Introduction
The best time to harvest seeds during their development and maturation is when they attain maximum quality. When is this? And how should seed quality be assessed and quantified to answer this question?
One simple, widely known, attractive (at first acquaintance) answer to the first question was the hypothesis that seed quality improves until physiological maturity (end of the seed-filling phase; Shaw and Loomis, Reference Shaw and Loomis1950; Box 1) and that thereafter seeds deteriorate because ‘nutrients are no longer flowing into the seed from the mother plant’ (Harrington, Reference Harrington and Kozlowski1972).
The hypothesis has been supported by a wide range of researchers (e.g. Eastin et al., Reference Eastin, Hultquist and Sullivan1973; Benedict et al., Reference Benedict, Kohel and Schubert1976; Maguire, Reference Maguire and Khan1977; Browne, Reference Browne1978; Delouche, Reference Delouche1980; Powell et al., Reference Powell, Matthews and Oliveira1984; Rasyad et al., Reference Rasyad, Van Sanford and TeKrony1990; TeKrony and Egli, Reference TeKrony, Egli, Ellis, Black, Murdoch and Hong1997). The attractiveness of the hypothesis when introduced was, I suggest, twofold. First, the very use of the term physiological maturity made the hypothesis appear irrefutable. Indeed, some of the term's more recent usage has divorced it from the original 1950 definition (Box 1). Secondly, this developmental stage coincides with the termination of assimilate supply to the seeds.
Box 1. Phases and stages of seed development
Seed-filling phase
After anthesis and seed set, there is an initial period of cell division and histodifferentiation as the embryo develops (Black et al., Reference Black, Bewley and Halmer2006). This appears as a lag phase in the relation between seed dry weight and duration from anthesis, often around 7–10 days, as there is no detectable change in weight. The seed-filling phase then commences with a constant or almost constant gradient until it ends and so can be represented by a straight line relation (e.g. Pieta Filho and Ellis, Reference Pieta Filho and Ellis1991a). After the end of the seed-filling phase, there is no further change in seed dry weight and this is represented by a plateau (maximum dry weight). Sometimes the beginning and end of seed filling is less abrupt, with a sigmoidal relation between seed dry weight and duration from anthesis (Egli, Reference Egli1998). In these cases too, the rate of seed filling can still be estimated as a constant (Egli, Reference Egli1998), with the end of seed filling when 95% of the asymptotic value is attained.
Physiological maturity
Physiological maturity is the end of the seed-filling phase (Shaw and Loomis, Reference Shaw and Loomis1950). Those agronomists introduced that term for this seed developmental stage to highlight that later agronomic interventions could not increase crop seed yield, because the funiculus loses functionality and so dry matter cannot be transferred from the mother plant to the seed. Harrington (Reference Harrington and Kozlowski1972) cited that definition in his hypothesis that this seed developmental stage is also that at which seed quality is greatest, because seed quality improves during the seed-filling phase but seeds deteriorate thereafter and so seed quality declines. In support of this observation regarding the apparent simultaneous end of seed weight increase and seed quality improvement, Harrington (Reference Harrington and Kozlowski1972) noted that after physiological maturity ‘nutrients are no longer flowing into the seed from the mother plant’.
Perhaps less well recognized, however, was the subsequent comment that ‘maximum dry weight may provide an index of physiological maturity, but not always’ (Harrington, Reference Harrington and Kozlowski1972). This led to some distortion or inconsistent use of the term. For example, physiological maturity was said to occur some days after maximum seed dry weight was attained in two Brassica seed crops (Still and Bradford, Reference Still and Bradford1998), contradicting the definition of Shaw and Loomis (Reference Shaw and Loomis1950). Nor did Black et al. (Reference Black, Bewley and Halmer2006) link their definition directly to the original seed developmental stage. They reduced the definition of physiological maturity from that of Harrington's, to the ‘stage of development at which a seed, or the majority of a seed population, has reached its maximum viability and vigour’. Nonetheless (given that the moisture status of seeds at the end of the seed-filling phase is similar to the mother plant), they maintained an indirect link to the earlier definition by adding ‘… not usually the stage of maturity at which seed should be harvested […] since seeds generally achieve physiological maturity at moisture contents that are too high …’. Finch-Savage and Bassel (Reference Finch-Savage and Bassel2016) also divorced the definition of physiological maturity from the original (end of the seed-filling phase) to define it solely as the point of maximum seed quality. They also noted that this occurs after mass maturity (see below) and usually before harvest maturity (see below).
Egli (Reference Egli1998), on the other hand, re-iterated the original definition of Shaw and Loomis (Reference Shaw and Loomis1950) and suggested that where sigmoidal relations between seed dry weight and duration from anthesis were detected then there were two values for physiological maturity: an estimated value provided by the intercept of the intrinsic linear relations; and an actual estimate when the asymptote was reached. He also noted that, because data are often not collected to calculate physiological maturity accurately, indirect (visual) indicators are often applied – such as the appearance of a black layer in maize (Zea mays L.) seeds (Egli, Reference Egli1998).
Mass maturity
Given that the definition of physiological maturity had become compromised and so misleading in seed science, the term mass maturity was proposed to designate the end of the seed-filling phase (Ellis and Pieta Filho, Reference Basso, Hoshino-Bezerra, Sartori, Buitink, Leprince and da Silva1992). For the avoidance of doubt, mass maturity is the seed developmental stage that Shaw and Loomis (Reference Shaw and Loomis1950) termed physiological maturity.
Maturation drying
The moisture content of seeds declines throughout their development and maturation. The decline is caused by the proportionally greater accumulation of assimilate than water (Egli, Reference Egli1990) during the seed-filling phase. The latter ends at mass maturity and the further decline in seed moisture content – the maturation-drying phase, which ends at harvest maturity – results from net loss in water from seeds. Mass maturity can be estimated by analysing serial results for seed weight and moisture content simultaneously (Pepler et al., Reference Pepler, Gooding and Ellis2006). Seeds in fleshy fruits also show net loss in water after mass maturity (e.g. Demir and Ellis, Reference Demir and Ellis1992b). Probert et al. (Reference Probert, Adams, Coneybeer, Crawford and Hay2007) describe this period as the post-abscission phase of seed development.
Harvest maturity
The term harvest maturity is somewhat imprecise, given that the maturity stage at which a seed crop is harvested varies amongst crops, and within crops amongst different farming systems in different parts of the world. Hence, for example, it is the ‘… stage of development which is seed or the majority of the seed population is best suited to harvesting in high quality and yield, considering its storage, its handling characteristics to minimise mechanical injury, and potential field losses …’ during harvest (Black et al., Reference Black, Bewley and Halmer2006). Kelly and George (Reference Kelly and George1998) recognized a diversity of seed harvest practices, but noted ‘If panicles are to be sun-dried, the harvest commences at 16 to 18% moisture’. This outcome is similar to the definition used here: excluding seeds within fleshy fruits, the seed developmental stage of harvest maturity is the end of the seed maturation phase by when seed moisture content has declined to values approaching equilibrium with the ambient environment. In the UK for starchy seed crops such as wheat (Triticum aestivum L.), for example, this is typically 14–16% moisture content (matching the traditional grain brittleness test – hard, but liable to break easily), but in oily seeds such as oilseed rape (Brassica napus L.) 8–10% moisture content.
If the hypothesis is correct, then why do seed producers (of most crops) delay harvest until later? Similarly, why are seeds of wild species rarely shed at this developmental stage? Indeed, seeds of Galanthus nivalis L. and Narcissus pseudonarcissus L. are shed early in their development and the embryos continue their development post-shedding in moist conditions with improvement to seed quality (Newton et al., Reference Newton, Hay and Ellis2013), but this is not common.
Harrington's hypothesis implies that in order to produce seeds of the best quality, producers should harvest seeds whilst their moisture content remains comparatively high, given that the moisture status of seeds at the end of the seed-filling phase approaches that of the mother plant; e.g. 42–49% moisture content in barley (Hordeum vulgare L.) and wheat (Triticum aestivum L.) (Ellis and Pieta Filho, Reference Ibrahim, Roberts and Murdoch1992). This requires great care in handling very moist seeds, particularly in those species where seeds are bruised by mechanical damage at such moisture contents (Moore, Reference Moore and Roberts1972), together with considerable drying ex planta.
Perhaps not surprisingly then, other researchers reported that seed quality continues to improve after (Wilson and Trawatha, Reference Wilson and Trawatha1991) or well after physiological maturity sensu stricto (e.g. Kameswara Rao et al., Reference Kameswara Rao, Appa Rao, Mengesha and Ellis1991; Pieta Filho and Ellis, Reference Pieta Filho and Ellis1991a, Reference Pieta Filho and Ellisb; Demir and Ellis, Reference Demir and Ellis1992a, Reference Demir and Ellisb, Reference Demir and Ellis1993; Ellis and Pieta Filho, Reference Pieta Filho and Ellis1992; Ellis et al., Reference Ellis, Hong and Jackson1993; Ellis and Hong, Reference Ellis and Hong1994; Zanakis et al., Reference Zanakis, Ellis and Summerfield1994; Hay and Probert, Reference Hay and Probert1995; Sanhewe and Ellis, Reference Sanhewe and Ellis1996; Sanhewe et al., Reference Sanhewe, Ellis, Hong, Wheeler, Batts, Hadley and Morison1996; Hay et al., Reference Hay, Probert and Smith1997; Sinniah et al., Reference Sinniah, Ellis and John1998a; Hay et al., Reference Hay, Smith, Ellis and Butler2010; Pereira Lima et al., Reference Pereira Lima, Buitink, Lalanne, Rossi, Pelletier, da Silva and Leprince2017; Basso et al., Reference Basso, Hoshino-Bezerra, Sartori, Buitink, Leprince and da Silva2018), or even later after seeds are shed from mother plants (e.g. Probert et al., Reference Probert, Adams, Coneybeer, Crawford and Hay2007; Newton et al., Reference Newton, Hay and Ellis2013) refuting Harrington's hypothesis. Given these contradictory reports, and so the potential of the use of the term physiological maturity to mislead with regard to seed quality, the term mass maturity was proposed to designate the end of the seed-filling phase (Box 1).
As well as direct evidence to reject the hypothesis, evidence that important maturation events occur within seeds after the vascular connection with the mother plant is lost (Galau et al., Reference Galau, Jakobsen and Hughes1991; Leprince et al., Reference Leprince, Pellizzaro, Berriri and Buitink2017) repudiated the earlier assumption that ending nutrient supply to seeds at physiological maturity inevitably terminates seed quality improvement. Oligosaccharides and low-molecular weight proteins have each been shown to accumulate within seeds in planta after physiological maturity, with each positively associated with seed quality improvement during seed development and maturation (Sinniah et al., Reference Sinniah, Ellis and John1998b). Proteins and RNA produced late in seed maturation are important for subsequent seed longevity (Chatelain et al., Reference Chatelain, Hundertmark, Leprince, Gall, Satour, Deligny-Penninck, Rogniaux and Buitink2012) and for the successful completion of the seed germination process upon imbibition after storage (Dirk and Downie, Reference Dirk and Downie2018).
There are circumstances where seed producers do act comparatively soon after the end of the seed-filling phase. Where seeds vary in maturity date and/or have a high propensity to shatter and shed, for example certain oilseeds or grass seed crops, the crop may be cut well before seeds mature and left as a swath in the field to mature further and to dry. The swath traps the shattered seeds, whilst cutting the whole crop hastens the maturation of less-developed seeds, and all seeds (previously shed or not) are subsequently threshed from the swath. In such cases, maturation drying of seeds occurs within the swath. Seed maturation has been shown to continue ex planta in environments which mimic those in planta, with seed quality improvement and, for example, the accumulation of oligosaccharides continuing ex planta (Hong et al., Reference Hong, Gedebo and Ellis2000). Indeed, in some cases the initial environment ex planta may benefit seeds harvested late in seed development and maturation: in rice (Oryza sativa L.) and some other crops, the quality of seeds harvested at high moisture content was improved considerably by high-temperature drying (Whitehouse et al., Reference Whitehouse, Hay and Ellis2015, Reference Whitehouse, Hay and Ellis2017, Reference Whitehouse, Hay and Ellis2018a, Reference Whitehouse, Owoborode, Adebayo, Oyatomi, Olaniyan, Abberton and Hayb). Evidence from ex planta studies also contradicts Harrington (Reference Harrington and Kozlowski1972), therefore.
Roberts (Reference Roberts1999) discussed his approach to seed science as a search for patterns. Such patterns can provide visual representations of our understanding, or hypotheses. Visual representations of several contrasting patterns of changes in seed quality, improvement and decline, during seed development and maturation are presented here in order to consider, challenge, and summarize advances in our understanding nurtured by Harrington's original, thought-provoking hypothesis.
Temporal patterns of seed quality development and decline for different criteria
The sensitivity of any particular test to assess seed quality has the potential to modify the extent of any variation detected by that test. Standard germination test environments to determine seed viability are designed to provide optimal conditions for germination, including breaking dormancy (ISTA, 2015). The results of the test have upper and lower boundaries (100% and 0%, respectively). Seedling emergence in the field occurs typically in sub-optimal environments, but is also constrained by the same numerical boundaries (Fig. 1a). Such data are binary in which one fraction of the seed population is able to germinate whilst the remaining individuals do not. Similarly in the harsher environment of the seedbed a smaller fraction of the seed population is able to produce seedlings. These are examples of population-based thresholds where the fraction of the population able to pass the threshold is a function of the seedbed environment (Ellis and Roberts, Reference Ellis and Roberts1981; Finch-Savage and Bassel, Reference Finch-Savage and Bassel2016; Bradford, Reference Bradford2018). Seed vigour is defined by reference to many variables including seed lot performance in the field (Perry, Reference Perry1978; ISTA, 2015). At the heart of the concept of seed vigour is the variability amongst seed lots in their ability to produce seedlings the harsher the seedbed environment, as exemplified by the seed-lot-by-environment interaction (Hegarty, Reference Hegarty1978). Such interactions can be explained, and quantified, by population-based thresholds which consider the progress of deterioration (ageing) in the individual seed and the consequences for the performance of different seed lots (Ellis and Roberts, Reference Ellis, Roberts and Hebblethwaite1980, 1981; Khah et al., Reference Khah, Ellis and Roberts1986; Ellis and Dolman, Reference Ellis and Dolman1988). The extent, or absence, of ageing in a seed lot is thus an important aspect of seed quality, sometimes described as physiological quality to distinguish it from variation resulting from mechanical, pest, or disease damage.

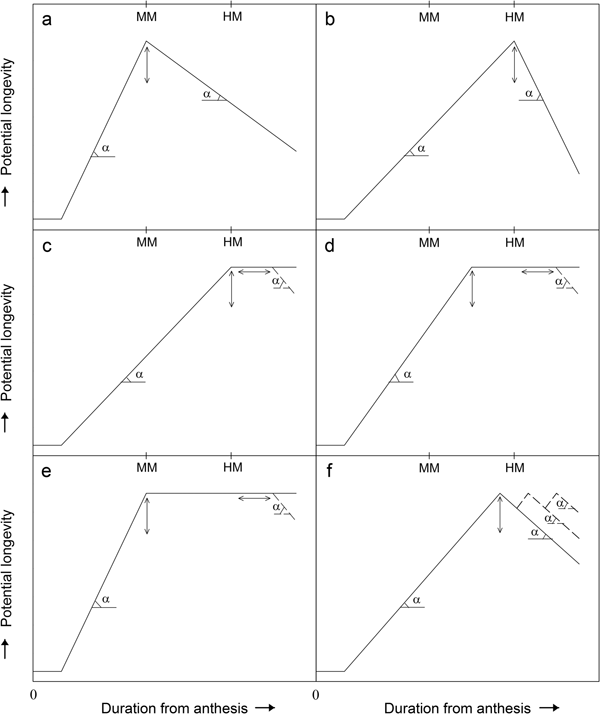
Fig. 1. Comparison of outline temporal patterns of seed quality development and decline during seed development and maturation (duration from anthesis) amongst three different methods of assessing seed quality: ability to germinate in standard laboratory tests (panel a, continuous line); emergence of seedlings from the field seedbed (panel a, dashed line); longevity in air-dry hermetic storage (panel b). These schematic patterns are abridged results for seeds of barley produced in 1988 (Pieta Filho and Ellis, Reference Pieta Filho and Ellis1991a, Reference Pieta Filho and Ellisb). MM, mass maturity; HM, harvest maturity.
Figure 1 provides a stylized, simplified representation of changes in seed quality in barley during seed development and maturation in one year where seed quality was assessed in each of three different ways (Pieta Filho and Ellis, Reference Pieta Filho and Ellis1991a, Reference Pieta Filho and Ellisb): ability to germinate in standard laboratory tests; field emergence; or longevity in air-dry storage. The upper limit provided by ability to germinate following desiccation and rehydration was reached comparatively early in development and then maintained for a considerable period of subsequent development and maturation in planta (32 days; Pieta Filho and Ellis, Reference Pieta Filho and Ellis1991a) until harvest maturity in this example (Fig. 1a). Seedbed environments are rarely optimal for germination and seedling emergence. This is shown in Fig. 1a, where the field emergence of seedlings was always less than the ability of seeds to germinate in laboratory germination tests. In addition, the period of seed development and maturation during which field emergence ability was greatest (11 days; Pieta Filho and Ellis, Reference Pieta Filho and Ellis1991b) was shorter than that for ability to germinate. That is, the more sensitive test of seed quality reduced the apparent period during which seeds, if harvested, showed maximum seed quality.
Seed longevity in air-dry storage is a continuous variable which provides another method of assessing changes in seed quality during seed development and maturation. It provided a pattern where seed quality improved during most of the study period, until maximum quality was attained at harvest maturity, and seed quality declined thereafter (Fig. 1b). Hence, the three different methods of assessment in Fig. 1 provided compatible, but not identical, results: all methods showed a simultaneous decline in quality immediately after harvest maturity, to a greater (longevity) or lesser (laboratory germination and field emergence) extent, after earlier improvement in quality. How early maximum quality was attained varied amongst the different assessment procedures, however: earliest for ability to germinate and latest for longevity. As a consequence, the duration of maximum quality in planta was shorter (longevity < field emergence < laboratory germination) the more sensitive the method of assessment. Hence, assessing seed longevity has considerable utility in investigations of seed quality development.
Variation in temporal patterns of seed quality development and decline
An inverted V portrays the temporal pattern of change in seed quality where seed quality improves until the end of the seed-filling phase and then declines as proposed by Harrington (Reference Harrington and Kozlowski1972). In this representation of his hypothesis, the two trend lines intersect at mass maturity (Box 1) to provide maximum seed quality at that developmental stage (Fig. 2a).

Fig. 2. Contrasting outline temporal patterns of seed quality (assessed by longevity in subsequent air-dry hermetic storage) development and decline during seed development and maturation (duration from anthesis) reported by various authors for different seed crops in different environments in planta: MM, mass maturity; HM, harvest maturity; α is the (variable) angle of change in seed quality (rate of increase or decrease in seed quality); vertical bidirectional arrows indicate that the value of maximum seed quality varies with environment and genotype; dashed lines of negative slope indicate decline in seed quality which begins after a variable duration of the maintenance of maximum seed quality; horizontal bidirectional arrows indicate that the timing of when such a decline may be observed varies with environment and genotype. Pattern (a) represents the hypothesis that seed quality improves during seed development and maturation until the end of the seed-filling phase and declines thereafter (Harrrington, Reference Harrington and Kozlowski1972); (b) that reported for the longevity of barley seeds produced in 1988 (Pieta Filho and Ellis, Reference Pieta Filho and Ellis1991a) where seed quality improved throughout seed development and maturation until harvest maturity, and then declined; (c) that reported for the longevity of barley seeds produced in 1989 (Pieta Filho and Ellis, Reference Pieta Filho and Ellis1991a) where high seed quality was maintained after the peak was first achieved; (d) that reported for the longevity of barley and wheat seeds produced in 1988 or 1989 (Pieta Filho and Ellis, Reference Ibrahim, Roberts and Murdoch1991a) where several cultivars reached peak quality during maturation drying and before harvest maturity and maintained their quality until harvest maturity or later; (e) that reported for Japonica rice in a comparatively hot environment where improvement in seed quality ended close to mass maturity with little further change in quality during maturation drying (Ellis et al., Reference Ellis, Hong and Jackson1993); (f) that reported for reported for wheat seeds subjected to rainfall and drying cycles where damage to seed quality from rainfall was reversed after drying in planta (Yadav and Ellis, Reference Yadav and Ellis2016).
In this and the subsequent stylized outline temporal patterns, the angle α indicates that the gradient of improvement or decline in seed quality over developmental time is affected by the seed production environment. For example, in wheat the former is a function of temperature (Sanhewe et al., Reference Sanhewe, Ellis, Hong, Wheeler, Batts, Hadley and Morison1996). In addition, as the angle is a function of environment, it may change during seed quality improvement and/or decline as the seed production environment varies from day to day. For example, in barley there was almost a pause (i.e. α tended to zero) in seed quality improvement for a short period in one year (Pieta Filho and Ellis, Reference Pieta Filho and Ellis1991a).
Pieta Filho and Ellis (Reference Pieta Filho and Ellis1991a) presented results for changes in barley seed longevity during seed development and maturation in 1988 which approximated to an inverted V, but where maximum seed quality coincided with harvest maturity (Fig. 2b). The weather during seed development and maturation in the following year, 1989, was warmer and drier (Pieta Filho and Ellis, Reference Pieta Filho and Ellis1991a). In that year also, barley seed longevity showed a consistent improvement until harvest maturity, but in contrast to the previous year the subsequent decline was delayed for 10–15 days and so the inverted-V temporal pattern was modified with a brief plateau at the peak (Fig. 2c). In tomato (Solanum lycopersicum), this plateau of maximum seed quality was of considerable duration, at least 40 days (Demir and Ellis, Reference Demir and Ellis1992b).
In similar research with several contrasting cultivars of each of barley and wheat, the results showed consistently that seed longevity continued to improve long after mass maturity (Ellis and Pieta Filho, Reference Ellis and Pieta Filho1992). In some cultivars the improvement continued until harvest maturity, and so matched the patterns in Fig. 2b and 2c. In others, however, maximum longevity was attained slightly earlier and so before harvest maturity (Fig. 2d). Similarly, results for different cultivars of soyabean (Glycine max L.) in two seasons matched the temporal pattern in either Fig. 2c or 2d (Zanakis et al., Reference Zanakis, Ellis and Summerfield1994).
Contrasting cultivars of rice in one temperature regime showed similar patterns where seed longevity continued to improve for about 20 days after mass maturity reaching maximum values shortly before harvest maturity (Ellis et al., Reference Ellis, Hong and Jackson1993), matching the temporal pattern in Fig. 2d. This was also the case for Indica and Javanica rice cultivars in a much warmer regime, whereas a Japonica cultivar failed to show further improvement in longevity from close to mass maturity onwards in that warmer regime. This genotype-by-environment interaction resulted in poor longevity in that case, but that poor value was maintained in planta for the subsequent 20–30 days before deterioration began to be detected (Fig. 2e). Note that in this particular genotype–environment combination, the Harrington hypothesis was met in one part: net seed quality improvement was limited to the seed-filling phase; but decline in seed quality in planta did not then commence.
The final temporal pattern presented (Fig. 2f) summarizes results from a 5-year investigation on the consequences of rainfall events for seed quality development in wheat. Rainfall events during seed development and maturation damaged seed quality immediately, but the damage was reversed after a short further period in planta during which seeds (re)dried (Ellis and Yadav, Reference Ellis and Yadav2016; Yadav and Ellis, Reference Yadav and Ellis2016). That effect was repeatable under successive rainfall events. Moreover, the effect was identified in planta in three phases of seed development and maturation: before mass maturity; between mass maturity and harvest maturity; and after harvest maturity. Hence, the trends of seed quality improvement and those of seed deterioration in Fig. 2 each represents net changes in seed quality: improvement and deterioration processes are both possible within each phase (Yadav and Ellis, Reference Yadav and Ellis2016).
Villiers and Edgcumbe (Reference Villiers and Edgcumbe1975) showed that repeated deterioration and improvement in seed quality occur ex planta also. They introduced the repair hypothesis to explain the improvement part of the cycle. Provided oxygen is available and germination is prevented (by dormancy), seed longevity ex planta can be considerable in very moist seeds (Ibrahim and Roberts, Reference Ibrahim and Roberts1983; Ibrahim et al., Reference Ibrahim, Roberts and Murdoch1983). High humidity or priming (Heydecker and Gibbins, Reference Heydecker and Gibbins1978) at harvest may also improve seed longevity in some circumstances (Powell et al., Reference Powell, Yule, Jing, Groot, Bino and Pritchard2000; Butler et al., Reference Butler, Hay, Ellis, Smith and Murray2009), but is detrimental in other circumstances (Argerich et al., Reference Argerich, Bradford and Tarquis1989; Tarquis and Bradford, Reference Tarquis and Bradford1992). Priming in early maturation (soon after mass maturity) did improve air-dry longevity in pepper (Capsicum annuum L.) but only acted to hasten seed improvement because priming later in seed development and maturation reduced longevity (Demir and Ellis, Reference Demir and Ellis1992a).
Discussion
These diverse temporal patterns of seed quality development and decline (Fig. 2) refute the hypothesis that seed quality is always greatest at mass maturity and then declines (Fig. 2a). A simple amendment of the original hypothesis to one where seed quality is instead greatest at harvest maturity before then declining (Fig. 2b) must also be rejected: it does occur, but there are further different temporal patterns of seed quality development and decline (Fig. 2).
The original hypothesis (Harrington, Reference Harrington and Kozlowski1972) was a conceptual framework. It consisted of several discrete hypotheses: (a) a phase of seed improvement which ends before seed deterioration then begins; (b) one single point during seed development and maturation, in all circumstances, when seed quality is maximal; (c) improvement in seed quality coincides temporally with seed filling perfectly; (d) deterioration always, and only, occurs post-seed filling; and hence, seed quality is maximal at the end of the seed-filling phase and then declines.
An improved conceptual framework would, I suggest, consist of the following: (a) seed improvement and deterioration processes can each cycle during seed development and maturation (Yadav and Ellis, Reference Yadav and Ellis2016), cycling sequentially if not simultaneously; (b) the relative sensitivity of rates of improvement and deterioration to environment (including seed moisture status) may differ, and this relativity may alter as seed development and maturation progress. For example, warmer temperatures in early seed development damage whereas warmer temperatures in late seed maturation benefit subsequent seed quality in wheat (Nasehzadeh and Ellis, Reference Nasehzadeh and Ellis2017); and developing rice seeds were most vulnerable to damage from high (and also low) temperature in the 7 or 14 days after anthesis (Martínez-Eixarch and Ellis, Reference Martínez-Eixarch and Ellis2015), but their longevity was improved by high-temperature drying in late maturation in contrast (Whitehouse et al., Reference Whitehouse, Hay and Ellis2018a).
As a consequence, as experimenters we estimate the net consequence of gross improvement and deterioration from assessments at any one seed developmental stage. Hence, the various plateaus (no change in seed quality over a particular duration in planta) shown in Fig. 2c–e represent periods where the rate of improvement more or less equals the rate of deterioration. Similarly the triple peaks in Fig. 2f provide a stable saw-tooth regulator effect: smoothing out observations over several days would give an impression of no change in seed quality. These considerations match the repair hypothesis of Villiers and Edgcumbe (Reference Villiers and Edgcumbe1975) for the post-harvest survival of seeds in aerated environments that are continuously or regularly at high moisture contents.
It follows that the period that maximum seed quality is maintained in planta can be extended in some seed production environments beyond just a brief period of ‘peak’ quality. In addition, precisely when maximum quality is attained during seed development and maturation can vary depending upon environment, considerably in the case of the Japonica rice example (Ellis et al., Reference Ellis, Hong and Jackson1993). That is, the genotype-by-environment interaction affects three important facets: the value of maximum seed quality; when it is first attained during seed development and maturation; and for how long it is maintained thereafter in planta.
These observations are relevant to investigations of the effect of environment on quality seed production; to the production of high-quality seeds; and to the breeding and selection of improved cultivars with better quality seed production. They are, moreover, pertinent to understanding the potential impacts of climate change on the production of quality seeds, especially in more marginal crop (and seed) production areas and with regard to the effects of brief episodes of extreme temperature, and to work to maximize the positive effects (e.g. cereal seed production at high latitudes; Sanhewe et al., Reference Sanhewe, Ellis, Hong, Wheeler, Batts, Hadley and Morison1996) and mitigate the negative effects (e.g. rice seed production at risk of flooding: Tejakhod and Ellis, Reference Tejakhod and Ellis2018) of climate change. In particular, increase in temperature has the potential to alter the temporal pattern of change in seed quality from that shown in Figure 2c towards that in Figure 2e, with consequent severe reduction in seed quality (Ellis et al., Reference Ellis, Hong and Jackson1993; Ellis and Hong, Reference Ellis and Hong1994), an agri-botanical-environmental tipping point of considerable possible severity for food security and ecological diversity alike.
Author ORCIDs
Richard H. Ellis, 0000-0002-3695-6894
Acknowledgements
I thank all my past collaborators for their many contributions to this topic and Dr James Ryalls for help preparing the figures.