INTRODUCTION
Enteric diseases pose a significant global health burden [1]. In developed countries, although such diseases are usually mild, the associated morbidity and cost are significant [Reference Mead2]. In Canada (population 33 million), it is estimated that about 70% of the population suffers from acute gastroenteritis annually [Reference Sargeant, Majowicz and Snelgrove3–Reference Thomas5], costing approximately $120 per capita per year [Reference Majowicz4, Reference Henson6].
Disability-adjusted life year (DALY) is a composite measure of morbidity and mortality and is regarded by the World Health Organization (WHO) as the measure of choice to quantify the burden of illness in the population [Reference Mathers, Lopez, Murray and Lopez7, Reference Murray8]. DALYs are being used by the WHO's Foodborne Disease Burden Epidemiology Reference Group (FERG) [9] and have also been employed in risk prioritization frameworks to guide priority setting in the area of foodborne diseases [Reference Lake10–Reference Kemmeren13]. In order to accurately estimate DALYs and other measures of burden of illness such as cost of illness, a significant amount of information on morbidity and mortality are required.
Large population studies and active surveillance are ideal data sources for burden-of-illness calculations as they quantify the incidence of diseases in the community and their different levels of severity. These types of studies discriminate between cases with mild symptoms that do not seek medical care, cases that visit a physician, that are admitted to the emergency room, that are hospitalized or that died from the disease. These studies are rare in Canada and are not always available for specific enteric pathogens. Therefore, estimates of burden of illness often start with data from notifiable disease systems, passive surveillance and national databases that are further corrected for under-reporting to estimate the true burden of illness. For this reason, although the data from these sources represent only the tip of the burden-of-illness pyramid, they can be a valuable source of information for burden-of-illness estimations.
In Canada, campylobacteriosis, salmonellosis and verotoxigenic Escherichia coli (VTEC) infections are reportable diseases, captured by the National Notifiable Diseases (NND) database from the Public Health Agency of Canada [14]. NND also captures information on age groups, sex and province or territory. The Canadian Institute for Health Information (CIHI) and the Canadian Vital Statistics registry maintain hospitalization and mortality databases, respectively, that record cases using the international statistical classification of diseases and related health problems (ICD) codes. These three different databases are seldom analysed together, partly because the latter two databases are only available upon request. Therefore, the objective of this study was to describe and integrate reported data to derive estimates of morbidity for key enteric pathogens of public health significance in Canada [Campylobacter spp., Salmonella spp., enterohaemorrhagic E. coli (EHEC), norovirus and Listeria spp.], along with associated sequelae [Guillain–Barré syndrome (GBS), haemolytic uraemic syndrome (HUS), and reactive arthropathies including Reiter's syndrome (RR)] to facilitate future calculations of burden-of-illness estimates.
METHODS
Data from a period of 4 years were extracted from three national databases, as follows.
Reported number of cases
The number of reported cases for Campylobacter spp., Salmonella spp. and VTEC by province and territory were obtained from the NND database via Notifiable Diseases On-line (Public Health Agency of Canada) [15] from 1 January 2001 to 31 December 2004.
Hospitalizations
The numbers of hospitalized cases for each of the five pathogens (Campylobacter spp., Salmonella spp., EHEC, norovirus, Listeria spp.) and three associated sequelae (GBS, HUS, RR) were obtained from CIHI's Hospital Morbidity Database (HMDB) [15–18] from 1 April 2001 to 31 March 2005. The acute HMDB contains national discharge statistics from all acute care facilities across Canada; this database does not include discharge data from chronic care, rehabilitation, or psychiatric facilities, day procedures (e.g. day surgeries), or emergency department visits. For each discharge event in the database, up to 25 possible diagnostic codes are captured.
For this study, a case was defined as a discharge who had one of the ICD codes listed in Table 1 recorded anywhere in the 25 possible diagnostic code categories. The variables extracted from the acute HMDB for each case were sex, age in years, principal diagnosis, associated diagnoses, length of hospital stay in days, and discharge disposition. Hospitalization data were summarized as total number of hospitalized cases for the 4 years of the study and mean annual hospitalization. The mean annual hospitalization incidence rate per 100 000 was calculated for all pathogens and sequelae by dividing the mean annual hospitalization number by the average Canadian population over the 4-year period [19]. For the three reportable diseases, the percentage of reported cases hospitalized was estimated by dividing the number of hospitalized cases by the number of reported cases for that particular pathogen during the 4 years of the study. The percentage of reported cases hospitalized and the mean annual hospitalization incidence rate were calculated overall and by sex and age categories (<1, 1–4, 5–9, 10–14, 15–19, 20–24, 25–29, 30–39, 40–59, >59 years). The distribution of the hospitalized cases (frequency of hospitalization) was calculated by dividing the number of hospitalized cases in a particular age category or sex group by the total number hospitalized cases.
Table 1. ICD-10Footnote * and ICD-9-CMFootnote † codes for select enteric pathogens and associated sequelae investigated in this study
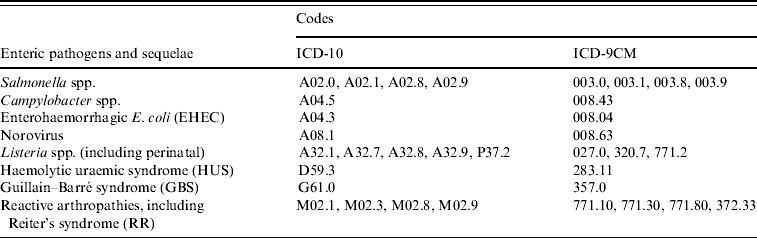
* International Statistical Classification of Diseases and Related Health Problems, 10th revision.
† International Statistical Classification of Diseases and Related Health Problems, 9th revision, Clinical Modification.
Deaths
The numbers of fatalities for each of the eight outcomes of interest were obtained from the Canadian Vital Statistics – Death Database (Statistics Canada, Health Statistics Division) [20] from 1 January 2001 to 31 December 2004. The Death Database (DD) is a comprehensive administrative survey that collects demographic and cause of death (as defined by the physician) information from all provincial and territorial vital statistics registries in Canada. The numbers of fatalities were extracted by year, sex, age category and cause of death (both as ICD-10 code and ICD-10 code description).
Adjustments for data completeness and representativeness
NND data were not available for the province of Saskatchewan in 2003 and 2004 (~3% of the Canadian population), and for Nunavut territory in 2004 (~0·1% of the Canadian population); therefore, to ensure comparability, no HMDB data on Campylobacter spp., Salmonella spp. and EHEC were extracted for these regions for these specific years. Additionally, we did not include any NND or HMDB data for the province of Quebec (~24% of the Canadian population), across the entire study period, since in Quebec during our study period, hospitalized cases were reported using ICD-9 coding (instead of ICD-10 or ICD-9-CM), which did not contain specific codes for the diseases of interest in this study (i.e. there is no specific code for Campylobacter spp. in the ICD-9 edition).
To most closely match the available outcome information, the average Canadian population used to calculate mean annual hospitalization incidence rates for the three reportable diseases excluded (i) Quebec, (ii) Nunavut and Saskatchewan for 2004, and (iii) Saskatchewan for 2003. For the calculation of mean annual hospitalization incidence rates for the two non-reportable diseases and the three associated sequelae, only the Quebec population was excluded, as hospitalization data for Nunavut and Saskatchewan were available.
Finally, NND reports cases as VTEC while the ICD reports as EHEC. Enterohaemorrhagic E. coli are Shiga-producing and verotoxin-producing E. coli (STEC/VTEC) and therefore VTEC reported cases from NND were utilized as a proxy to calculate EHEC estimates.
Statistical analysis
A χ2 test was used to test whether the frequency of hospitalization for all eight outcomes varied with age category and sex compared to the total Canadian population (adjusted for data completeness). The non-parametric Kruskal–Wallis test was used to test for differences in the length of hospital stay by age. Basic statistical measures (mean and median) were calculated for the length of hospital stay. All statistical analyses were done in Minitab15® (USA).
RESULTS
Reported number of cases
During the 4 years of the study, 32 702 cases of campylobacteriosis, 17 459 cases of salmonellosis and 3751 cases of VTEC were reported in Canada (excluding Quebec) (Table 2). This translated respectively into an average of 34·9, 18·6 and 4·0 cases/100 000 population per year (comparable to reported rates that include Quebec; data not shown).
Table 2. Total number of cases and percentage of reported cases hospitalized by age category and sex for the three reportable enteric pathogens in Canada, 2001–2004
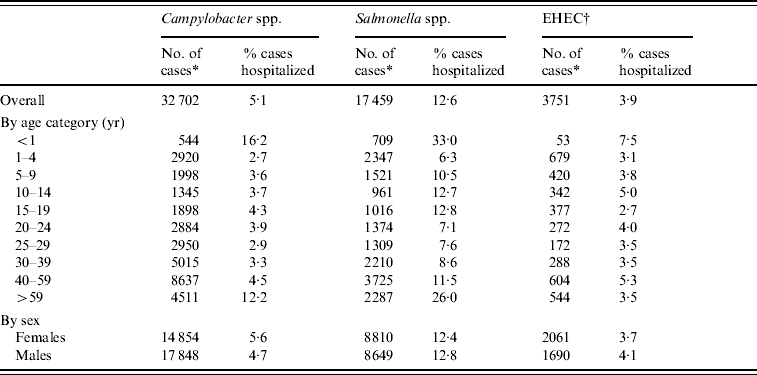
EHEC, Enterohaemorrhagic E. coli.
* Reported cases from the National Notifiable Diseases (NND) database excluding cases from Quebec.
† The number of cases from NND are reported as VTEC and used as proxy to calculate the estimates for EHEC.
Hospitalizations
It was estimated that about 13% of reported Salmonella spp. cases were hospitalized, more than twice the percentage of campylobacteriosis and EHEC cases (VTEC cases used as proxy to calculate EHEC proportions). The percentage of reported cases hospitalized was greater for the <1-year-old age category for all three pathogens and for those aged >59 years for Salmonella spp. and Campylobacter spp. (Table 2).
Table 3 summarizes the hospitalization data for the eight selected outcomes in terms of absolute numbers, mean annual hospitalization incidence rates and frequency of hospitalization by age category and sex (expressed as percentage of the total hospitalized cases). GBS and salmonellosis had the highest mean annual hospitalization incidence rate per 100 000 for the 4 years of the study, followed by Campylobacter spp. and norovirus. There were more females hospitalized than males for norovirus (58·4% female, P<0·0001) and HUS (54·3% female, P=0·02), and more males hospitalized than females for GBS (54·9% male, P<0·0001) and RR (69·3% male, P<0·0001). There was no difference between the proportion of males and females hospitalized for Listeria spp., Salmonella spp., Campylobacter spp. and EHEC.
Table 3. Summary of hospitalized cases of select enteric pathogens and associated sequelae in Canada, 2001–2004Footnote *

EHEC, Enterohaemorrhagic E. coli; GBS, Guillain–Barré syndrome; HUS, haemolytic uraemic syndrome; RR, reactive arthropathies, including Reiter's syndrome.
* Fiscal year (April 2001–March 2005).
† Including perinatal.
The frequency of hospitalization varied with age category for all eight conditions (P<0·001). Hospitalizations increased with age for GBS and RR, but decreased with age for HUS. For EHEC, the frequency of hospitalization was higher for age categories 1–4 years and >40 years. For Salmonella spp., Campylobacter spp., norovirus and Listeria, the frequency of hospitalization was highest in those aged <5 and >59 years (Table 3).
Overall, the hospitalized cases varied in age from 0 to 101 years. The mean hospitalization age varied from 27 (HUS) to 59 (norovirus) years. Generally, hospitalized cases of EHEC and HUS were younger, while hospitalized cases of Listeria, norovirus and GBS were older (Table 3).
The number of days spent in hospital also varied widely. Patients diagnosed with listeriosis (mean stay 23 days), norovirus (mean stay 17 days), GBS (mean stay 20 days) and HUS (mean stay 13 days) stayed longer in hospital than patients diagnosed with campylobacteriosis, salmonellosis, EHEC infection or RR (mean stays ⩽7·2 days). The number of days hospitalized (Table 3) ranged from a minimum of 1 day (all eight conditions) to a maximum of 760 days (listeriosis). The median days in hospital varied with age for all conditions, with the exception of EHEC (P=0·07). The median days in hospital increased with age, and was higher for patients aged >59 years for all conditions except EHEC and RR. In addition, the median days in hospital was also higher for those aged <1 and 20–24 years (listeriosis, HUS), and 25–29 years (listeriosis; data not shown).
Salmonella spp., Campylobacter spp., GBS and EHEC, were recognized as the primary diagnosis (with or without secondary diagnoses) in >60% of the cases (Fig. 1). In contrast, over 50% of cases of Listeria, norovirus, HUS, and RR were secondary diagnoses associated with other conditions.
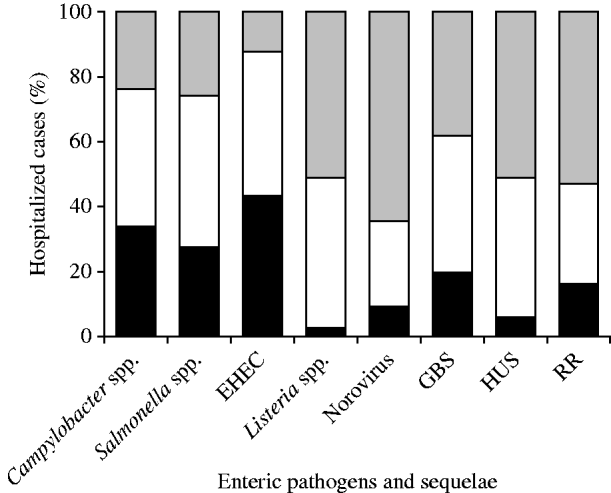
Fig. 1. Percentage of hospitalized cases per principal/associated diagnosis, for selected enteric pathogens and associated sequelae in Canada, 2001–2004. Fiscal year (April 2001–March 2005). EHEC, Enterohaemorrhagic E. coli; GBS, Guillain–Barré syndrome; HUS, haemolytic uraemic syndrome; RR, reactive arthropathies, including Reiter's syndrome. ▪, Primary and only diagnosis; □, primary plus other(s) diagnosis; ![]() , secondary diagnosis.
, secondary diagnosis.
Eighty-six percent or more of hospitalized cases of Campylobacter spp., Salmonella spp., EHEC and RR were discharged to the home setting without support services. In contrast, ~64% of norovirus and HUS cases, and <50% of Listeria and GBS cases, were discharged home without support services. About 12% of Listeria and norovirus, 8% of GBS, 6% of HUS, 5% of Campylobacter spp., RR and Salmonella spp. and 1% of EHEC hospitalized cases were sent home with support services. In addition, ~37% of GBS, 23% of Listeria, 25% HUS, 18% norovirus and 11% of EHEC hospitalized cases were transferred to either a long-term care facility, another facility providing in-patient hospital care or another type of institution. Fewer than 6·5% of RR, Salmonella spp. or Campylobacter spp. hospitalized cases were transferred to another institution. Overall, <1% of hospitalized cases signed out (2% for RR).
Deaths
The highest mean annual number of deaths was reported for GBS and norovirus (Table 4). No deaths were reported for EHEC during the 4-year study period, and the mean annual number of deaths was <1 for RR and Campylobacter spp. (Table 4). Twenty-five percent of the deaths due to HUS were in children aged <15 years (4/16) and 19% in children aged <4 years (3/16) (data not shown).
Table 4. Number of deaths for select enteric pathogens and associated sequelae in Canada, 2001–2004
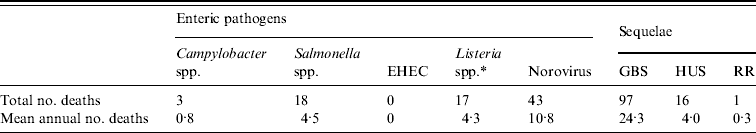
EHEC, Enterohaemorrhagic E. coli; GBS, Guillain–Barré syndrome; HUS, haemolytic uraemic syndrome; RR, reactive arthropathies, including Reiter's syndrome.
* Including perinatal.
DISCUSSION
No surveillance tool or database accurately captures the total burden of infectious gastroenteritis as gastrointestinal diseases are vastly under-reported [Reference Thomas5, Reference Fleury21]. Although notifiable report systems and national databases represent only a portion of the burden-of-illness pyramid, they are still a useful source of data, particularly when analysed collectively. Therefore the objective of this study was to describe and integrate existing data sources to derive better estimates of morbidity for key enteric pathogens and their associated sequelae, in order to facilitate the calculation of burden-of-illness estimates for Canada. We did not attempt to compare the present results with the international literature as surveillance systems are country specific and comparison are often difficult and complex.
Healthcare is a provincial or territorial responsibility in Canada, and primary data are collected at this level. The databases utilized in this study posed challenges and limitations to the estimation of national morbidity and mortality values, as regional data were either collected in different formats or not collected/reported in specific periods, requiring calculations to be adjusted to accommodate data gaps. Nunavut and Saskatchewan represent a relatively small portion of the Canadian population and the absence of data from these regions for 1 or 2 years probably did not affect the estimated rates at a national level. The lack of data from Quebec was a more significant gap as Quebec comprises ~24% of the Canadian population. However, it is important to mention that when comparing the NND incidence rate for the three reportable diseases for Quebec and Canada, there were no major differences (data unknown). Quebec age-standardized hospitalization rates reported by a study that evaluated hospitalization due to acute gastroenteritis in Canada from 1995 to 2004, were also similar to the age-standardized hospitalization rates for the whole of Canada [Reference Fleury21]. For these reason, we believe that excluding Quebec data from this analysis, although not ideal, allowed us to obtain specific data on relevant enteric pathogens and sequelae without compromising the representativeness of the estimated rates. Because provinces and territories are not identified in DD, the number of deaths reported reflects the data for the entire country.
In the current study, most (62·7%) hospitalizations due to norovirus were in patients aged >59 years and ~91% of the cases had more than one diagnosis. Out of the cases with more than one diagnosis, 65% were aged >59 years, which might explain the high proportion of norovirus cases in the older age group as well as the unusual long hospitalization period as comorbidities might increase the susceptibility of individuals to infection and might also extend their recovery period.
The low mean annual hospitalization incidence rate of listeriosis in patients aged >59 years was unexpected. This result can probably be explained by the fact that the acute HMDB does not include cases from chronic care and rehabilitation facilities and therefore, HMDB might under-report listeriosis cases diagnosed in older age groups. The low hospitalization rate for EHEC was unexpected. It is likely that some EHEC cases were coded under ‘Other Intestinal E. coli Infections’ (A04.4, 008.00 and 008.09). Therefore, using strictly the EHEC ICD code (A04.3 and 008.04) could have underestimated the hospitalization rate for EHEC. Unfortunately, it is difficult to determine the proportion of ‘Other Intestinal E. coli Infections’ that are truly EHEC cases. If a large portion of them were truly EHEC, then the total count and hospitalization rate would be significantly higher.
No death associated with EHEC was captured in the DD in the 4 years of the study. However, HMDB discharge disposition data showed that in the 4 years of the study, three cases of EHEC were discharged as dead, which would represent a case-fatality ratio of 0·08% (3/3751) for reported cases. Data from several outbreaks in the USA have reported case-fatality ratios of 0·5% for all E. coli O157:H7 cases and 0·2% for E. coli O157:H7 non-HUS cases [Reference Rangel22]. In Ontario, from 1997 to 2001, 0·6% of the VTEC cases died as a result of the infection [Reference Lee and Middleton23]. Thus, the absence of deaths in the DD may be, in part, an artefact of under-reporting. For all pathogens and sequelae, the number of discharges ‘as dead’ was greater than the number of deaths reported by the DD. It is probable that the number of deaths for each pathogen is between the numbers derived from DD and HMDB discharge data, as DD probably under-reports deaths and HMDB discharge data probably overestimates them because of uncertainty as to whether the cause of death was due to the infection with the pathogen in question. Thus, the results presented here could serve to reflect a range of uncertainty in future burden and DALY calculations.
In the absence of incidence data for severe conditions, known to have high hospitalization rates such as HUS and GBS, the mean annual hospitalization incidence rates could probably be used as a good approximation to estimate the burden of these conditions in the community. Assuming the mean annual hospitalization incidence rate for GBS (2·6 cases/100 000) as a proxy for its incidence rate, we estimated the number of GBS cases associated with Campylobacter spp. infection based on cultural and serological studies conducted in USA. These studies showed that 20–40% of GBS cases had antecedent Campylobacter spp. infections [Reference Buzby, Roberts and Allos24, Reference Mishu25], therefore it is likely there are between 126 and 251 cases of Campylobacter-associated GBS in Canada annually.
To our knowledge this is the first study performed at a national level in Canada reporting data from HMBD and DD and used to estimate morbidity for five common enteric pathogens and their associated sequelae. In addition, it derives estimates for the number of Campylobacter-associated GBS in Canada.
It is important to note that none of the estimates reported here take into consideration under-reporting. Moreover, the databases only capture those ill enough to require hospitalization and therefore the distributions and statistical differences observed for age and sex mean hospitalization incidence rates might not always mimic the incidence rate distribution by age and sex present in the community as these databases are subject to report bias. Under-reporting is a major factor when calculating the burden of disease and can adjust reported data to reflect a more accurate burden of illness. In Canada it has been estimated that on average for every reported case of VTEC, Salmonella spp. and Campylobacter spp., 10–47, 13–37 and 23–49 cases, respectively, actually occur [Reference Thomas26].
This paper provides a starting point from which the burden of various enteric pathogens in Canada can be estimated. In particular, these data provide the basis for future calculations of DALYs, a recognized metric useful for capturing disease burden, which have not yet been calculated for enteric diseases in Canada. Such an understanding of disease burden is crucial to advocate for public health interventions and policies that are evidence- and science-based.
ACKNOWLEDGEMENTS
This study used data and information provided by the Canadian Institute for Health Information. However, the analyses, conclusions, opinions and statements expressed herein are those of the authors, and not necessarily those of the Canadian Institute for Health Information. In addition, the study also used data provided to the Public Health Agency of Canada, from the Canadian Vital Statistics databases at Statistics Canada with the knowledge and consent of the provincial and territorial vital statistics registries which supply the data to Statistics Canada. Their cooperation is gratefully acknowledged. Financial support for this research from the Natural Sciences and Engineering Research Council (Canada) is gratefully acknowledged. The authors thank Merrilyn Allegakone from Health Canada for support with the databases.
DECLARATION OF INTEREST
None.







