The effect of blood trans-fatty acid (TFA) levels on human diseases has recently aroused considerable attention(Reference Shearer, Pottala and Spertus1–Reference Moyers, Farzaneh-Far and Harris3). Chavarro et al. (Reference Chavarro, Stampfer and Campos4) reported that the whole-blood TFA level was associated with an increased risk of non-aggressive prostate tumour. Chajes et al. (Reference Chajes, Thiebaut and Rotival5) showed that a high serum level of TFA contributed to the risk of invasive breast cancer in women. Benatar et al. (Reference Benatar, Gladding and White6) proposed that plasma total TFA may be associated with vascular disease and increased C-reactive protein in patients with severe coronary artery disease. Meanwhile, Lemaitre et al. (Reference Lemaitre, King and Sotoodehnia7) found that a high erythrocyte membrane TFA level was correlated with an increased risk of sudden cardiac arrest.
It is well accepted that TFA are highly associated with CHD risk, and different kinds of TFA isomers play different roles in CHD events(Reference Oomen, Ocke and Feskens8, Reference Sun, Ma and Campos9). Industry-originated TFA, such as trans-16 : 1n-9, trans-18 : 1n-9 and trans-18 : 2n-6n-9, are considered to have deleterious effects on cardiovascular health(Reference Hunter, Zhang and Kris-Etherton2, Reference Smith, Robinson and Nam10, Reference Bendsen, Stender and Szecsi11), while TFA from ruminant sources are associated with a slightly neutral risk(Reference Mozaffarian12), because trans-16 : 1n-7 and trans-18 : 1n-7, the dominant TFA isomers in milk, can be biotransformed to conjugated linoleic acid (CLA) such as 9-cis, 11-trans-18 : 2n-6 CLA by Δ9-desaturase, which is conducive to anticancer, anti-diabetic and anti-CHD effects(Reference Rungapamestry, McMonagle and Reynolds13). Therefore, the effects of TFA on CHD vary in differently originated TFA, and it may be irrational to estimate the effects of TFA simply by considering the total amount of TFA levels. However, if there were combined parameters, taking into account both industry-originated TFA and ruminant-sourced TFA, the effects of TFA on CHD could be estimated according to different TFA isomers and their own physico-chemical characteristics.
In the present study, we explored a TFA index that unifies the disparate impacts of different TFA isomers on CHD by discriminating the different effects into one equation. Meanwhile, a 10-year CHD risk probability (RP) was also calculated in the present cross-sectional study to represent different pathological severities and potential risks of CHD. The index may be used to confirm the presence of a true association between TFA and CHD. The aims of the present study were to verify whether the TFA index was associated reliably with the 10-year CHD RP and to identify which study sample (whole blood, serum or erythrocyte membrane) could truly reflect the change in body TFA levels.
Methods
Ethical statement
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Institutional Ethics Committee, the First and Second Affiliated Hospital, Nanchang University, China. Written informed consent was obtained from all subjects/patients.
Patients
In the present study, we divided CHD into two relatively independent clinical pathological forms: a severely harmful acute coronary syndrome (ACS), which is manifested by myocardial infarction and unstable angina, and a chronic/stable form known as chronic coronary artery disease (CCAD), which has a phenotype of stable angina and ischaemic cardiomyopathy(Reference Cassar, Holmes and Rihal14). The ACS and CCAD patients were selected to represent an acute and a chronic CHD pathology, respectively. A high-risk population (HRP) and healthy volunteers (HV) served as controls for CHD patients in the ACS and CCAD groups. Thus, the four groups represented different pathological severities and potential risks of CHD.
A cross-sectional survey on six large physical populations of 12 380 individuals was conducted at the First and Second Affiliated Hospitals, Nanchang University between 2007 and 2010. Excluding confounding factors and other diseases, 2713 individuals were screened in the ACS (n 581), CCAD (n 631), HRP (n 659) and HV (n 842) groups to represent different pathological severities and potential risks of CHD. The ACS was diagnosed on the basis of pre-specified criteria for acute myocardial infarction or unstable angina(Reference Alpert, Antman and Apple15, Reference Khot, Jia and Moliterno16). The CCAD patients were selected on the basis of the objective clinical and diagnostic criteria of the American Heart Association(Reference Cassar, Holmes and Rihal14, Reference Gibbons, Abrams and Chatterjee17). The HRP of CHD was classified on the basis of the International Statistical Classification of Diseases codes and previous studies(Reference O'Rourke, Brundage and Froelicher18, Reference Niu, Zhao and Zhu19). Exclusion criteria included: (1) inadequate multi-detector computed tomography imaging, due to heavily calcified lesions by visual estimation; (2) a culprit lesion in the left main coronary artery; (3) atrial fibrillation; (4) malignant disease; (5) dialysis; (6) diabetes mellitus; (7) renal insufficiency.
Blood sample preparation
After an overnight fasting of the participants, blood (5 ml) was drawn from the antecubital vein into vacutainer tubes containing ethylene diamine tetraacetic acid. Serum was separated from erythrocytes by centrifugation at 2000 g at 4°C for 10 min. Erythrocytes were washed three times with ice-cold isotonic saline to remove the buffy coat. Membranes were isolated by a modification of Burton's method(Reference Burton, Ingold and Thompson20). Briefly, packed cells were lysed with cold distilled water, centrifuged at 20 000 g at 10°C for 20 min, and washed several times to eliminate Hb residues. Whole-blood, serum and erythrocyte membrane samples from each participant were stored at − 80°C under liquid N2 until lipid extraction.
Extraction and analysis of fatty acids
Lipids were extracted by chloroform–methanol (1:1) and methylated by sodium methoxide as described previously(Reference Cruz-Hernandez, Deng and Zhou21). Fatty acid methyl esters were analysed by a gas chromatograph (GC 6890 N; Agilent) equipped with a flame ionisation detector, an autosampler injector (7683-B; Agilent) and a fused silica capillary column (CP-Sil 88, 100 m × 0·25 mm inner diameter, 0·20 μm film thickness; Varian). The temperature was held at 45°C for 4 min, and then ramped to 175°C at a flow rate of 13°C/min, held for 27 min, and, finally, increased to 215°C at a flow rate of 4°C/min, held for 35 min.
The levels of five TFA (trans-16 : 1n-7, trans-16 : 1n-9, trans-18 : 1n-7, trans-18 : 1n-9 and trans-18 : 2n-6n-9) were calculated from the GC results using normalisation and internal standard methods, as described previously(Reference Zghibeh, Gopal and Poff22). The results were compared with standard fatty acid methyl esters (GLC-463; Nu-Chek Prep, Inc.), with 21 : 0 fatty acid methyl esters added. Fatty acid profiles were expressed as the percentage of TFA. The TFA index was defined by the following equation:
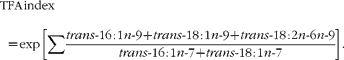 $$TFA\,\,\,index = \,exp\,\left [\sum \frac { trans \hyphen 16\,:\,1 n \hyphen 9 + trans \hyphen 18\,:\,1 n \hyphen 9 + trans \hyphen 18\,:\,2 n \hyphen 6 n \hyphen 9}{ trans \hyphen 16\,:\,1 n \hyphen 7 + trans \hyphen 18\,:\,1 n \hyphen 7}\right ]. $$<label>(1)</label>
$$TFA\,\,\,index = \,exp\,\left [\sum \frac { trans \hyphen 16\,:\,1 n \hyphen 9 + trans \hyphen 18\,:\,1 n \hyphen 9 + trans \hyphen 18\,:\,2 n \hyphen 6 n \hyphen 9}{ trans \hyphen 16\,:\,1 n \hyphen 7 + trans \hyphen 18\,:\,1 n \hyphen 7}\right ]. $$<label>(1)</label>
In this equation, the numerator adds the levels of industrial TFA (trans-16 : 1n-9, trans-18 : 1n-9 and trans-18 : 2n-6n-9) together, and the denominator adds the levels of ruminant TFA (trans-16 : 1n-7 and trans-18 : 1n-7) together. An exponential function was used to make the index stronger and more obvious as the TFA index.
Characteristic data
Serum lipids (TAG, total cholesterol and HDL), apoA (mainly included apoAI), apoB (included both apoB100 and apoB48) and lipoprotein α were measured in the hospital clinical laboratory with an automatic biochemistry analyser (Beckman CX9). Levels of LDL were calculated by the Friedewald equation. Other standard risk factors, including age, sex, BMI, tobacco use, duration of diabetes mellitus, systolic/diastolic blood pressure, waist circumference and history of diagnosed hypertension, were collected as variables (Table 1).
Table 1 Characteristics of study participants (n 2713) (Mean values and standard deviations; number of patients and percentages)

ACS, acute coronary syndrome; CCAD, chronic coronary artery disease; HRP, high-risk population; HV, healthy volunteers; TC, total cholesterol; Lp-α, lipoprotein α.
a,b,cValues within a row with unlike superscript letters were significantly different (P< 0·05 or P< 0·001).
* Blood lipids tested include: TAG; TC; HDL-cholesterol (HDL); LDL-cholesterol (LDL); apoA (mainly including apoAI); apoB (including both apoB100 and apoB48); Lp-α.
Statistical analysis
The 10-year RP of CHD was calculated on the basis of the Cox proportional hazards multivariate model formulation and the Framingham risk equation(Reference Kinlay, Oconnell and Evans23). The risk score was defined as
Furthermore, the 10-year CHD RP was calculated from the following equation:
where RP(group) is the CHD probability within the next 10 years of each group (ACS, CCAD, HRP and HV); S 0(t) is the hazard function at time t; ![]() $$X _{1}, X _{2}, X _{3},\ldots , X _{ i } $$ are independent risk factor variables for each individual;
$$X _{1}, X _{2}, X _{3},\ldots , X _{ i } $$ are independent risk factor variables for each individual; ![]() $$M _{1}, M _{2}, M _{3},\ldots , M _{ i } $$ are the average levels of risk factors in each group;
$$M _{1}, M _{2}, M _{3},\ldots , M _{ i } $$ are the average levels of risk factors in each group; ![]() $$\beta _{1}, \beta _{2}, \beta _{3},\ldots , \beta _{ i } $$ are the partial regression coefficients of different risk factors.
$$\beta _{1}, \beta _{2}, \beta _{3},\ldots , \beta _{ i } $$ are the partial regression coefficients of different risk factors.
The estimated partial regression coefficients, hazard ratio and their corresponding 95 % CI are shown in Table 2; the descriptive characteristics are shown in Table 1. These parameters and data were substituted into equation 2 and the risk score for the ACS group was calculated as ![]() $$f _{(ACS)}( X , M ), $$ which, in turn, was substituted into equation 3 and the average 10-year RP(ACS) was calculated as
$$f _{(ACS)}( X , M ), $$ which, in turn, was substituted into equation 3 and the average 10-year RP(ACS) was calculated as ![]() $$1 - 0\cdot 9876^{\,exp\,(\, f _{(ACS)}[ X , M ])} $$. In the same way, the average 10-year RPCCAD of the CCAD group was calculated as
$$1 - 0\cdot 9876^{\,exp\,(\, f _{(ACS)}[ X , M ])} $$. In the same way, the average 10-year RPCCAD of the CCAD group was calculated as ![]() $$1 - 0\cdot 9821^{\,exp\,(\, f _{(CCAD)}[ X , M ])} $$, the average 10-year RP(HRP) of the HRP group as
$$1 - 0\cdot 9821^{\,exp\,(\, f _{(CCAD)}[ X , M ])} $$, the average 10-year RP(HRP) of the HRP group as ![]() $$1 - 0\cdot 9781^{\,exp\,(\, f _{(HRP)}[ X , M ])} $$, the average 10-year RP(HV) of the HV group as
$$1 - 0\cdot 9781^{\,exp\,(\, f _{(HRP)}[ X , M ])} $$, the average 10-year RP(HV) of the HV group as ![]() $$1 - 0\cdot 9753^{\,exp\,(\, f _{(HV)}[ X , M ])} $$ (For the equation calculation process, see the Supplementary material, available online).
$$1 - 0\cdot 9753^{\,exp\,(\, f _{(HV)}[ X , M ])} $$ (For the equation calculation process, see the Supplementary material, available online).
Table 2 Partial regression coefficients of the hazard score in the Cox model analysis (Mean values and standard deviations; hazard ratios (HR) and 95 % confidence intervals)

ACS, acute coronary syndrome; CCAD, chronic coronary artery disease; HRP, high-risk population; HV, healthy volunteers; RC, regression coefficient; TC, total cholesterol; LDL-C, LDL-cholesterol.
* Sex = 1 if woman, 0 if man.
† Tobacco use = 1 if yes, 0 otherwise.
The assumption of proportional hazards and the calibration were adjusted for confounding factors such as baseline age, sex, smoking and LDL, and verified by the Hosmer and Lemeshow test(Reference Lin, Wei and Ying24, Reference Harrell, Lee and Mark25). The proportional hazards assumption was considered to be valid when the difference in the P value was < 0·05.
SPSS for Windows version 18.0 was used to calculate the regression equations and correlation coefficients between the TFA index and the 10-year CHD-RP for the different groups. Bonferroni correction was performed for ANOVA and correlation analysis to verify statistically significant differences with a P value < 0·05.
Results
Trans-fatty acid profile and index
The erythrocyte membrane, serum and whole-blood TFA profiles are listed in Table 3. Only in erythrocyte membranes were there significant differences in the levels of all TFA isomers, as well as the TFA index among the different groups. The levels of industry-generated isomers (trans-16 : 1n-9, trans-18 : 1n-9 and trans-18 : 2n-6n-9) were the highest in the ACS group, whereas the levels of ruminant-originated isomers (trans-16 : 1n-7 and trans-18 : 1n-7) were the highest in the HV group, and the TFA index showed a significant progressive decrease from the ACS to the HV group. In serum, only the levels of trans-18 : 2n-6n-9 were significantly different between these groups; although the TFA index was high in the ACS group, there was still no significant difference. In whole blood, the levels of both trans-18 : 1n-9 and trans-18 : 2n-6n-9 were significantly higher in the ACS group than those in the other groups, but no difference existed in the other groups. In addition, the total industrial and ruminant TFA levels showed no significant differences between the groups.
Table 3 Erythrocyte membrane, serum and whole-blood trans-fatty acid (TFA) profiles in each group* (Mean values and standard deviations)

ACS, acute coronary syndrome; CCAD, chronic coronary artery disease; HRP, high-risk population; HV, healthy volunteers.
a,b,c,dMean values within a column with unlike superscript letters were significantly different (P< 0·05 or P< 0·001).
* Values are given as the percentages of individual fatty acids relative to the total chromatogram area (FA%).
In the TFA index equation, the total industrial TFA levels in the numerator exhibited an increasing trend from the HV to the ACS group, while the total ruminant TFA levels in the denominator showed a decreasing trend. To unify the incompatible trend of decreased ruminant TFA and increased industrial TFA from the HV to the ACS group, we divided the total industrial TFA levels by the total ruminant TFA levels. This index value represents a coincidental trend of TFA change. In all the four groups, only the erythrocyte membrane TFA index manifested a significant difference. The TFA index in the erythrocyte membrane fraction progressively decreased from 7·12 (sd 1·23) to 5·06 (sd 1·03), 3·11 (sd 0·89) and 1·92 (sd 0·66) in the ACS, CCAD, HRP and HV groups, respectively (P< 0·001).
CHD risk probability
The descriptive characteristics of traditional CHD risk factors in each group are described in Table 1. Sex, age, tobacco use and BMI were significantly different between the groups, while waist circumference, weight and education status were not. As for the blood lipids, only total cholesterol, TAG and LDL showed significant differences between the groups. The estimated partial regression coefficients, hazard ratio and their corresponding 95 % CI are shown in Table 2. The risk scores in the four groups were determined as follows: ACS, ![]() $$f _{(ACS)}\,( X , M ) = 31\%; $$ CCAD,
$$f _{(ACS)}\,( X , M ) = 31\%; $$ CCAD, ![]() $$f _{(CCAD)}( X , M ) = 21\%; $$ HRP,
$$f _{(CCAD)}( X , M ) = 21\%; $$ HRP, ![]() $$f _{(HRP)}( X , M ) = 13\%; $$ HV,
$$f _{(HRP)}( X , M ) = 13\%; $$ HV, ![]() $$f _{(HV)}( X , M ) = 4\% $$.
$$f _{(HV)}( X , M ) = 4\% $$.
Correlation between trans-fatty acid index and 10-year risk probability
The comparison between the erythrocyte membrane TFA index and the 10-year CHD RP showed a strong linear correlation (R 2 0·9981, P< 0·001; Fig. 1). In contrast, the comparison between the serum or whole-blood TFA index and the 10-year CHD RP showed no significant correlation (P>0·05 and P>0·01, respectively; Figs. 2 and 3). In each group (HV, HRP, CCAD and ACS), the average erythrocyte membrane TFA index and the 10-year CHD RP coincided with the regression line.

Fig. 1 Linear correlation between the erythrocyte membrane trans-fatty acid (TFA) index and the 10-year CHD risk probability (RP) (y= 17·122x+1·1365 and R 2 0·9981). Values (Δ) are average TFA index and RP in the healthy volunteers (HV, n 842), high-risk population (HRP, n 659), chronic coronary artery disease (CCAD, n 631) and acute coronary syndrome (ACS, n 581) groups, respectively, with standard deviations represented by vertical bars.

Fig. 2 Linear correlation between the whole-blood trans-fatty acid (TFA) index and the 10-year CHD risk probability (RP) (y= 14·365x+21·141 and R 2 0·5607). Values (Δ) are average TFA index and RP in the healthy volunteers (HV, n 842), high-risk population (HRP, n 659), chronic coronary artery disease (CCAD, n 631) and acute coronary syndrome (ACS, n 581) groups, respectively, with standard deviations represented by vertical bars.

Fig. 3 Linear correlation between the serum trans-fatty acid (TFA) index and the 10-year CHD risk probability (RP) (y= 0·104x+21·81 and R 2 0·012). Values (Δ) are average TFA index and RP in the healthy volunteers (HV, n 842), high-risk population (HRP, n 659), chronic coronary artery disease (CCAD, n 631) and acute coronary syndrome (ACS, n 581) groups, respectively, with standard deviations represented by vertical bars.
Discussion
Erythrocyte membrane trans-fatty acids reflect the change in body trans-fatty acids in CHD
The levels of all of the erythrocyte membrane TFA isomers differed significantly between the groups. In contrast, only trans-18 : 1n-9 and trans-18 : 2n-6n-9 differed significantly between the whole-blood and serum samples. The probable reasons for this difference were as follows: on the one hand, the serum fatty acid level reflected fat intake over the past few days due to the immediate penetration of dietary fat into the serum(Reference Sepulveda, Tanhehco and Frey26), and it is hard to get much change in fatty acid profile during a few days, especially TFA; on the other hand, the fatty acid level of erythrocyte membrane reflected chronic lipid storage, as well as the average level of body lipids over the previous 4–12 weeks(Reference Katan, Deslypere and vanBirgelen27), and there was no fatty acid synthesis, chain elongation or desaturation in the membrane(Reference Pittman and Martin28). Therefore, erythrocyte membrane TFA reflected the change in body TFA levels in CHD(Reference Oomen, Ocke and Feskens8).
Trans-fatty acid index reflects trans-fatty acid hazard on CHD
The five TFA isomers in the erythrocyte membrane included two n-9 TFA (trans-16 : 1n-9 and trans-18 : 1n-9), two n-7 TFA (trans-16 : 1n-7 and trans-18 : 1n-7) and one double-bonded TFA (trans-18 : 2n-6n-9). The two n-9 TFA and trans-18 : 2n-6n-9 primarily originate from partially hydrogenated vegetable oils (‘industrial’ TFA)(Reference Micha, King and Lemaitre29). The two n-7 TFA are generally ruminant-sourced(Reference Micha, King and Lemaitre29), although trans-18 : 1n-7 may also come from an industrial source. The levels of n-9 TFA and trans-18 : 2n-6n-9 were significantly higher in ACS, which is consistent with the proven detrimental impact of industrial TFA in the promotion and induction of CHD events from the Nurses' Health Study in 32 826 participants and 6 years of follow-up(Reference Sun, Ma and Campos9). Another study investigating TFA and sudden cardiac death among 86 762 women has shown that the levels of trans-18 : 2 and trans-16 : 1n-9 were positively associated with myocardial infarction (P< 0·001) after adjusting for established risk factors and other confounders, and that the trans-18 : 2 isomer may play a greater role in sudden cardiac death among individuals with clinically manifest atherosclerosis(Reference Chiuve, Rimm and Manson30).
Remarkably, in contrast, n-7 TFA levels were high in the HV group while low in the ACS group, which may result from the ruminant-sourced generation. Ruminant trans-16 : 1n-7 was associated with trans-18 : 1n-7 and may be converted into trans-18 : 1n-7 by the carbochain increase(Reference Or-Rashid, Wright and McBride31). As a major TFA of ruminant fat, trans-18 : 1n-7 is produced in the rumen and converted in tissues to 9-cis, 11-trans-18 : 2n-6 CLA by Δ9-desaturase with an average conversion rate of 19 %(Reference Turpeinen, Mutanen and Aro32). 9-cis, 11-trans-18 : 2n-6 CLA could positively modulate HDL-cholesterol metabolism and enhance reverse cholesterol transport, and prevent the progression of atherosclerosis in humans(Reference Komori, Arai and Kashima33). Hence, the effect of ruminant TFA on CHD may be neutral or somewhat favourable due to the indirect benefit of 9-cis, 11-trans-18 : 2n-6 CLA(Reference Gomez-Cortes, Tyburczy and Brenna34). However, the industrial TFA are harmful. It has been reported that the consumption of industrial trans-18 : 1n-9 by LDL receptor-deficient (LDL − / − ) mice stimulated atherosclerotic development(Reference Bassett, McCullough and Edel35), while consumption of a diet enriched in trans-18 : 1n-7 reduced cholesterol-induced hyperlipidaemia and atherosclerosis and thus protected against atherosclerotic lesions(Reference Bassett, Edel and Patenaude36). Chronic trans-18 : 1n-7 supplementation also significantly abated dyslipidaemia in both the food-deprived and postprandial states in JCR:LA-cp rats due to reductions in intestinal chylomicrons and hepatic de novo lipogenesis pathways(Reference Wang, Jacome-Sosa and Ruth37). Another study investigating the effects of ruminant-derived TFA on immune function in a model of the metabolic syndrome (JCR:LA-cp rats) has shown that vaccenic acid might protect from CVD due to an anti-inflammatory action(Reference Blewett, Gerdung and Ruth38). Although ruminant TFA have been shown to elicit neutral or less detrimental effects, further studies in human subjects are still needed to prove the protective effects.
To unify the inverse trend of decreased ruminant TFA and increased industrial TFA from the HV to the ACS group, we divided the total industrial TFA levels by the total ruminant TFA levels. This index value represents a coincidental trend of TFA change. An exponential function was used to make the index stronger and more obvious as the TFA index. The TFA index could estimate the true objective change in TFA levels comprehensively by unifying the disparate effects of different TFA on the body; accordingly, the hazardous level of all TFA isomers on CHD could be evaluated. In addition, it was interesting to note that none of the five TFA isomers showed any significant differences except the TFA index (P< 0·001) between the ACS and CCAD groups, indicating that the TFA index could discriminate the acute CHD symptoms of the ACS from the chronic CHD symptoms of CCAD.
Trans-fatty acid index is associated positively with 10-year CHD risk probability
The average TFA index in erythrocyte membranes differed between the groups and increased progressively from the HV to the ACS group. The 10-year CHD RP were 4, 13, 21 and 31 % in the HV, HRP, CCAD and ACS groups, respectively. These values generally reflect the potential probability and pathological severity of CHD. A strong linear correlation was seen between the average TFA index and the 10-year CHD RP (R 2 0·9981; Fig. 1), which suggests that as the erythrocyte membrane TFA level goes up, CHD may get worse.
CHD incidence could be influenced directly or indirectly by TFA through TAG accumulation, vasodilation, inflammation, PG translation and/or platelet aggregation(Reference Oomen, Ocke and Feskens8, Reference de Lorgeril and Salen39). Trans-fatty acids elicit an unfavourable effect on the lipoprotein profile by stimulating cholesteryl ester transfer protein activity (r 0·58, P< 0·005), increasing the LDL level and decreasing the HDL level (r −0·57, P< 0·01). These changes may contribute to a more atherogenic lipoprotein profile(Reference Abbey and Nestel40–Reference Williams42). A high intake of TFA could adversely affect endothelial function; this would partially explain why the positive relationship between trans-fats and cardiovascular health took precedence over the adverse effects of trans-fats on lipids and lipoproteins(Reference Harvey, Arnold and Rasool43, Reference Bionaz, Thering and Loor44). In addition, a recent study has suggested that trans-18 : 1n-9 maintains the levels of vascular cell adhesion molecule-I and intercellular cell adhesion molecule-I up-regulated by TNF-α or lipase. This kept the human brain microvascular endothelial function at the stimulated phenotype, which could promote CHD(Reference Sanadgol, Mostafaie and Bahrami45).
In summary, the erythrocyte membrane TFA index was proposed as a method to unify the content changes in different TFA isomers and their effects on CHD in one equation. The TFA index manifested a strong and positive linear correlation with the 10-year CHD RP. Although the present study might be limited to the variety of TFA isomers analysed, the results should contribute to further studies on the relationship between TFA and CHD.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114513000196
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (30972482), the Academic Leader Program of Jiangxi Province (2008DD00900), the Postgraduate Innovation Funds of Jiangxi Province (YC09A029), the PhD Subject Fund from the Department of Education (20113601120004) and the Natural Science Fund from the Department of Science and Technology of Jiangxi Province (20114BAB214016), China. We deeply appreciate the participation of our colleagues and co-workers at the Department of Cardiology, the First Affiliated Hospital, Department of Cardiology, Medical College, the Second Affiliated Hospital, Nanchang University, and especially the contribution of the research participants to the study. Z.-Y. D. and X.-R. L. initiated, designed, coordinated and, among others, conducted the initial draft board study. J.-N. H., Y.-W. F., R. L. and J. L. carried out the analysis of TFA. J.-T. P., H. S., Q. P. and W.-F. L. screened the patients and collected the clinical data. X.-R. L drafted and completed the manuscript, which was further edited by all the co-authors. All the authors declare that they have no conflicts of interest in relation to the present study.








