Dengue fever is an acute human viral infection caused by a flavivirus [dengue virus (DV)], and it is characterized by fever, severe headache, orbital pain, and general malaise. Dengue haemorrhagic fever (DHF) is a severe expression of the disease, characterized by haemorrhages and an increase in vascular permeability which frequently leads to death. Dengue is an important health problem in most tropical and subtropical countries [Reference Gulber and Kuno1]. In Mexico during 2005–2006, a total of 46 853 laboratory-confirmed cases were reported, 9430 of which were DHF [2].
Primate species have been found to have clinically unapparent DV infections. Besides primates that are expensive to obtain and protected by animal welfare organizations, suckling mice are currently used as an experimental model for dengue infections. Nevertheless, mice susceptibility depends on age and virus adaptation [Reference Gulber and Kuno1]. The lack of an economical and available laboratory animal model, among other factors, may have hampered the successful development of an effective vaccine.
The order Chiroptera is known as being the origin of most of the members of the Lyssavirus genus, including the classical rabies virus. Members of the order Chiroptera have been described as natural hosts of emergent viruses affecting humans, e.g. the Hendra and Nipah paramyxoviruses, Ebola virus, and SARS (severe acute respiratory syndrome) coronavirus [Reference Calisher3].
Bats represent the second most abundant and diverse mammal order in Mexico, with at least 138 species [Reference Romero Almaraz, Aguilar Setién and Sánchez Hernández4]. In a previous study, the prevalence of antibodies against rabies and La Piedad Michoacán paramyxovirus (LPMV) in several species of non-haematophagous bats in Mexico was studied [Reference Salas Rojas5]. The susceptibility of vampire bats (Desmodus rotundus) to rabies virus and their immunization against this disease has been studied using animals maintained in captivity [Reference Aguilar Setién6], demonstrating that they are effective models for the research of viral diseases.
It is possible that some bat species are susceptible to DV. In 1998, Zhang et al. [Reference Zhang, Yang and Li7] detected DV by reverse transcriptase–polymerase chain reaction (RT–PCR) in bats captured in Hainan, China. Later, Platt et al. [Reference Platt8] reported antibodies against DV in bats captured in Costa Rica and Ecuador. Studies on the susceptibility of bats to DV are scarce and incomplete. In populated areas, DV circulates among humans by Aedes mosquito vector transmission with no other known mammal reservoir. Many species of bats cohabit with humans in populated areas; however, their role as probable reservoirs remains hypothetical.
Between 2005 and 2006, 2339 human cases of DV infection were reported in Colima and Jalisco on the central Pacific coast of Mexico, and 7272 human cases in Veracruz on the Gulf coast of Mexico [2]. The strong DV activity in these areas led us to investigate bats for the presence of DV.
The study was performed in five localities on the Pacific coast, in Colima (18–19 July 2005) and Jalisco (21–23 July 2005); and in seven localities on the Gulf of Mexico's coast, in Veracruz (30 January to 3 February 2006, and 7–10 August 2006) (Fig. 1).
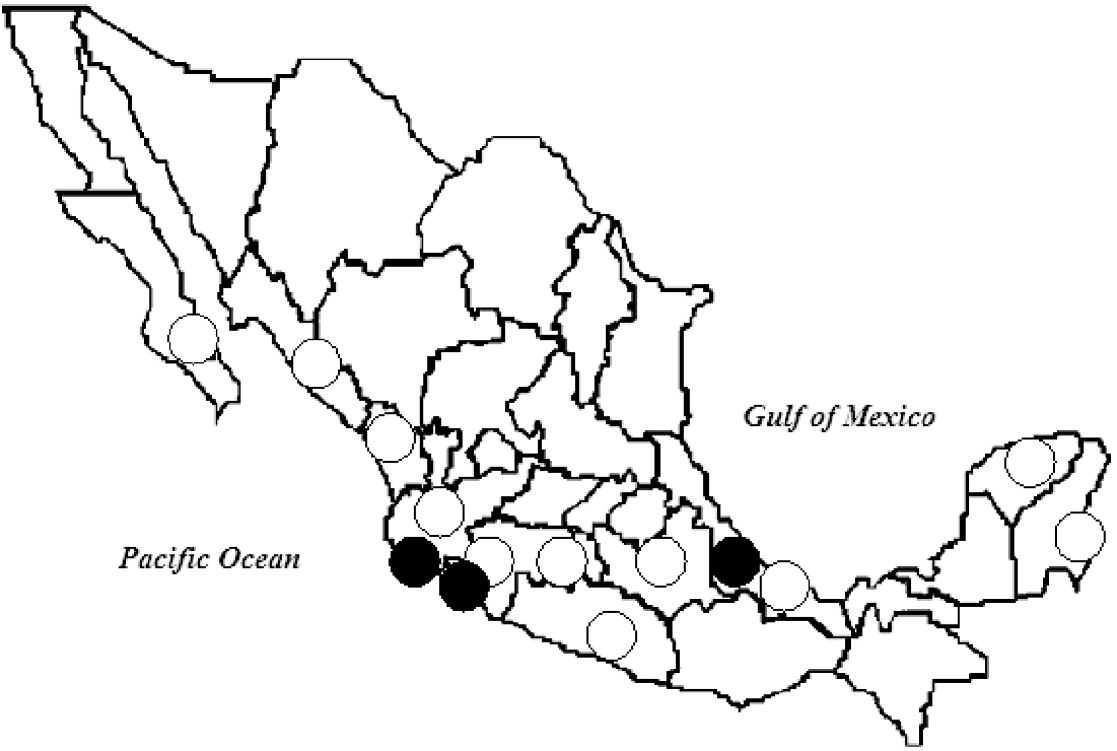
Fig. 1. Sites of important dengue virus outbreaks (○) and bat sampling (•), 2005–2006. (Source: Secretaría de Salud, Mexico (Health Ministry) [Reference Salas Rojas5].)
Between 2005 and 2006, municipalities close to the capture areas (in a perimeter ranging from 1 km to 80 km) reported rates of DV infection in humans between 1097 and 253/100 000 population in Jalisco; between 392 and 120/100 000 in Colima, and between 904 and 95/100 000 in Veracruz, showing a strong DV activity in the study period [2] (Fig. 1).
Bats were collected at night with mist nets, 2·6 m high and 12 m long (38-mm mesh). During the day, we searched for roosts in houses, sheds, and culverts under roads.
All captured specimens were collected following the recommendations of the Animal Care and Use Committee. In total, 149 trapped animals were sacrificed, and 31 animals were released after samples were taken. We preserved voucher specimens using standard procedures. For most captured specimens, we gathered information of their natural history and reproductive condition. The skins, skulls and/or skeletons of the specimens were preserved for taxonomic studies. Blood, brain, liver, heart, tongue, and kidneys from some specimens were kept for virological study. Blood samples for serological study were obtained from the marginal vein of the wing or by cardiac puncture [Reference Salas Rojas5, Reference Aguilar Setién6]. The collected specimens were deposited at the National Mammals Collection of Mexico (CNMA) at the Instituto de Biología of the Universidad Nacional Autónoma de México. The serum samples, tissues, and brains were stored at the Coordinación de Investigación en Salud, Unidad de Investigación Médica en Inmunología, Instituto Mexicano del Seguro Social under refrigeration at −70° C. It is important to note that not all specimens and samples were submitted to all the tests. Due to their small size, some species did not render a sufficient amount of serum nor tissue for all tests. From the released specimens only blood samples were taken. Considering the high variability in size of the specimens collected, the volume of blood drawn from the bats differed according to genus. For instance: <0·1 ml in genus like Myotis and Pteronotus (weighing 4–6 g) and 2 ml for Artibeus (weighing 80–120 g).
The ELISA test was performed using commercial plates (Nunc MaxiSorp®, cat. no. 473768, Roskilde, Denmark) sensitized with four DV serotypes (Den-1, Hawaii strain; Den-2, NGC strain; Den-3, H-87 strain, and Den-4, H241 strain) from our virus collection, replicated in cell cultures and purified by sucrose gradients according to standardized procedures. Serum samples (diluted 1:20 in PBS) were added and incubated for 2 h at 37°C. After washing, protein A from Staphylococcus aureus labelled with peroxidase (Protein A-Peroxidase, cat. P8651; Sigma, St Louis MO, USA) and diluted 1:2000, was used for binding to bat immunoglobulin. After adding o-phenylenediamine as a substrate, the developed colour was read at 490 nm. Assays were performed by duplicate and repeated at least twice. The cut-off value was considered at an optical density of 0·200 based on a pool of human sera negative for antibodies against DV. Taking in consideration that this was a preliminary study, we did not know whether the virus would affect the studied Chiroptera, therefore it was impossible to have negative or positive Chiroptera reference sera prior to this study. Using human sera the ELISA has 75% specificity and a sensitivity of 93%.
To compare the binding capacity of protein A to bat and human immunoglobulins (Igs), a constant amount of vampire bat (D. rotundus) Igs (50 μg/well) obtained from animals maintained in captivity and the same amount of human Igs were used to sensitize ELISA plates; logarithmic (base 2) dilutions of protein A peroxidase conjugate were added. The final results were compared and correlated (r value).
Viral RNA was extracted from bat heart using TRIzol® reagent (Invitrogen Life Technologies, Gaithersburg, MD, USA) according to the manufacturer's instructions. Total RNA was used for RT reaction and performed with M-MLV reverse transcriptase and random primers to obtain cDNA. The PCR reaction was performed as described by Lanciotti et al. [Reference Lanciotti9]. Briefly, we used primers that amplify a conserved region (511 bp) of the DV genome, which presents different sequences among serotypes 1, 2, 3 and 4. Then, a nested PCR was performed using specific primers for each serotype [Reference Lanciotti9]. Positive PCR controls were plasmids containing the insert of a 511-bp fragment of each serotype and C6/36 cells infected with DV. C6/36 non-infected cells and heart tissue from BALB/C laboratory mice were used as negative controls.
It has been demonstrated that the dengue virus non-structural 1 protein (DV NS1) is produced during the acute phase of dengue infection and correlates with virus replication [Reference Alcon10]. After obtaining DV-positive bats by RT–PCR, we wanted to find out if this protein could be detected in bat sera. The detection of DV NS1 protein was performed using the commercial kit PlateliaTM Dengue NS1 AG (Bio-Rad, France). This test uses a capture sandwich-like ELISA system. If NS1 antigen is present an immune-complex monoclonal antibody MAb-NS1-MAb/peroxidase will be formed. The presence of NS1 protein is determined by comparing the optical density reading of the sample to the optical density of the cut-off control serum. According to the manufacturer, this kit is specific for detecting DV NS1 protein and does not cross-react with other flaviviruses. This test detects NS1 not only in serum and plasma, but also in any medium in which NS1 is dissolved. We confirmed that using this test the NS1 protein can be detected in DV-infected cell line supernatants from Aedes aegypti (C636). This test has 100% specificity and a sensitivity of 91%.
We examined 162 bat samples representing five families: Emballonuridae, Mormoopidae, Phyllostomidae, Natalidae and Vespertilionidae, 12 genera, and 19 species. From 19 captured species, eight species were frugivorous, seven insectivorous, three nectarivorous, and one hematophagous. In Colima and Jalisco, collection occurred during the wet season, while in Veracruz it took place during both dry and wet seasons.
The assays performed to test the affinity of protein A to bat Igs showed a good correlation (r=0·921), although binding ratio was on average 25% less intense when compared to human Igs.
Nine individuals from four species were seropositive by ELISA: three insectivores, Myotis nigricans (four positives/12 examined) from Veracruz, Pteronotus parnellii (3/19) from Jalisco, and Natalus stramineus (1/4) from Colima, and one frugivore Artibeus jamaicensis (1/35) from Jalisco (12·86% seroprevalence in positive species) (Table).
Table 1. Synopsis of bat species testing positive for antibodies against DV, DV NS1 protein, and DV type 2 by RT–PCR, and site of capture
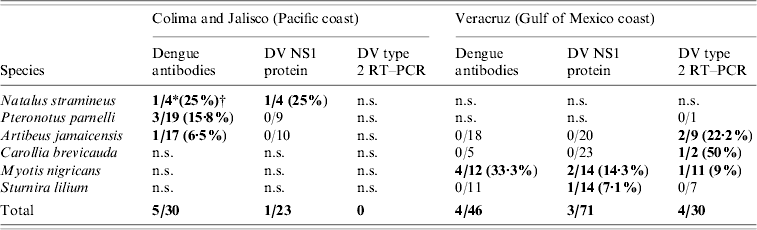
DV, Dengue virus; RT–PCR, reverse transcriptase–polymerase chain reaction; n.s., not sampled.
Bold values indicate positive results.
* Number of positive individuals/tested individuals.
† Positive percentage.
In Veracruz only M. nigricans was found positive. These positive Myotis bats came from the same roost.
Forty heart tissue samples were processed and analysed by RT–PCR for detection of DV serotypes 1, 2, 3 and 4 [Reference Lanciotti9]. All these samples were from the last batch collected in Veracruz (7–10 August 2006 – rainy season). Only DV serotype 2 was detected by RT–PCR in heart tissue from four bats belonging to three different species: one insectivore, M. nigricans (one positive/11 examined, 9%), and two frugivores, A. jamaicensis (2/9, 22·2%) and Carollia brevicauda (1/2, 50%) (Table). All RT–PCR positive animals were tested by ELISA to detect antibodies against DV, obtaining only negative results.
In total, 162 sera were processed for DV NS1 protein detection, with only four resulting positive. These belonged to three species: three insectivorous M. nigricans (two positive/14 examined, 14·3%), and N. stramineus (1/4, 25%), and one frugivore Sturnira lilium (1/14, 7·1%) (Table 1), M. nigricans and S. lilium were captured in Veracruz, and N. stramineus was captured in Jalisco. All DV NS1-positive animals were tested by ELISA in order to detect antibodies against DV and by RT–PCR to detect the virus. One M. nigricans proved positive to DV serotype 2 by RT–PCR and showed no antibodies against DV by ELISA. The other DV NS1-positive sample from M. nigricans resulted in a positive ELISA (low titre), but negative by RT–PCR. The NS1-positive N. stramineus sample gave a positive result when tested by ELISA (low titre), but negative to RT–PCR. NS-1-positive S. lilium showed negative results for both tests.
Dengue, the most important arthropod-borne viral human disease worldwide, has the Aedes mosquito as a vector and non-human primates as amplifying reservoirs. Although published reports are rare, there is reported evidence that bats could be naturally infected with DV [Reference Zhang, Yang and Li7, Reference Platt8]. In the present study, we report evidence that might support the presence of DV in bats from the Pacific and Gulf Coasts of Mexico, where dengue fever is endemic and an abundance of human cases were reported during 2005–2006 [2]. DV was detected by RT–PCR in bats from the Gulf Coast (Veracruz), captured during the rainy season. In addition to this, the detection of DV NS1 protein in a RT–PCR-positive bat suggests an active infection.
Anti-DV antibodies were detected by ELISA at 12·86% seroprevalence in positive species: A. jamaicensis, N. stramineus, M. nigricans, and P. parnellii (Table 1). Since this preliminary study was not directed to a particular genus, seroprevalence with 95% confidence intervals was not evaluated. Testing for the presence of neutralizing antibodies by the plaque reduction neutralization test (PRNT) was not performed in the present study due to the limited amount of serum obtained from most individuals.
Serotype 2 DV was detected by specific DV RT–PCR in tissues from four bats representing three species: M. nigricans, A. jamaicensis, and C. brevicauda (Table 1). Two out of these three genera: Artibeus and Carollia, were previously reported with antibodies against DV in 2001 [Reference Platt8].
In China's Hainan Island, where dengue is endemic, the presence of the DV genome was detected in 20/35 tested bats. DV was also reported in A. aegypti mosquitoes from that same region, while similar examinations of bat samples and mosquitoes from non-endemic areas were all negative [Reference Zhang, Yang and Li7].
M. nigricans, N. stramineus, and S. lilium all resulted positive to DV NS1 protein (Table 1). M. nigricans individuals were also positive to anti-DV antibodies and to DV type 2 by RT–PCR. The only N. stramineus individual positive to DV NS1 protein was simultaneously positive to anti-DV antibodies, although in low titres. Detection of anti-DV antibodies is commonly used for routine diagnostic testing of human samples; however, antibodies appear after the onset of clinical symptoms. On the other hand, it has been demonstrated that DV NS1 protein appears early (from the first day and up to 9 days after the onset of fever) and denotes an active infection [Reference Alcon10]. All individuals testing positive to DV NS1 protein in this work had no anti-DV antibodies or low titres. Similarly, those bats positive to DV type 2 by RT–PCR did not present anti-DV antibodies at all. The evidence of a probable DV infection in positive RT–PCR and DV NS1 protein bats is logically supported by their negative or low antibody titres, a common feature in prime infections.
Only 14 M. nigricans bats were captured, four of which tested positive for the presence of anti-DV antibodies, one other individual was positive to DV NS1 protein, and one other simultaneously presented DV type 2 by RT–PCR and DV NS1 protein, showing positive individuals for the three tests that were used in the present study (Table 1). This is the first time that the Myotis genus is reported as positive for DV, showing the highest percentage of positive individuals when all the applied tests are taken into consideration (Table 1).
It is important to point out that indirect evidence of DV presence in bats described in this study does not confirm an active infection and/or circulation of this virus in the captured individuals. Virus isolation from bats and controlled laboratory infections are necessary to justify the conclusion that wild bats are susceptible.
All geographic areas where evidence of DV presence in bats has been reported: Hainan Island in China [Reference Zhang, Yang and Li7]; Costa Rica and Ecuador [Reference Platt8], and the Gulf and Pacific coasts of Mexico in the present study, are areas of endemic dengue fever (Fig. 1). We believe this to be an interesting fact since, as previously mentioned, human DV infection rates ranged between 95 and 1097/100 000 population in the areas and period of capture.
Species reported here with indirect evidence of DV are found in populated areas, especially frugivorous bats such as Artibeus and Sturnira, which share their habitats with humans, and where fruit trees are cultivated, and Myotis which tend to use households, sheds, and culverts. In the present study, positive Myotis bats were captured 100 m distance from areas of human habitation. It is important to point out that type 2 DV outbreaks in humans and sampling took place simultaneously in Veracruz in 2006. Moreover, it is of interest to note that type 2 DV was also identified in all positive bats captured in that same area and period.
Individuals testing positive to any of the three tests applied in this work were frugivorous bats (Artibeus, Carollia, Sturnira) or insectivorous bats (Myotis, Natalus, Pteronotus). The diet of insectivore bats may contribute to infection by mosquito-borne viruses. Through the ingestion of virus-infected mosquitoes, insectivorous bats could theoretically become infected by the oral route, however, this remains an unproven hypothesis. Frugivorous bats, however, would have to be infected by a mosquito bite. Further studies are needed in order to clarify if Aedes mosquitoes could infect bats with DV and if normal Aedes mosquitoes could obtain the virus from them. It is well known that A. aegypti is the main vector of DV. However, other Aedes species could be possible vectors of dengue, such as A. albopictus, which has been detected with DV in Mexico [Reference Ibáñez-Bernal11].
All bats positive by the specific DV RT–PCR test were captured during the rainy season, when mosquito populations are high, a condition that may favour the hypothetical infection. Nevertheless, a larger sample of possibly susceptible species, as well as the mechanisms of infection, should be studied. These preliminary findings have led us to attempt to experimentally infect the positive species reported in the present study (study in progress).
The presence of anti-DV antibodies and/or DV by RT–PCR in the species captured in the present study might suggest their susceptibility to DV infection. Flaviviruses that are antibody cross-reactive with DV have been previously described in bats. Thus, a probable cross-reaction with other flaviviruses should not be ruled out. Nevertheless the RT–PCR test developed by Lanciotti et al. [Reference Lanciotti9] is considered a highly specific test for DV detection. Considering the results that we obtained, we believe that even if bats do not act as amplifying reservoirs, it is plausible to think that they might play a role conserving DV in nature (probably in low titres).
ACKNOWLEDGEMENTS
We acknowledge the skilful field assistance of: B. Garcia Lacy, G. Galvez, E. Tesoro Cruz, L. Perea, K. Gordillo. This work was financed by the Consejo Nacional de Ciencia y Tecnología, México (grant no. IMSS-CONACYT 2004-CO1-036). We also thank Dr Francisco Trigo, Head of the Veterinary Medicine Faculty at UNAM, for allowing us to use the facilities of the Clarin ranch in Veracruz. Collecting permit by SEMARNAT Mexico in 2005 (SGPA/DGVS/10415) and in 2006 (SGPA/DGVS/06541).
DECLARATION OF INTEREST
None.




