity of antigen allows control of all variables associated with antigenic exposure, the only possible variation being the ability of the immune system of the individual to respond to the antigenic challenge. This allows for an assessment of the integrated immune response to a ‘model infection’ that may predict the immune response when the subject is exposed to wild-type pathogens(Reference Albers, Antoine and Bourdet-Sicard4). The exact clinical benefit of these probiotic strains needs, however, to be investigated in separate studies.
Effects on innate immune parameters were not seen in the present study, which is somewhat surprising. An effect of various probiotic strains on different innate immune markers has been shown in other clinical studies(Reference Schiffrin, Rochat and Link-Amster9, Reference Olivares, Diaz-Ropero and Sierra14, Reference Cox, Pyne and Saunders22, Reference Rautava, Arvilommi and Isolauri35). However, a typical viral challenge of the immune system stimulates a cascade of immunological functions, with an increase in cytokines, natural killer activity and phagocytosis during the first days of infection which are replaced gradually with viral antigen-specific T-cell responses(Reference Calder36, Reference Burleson and Burleson37). It is therefore possible that effects of the probiotics on these responses were not seen because the timing of measurement of these responses was well beyond their peak timing. A future study could include more frequent blood sampling to investigate the potential effects on these earlier immune responses further.
The fact that both IgG1 and IgG3 were significantly increased after the probiotic supplementation in the present study suggests that the activities of both T-helper (Th)1 and Th2 lymphocytes are promoted, as IgG1 and IgG3 are considered to be more indicative of Th2 and Th1 functionality, respectively. Notably, besides being preferentially correlated with Th1 and Th2 T-cell subsets, IgG1 and IgG3 are also associated with the optimal activation of complement and phagocytosis by macrophages, respectively(Reference Kalia, Sarkar and Gourley38). Because complement and phagocytosis work synergistically to eliminate pathogens, the fact that supplementation with these probiotics enhances production of both types of antibody subclasses further supports the potential beneficial effects of BB-12® and L. casei 431®.
The observation that all the subclasses of antibodies – with the exception of IgM – were augmented indicates that the immune response enhanced by supplementation with these probiotics is a secondary response, which is the type of immune response expected upon an influenza vaccination where most subjects have previously encountered the antigen(Reference Cox, Brokstad and Ogra39). This confirms that the dietary supplementation stimulates antigen-specific responses directed towards the antigen to which the immune system has been exposed. Influenza-specific memory B-cells alone were restimulated by the vaccine; the effect of the restimulation on these cells was specifically strengthened by the probiotics. Additionally, analysis of tetanus-specific IgG concentrations was included as a safety parameter, which confirmed that supplementation with BB-12® and L. casei 431® only elicited antigen-specific responses without resulting in unspecific, generalised immune activation.
The mechanism by which the probiotic strains used in the present study act to improve some aspects of the host's immune system remains unclear. The improvement in anti-vaccine antibody response did not seem to correlate with significant changes in the other immune markers measured here. However, as mentioned above, the timing of measurement of these other immune markers may not have been optimal. Even so, it is not readily apparent how changes in the gut microbiota have an impact on the host's systemic immune response. This can be explained by two possible mechanisms, both involving an interaction with immune cells within the host's gut-associated lymphoid tissue(Reference Thomas and Versalovic40). First, changes in the microbiota could result in an altered concentration of signalling molecules, such as a SCFA or a peptide, within the gut lumen that directly affect the activity of the host's immune cells. Second, a direct contact could be made between the host's immune cells and the gut bacteria, and this interaction could alter the host's immune cell activity. Whichever mechanism is involved, the modifications in the activity of the host's immune cell or cells within the wall of the gastrointestinal tract must then be transferred systemically. This is possible because of recirculation of immune cells between body compartments, including the gut-associated lymphoid tissue, blood and lymph. Since increased anti-vaccine antibody concentrations reflect the B-cell output of Ig, any cell type involved in the immune reactions that lead up to that point could be influenced by the probiotics. Thus, potential target cells are dendritic cells and other antigen-presenting cells, T-cells and B-cells. Further studies will be needed to better define the mechanisms by which probiotics affect the host's immune response.
Conclusion
Results of the present study show that the consumption of either of the probiotic strains BB-12® or L. casei 431® significantly increases antigen-specific immune responses in healthy individuals receiving an influenza vaccination. The elicitation and strengthening of multiple and complementary effector mechanisms demonstrated in the present study are considered to be associated with optimal protection against mucosally transmitted pathogens, such as the influenza virus. The data also confirmed that dietary supplementation with these two probiotic strains results in the elicitation of antigen-specific responses alone and not in a potentially harmful generalised immune activation, as shown by the lack of effect on the plasma titres of tetanus-specific antibodies. Dietary supplementation with BB-12® or L. casei 431® may thus be a safe and effective means to improve immune function by augmenting the response to challenges.
Acknowledgements
This clinical study was sponsored by Chr. Hansen A/S. M. C., P. C. C. and D. E. contributed to the design of the study. G. R. and A. C. were responsible for subjects' visits and data collection. All authors contributed to the interpretation of the study results and provided input to the manuscript. Conflicts of interest: L. J. and D. E. are employed at Chr. Hansen A/S. M. C. and P. C. C. perform consultancy work for Chr. Hansen A/S. We thank Maria Vittoria Cossu, Department of Infectious Diseases, Luigi Sacco Hospital who conducted all subjects' visits and study documentation, Professor Emilio Clementi, Chair of Pharmacology, University of Milano and Unit of Clinical Pharmacology, Luigi Sacco Hospital, and Francesca Mazza, MSc, Unit of Clinical Pharmacology, Luigi Sacco Hospital who handled the preparation of the blood and saliva samples. Sprim Italy handled the operational conduct of the study. All laboratory analyses were done at DaAn Central Laboratories in Shanghai, China. Signifikans A/S, Vedbæk, Denmark performed the statistical analyses.






