Phospholipids (PL) are multifunctional, with phosphatidylcholine (PC) being the most important and abundant class in regular PL sources( Reference Feng, Cai and Zuo 1 ). In mammals, PL are recognised as promising compounds that are useful in the treatment of fatty liver disease( Reference Cohn, Wat and Kamili 2 ). Numerous studies have demonstrated that dietary PL can markedly reduce hepatic lipid levels, subsequently alleviating fatty liver disease( Reference Lee, Kim and Kim 3 – Reference Liu, Shi and Liu 11 ). Furthermore, lipid-lowering effects of PL have been suggested to be related with suppression of lipid synthesis and/or enhancement of fatty acid oxidation( Reference Buang, Wang and Cha 6 , Reference Kabir and Ide 8 , Reference Liu, Xue and Liu 10 , Reference Liu, Shi and Liu 11 ). In addition, a series of in vitro and in vivo studies suggested that when intracellular PC production was inhibited the secretion of VLDL would be attenuated accordingly( Reference Fast and Vance 12 – Reference Jacobs, Lingrell and Zhao 15 ). In fish, although it has been reported that supplementation of PL to the diet could prevent lipid accumulation in liver as well( Reference Feng, Cai and Zuo 1 , Reference Lu, Zhao and Zhao 16 , Reference Salhi, Hernández-Cruz and Bessonart 17 ), mechanisms related are still elusive.
Large yellow croaker (Larimichthys crocea) is a commercially important fish species in China because of its delicious taste. In the past decade, high-lipid diet has been extensively used in the culture of this fish species owing to its protein sparing effect( Reference Hemre and Sandnes 18 ). However, high-lipid diet can induce abnormal lipid accumulation in fish liver, giving rise to increased inflammation and disturbed metabolism( Reference Wang, Yan and Xu 19 , Reference Yan, Liao and Wang 20 ), which are similar to those observed in mammals with fatty liver. In addition, the process of hepatic lipid metabolism in large yellow croaker was essentially similar to those of other fish species and mammals( Reference Yan, Liao and Wang 20 ). Therefore, large yellow croaker is an appropriate comparative natural model to investigate the mechanisms about how PL regulates hepatic lipid deposition in fatty liver induced by high-lipid diet.
Methods
The protocols for animal care and handling used in this study were approved by the Institutional Animal Care and Use Committee of Ocean University of China (permit no.: 20001001).
Animals, diets and feeding trial
Large yellow croaker juveniles were bought from a local farm (Aquatic Fingerlings Limited Company of Xiangshan Harbour). The fish were reared in floating sea cages (3·0×3·0×3·0 m) for 2 weeks for acclimation to the experimental conditions. At the beginning of the experiment, the fish were fasted for 24 h and weighed after being anaesthetised with eugenol (1:10 000) (Shanghai Reagent). Then, fish with similar weight (approximately 8 g) were randomly assigned to four groups (three cages per group with 60 fish/cage (1·5×1·5×1·5 m)): low lipid and low phospholipid (LL-LP) group, fed the control diet (LL-LP diet, containing 12 % lipid and 1·5 % PL); low-lipid and high-PL (LL-HP) group, fed the LL-HP (containing 12 % lipid and 8 % PL); high-lipid and low-PL diet (HL-LP) group, fed the high-lipid and low-PL diet (HL-LP diet, containing 20 % lipid and 1·5 % PL); and high-lipid and high-PL diet (HL-HP) group, fed the high-lipid and high-PL diet (HL-HP diet, containing 20 % lipid and 8 % PL). In the present study, fish oil, soyabean oil and soyabean lecithin were used as the main lipid sources. Fishmeal, casein and soyabean meal were used as the main protein sources. Formulation and chemical composition of the experimental diets are shown in Table 1. The fish were hand-fed to apparent satiation twice daily (05.00 and 17.00 hours). Feeding trial lasted for 70 d during which the water temperature ranged from 21 to 28·5°C; the salinity ranged from 28 to 32 ‰ and the dissolved oxygen level ranged from 6·7 to 7·8 mg/l.
Table 1 Formulation and chemical composition of the experimental diets (g/kg dry diet)
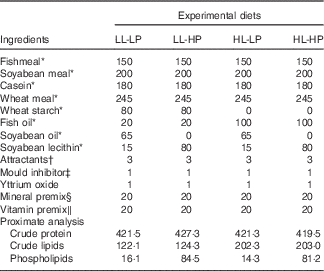
LL-LP, low lipid and low phospholipid; LL-HP, low lipid and high phospholipid; HL-LP, high lipid and low phospholipid; HL-HP, high lipid and phospholipid.
* All of these ingredients were supplied by Great Seven Biotechnology Co.
† Attractants: glycine and betaine.
‡ Mould inhibitor: contained 50 % calcium propionic acid and 50 % fumaric acid.
§ Vitamin premix (mg or g/kg diet): cholecalciferol, 5 mg; retinol acetate, 32 mg; thiamin 25 mg; vitamin B12 (1 %), 10 mg; riboflavin, 45 mg; pyridoxine HCl, 20 mg; ascorbic acid, 2000 mg; α-tocopherol (50 %), 240 mg; vitamin K3, 10 mg; pantothenic acid, 60 mg; inositol, 800 mg; niacin acid, 200 mg; folic acid, 20 mg; biotin (2 %), 60 mg; choline chloride (50 %), 4000 mg; microcrystalline cellulose, 12·47 g.
‖ Mineral premix (mg or g/kg diet): CuSO4.5H2O, 10 mg; Ca(IO3)2.6H2O (1 %), 60 mg; CoCl2.6H2O (1 %), 50 mg; FeSO4.H2O, 80 mg; MgSO4.7H2O, 1200 mg; MnSO4.H2O, 45 mg; NaSeSO3.5H2O (1 %), 20 mg; ZnSO4.H2O, 50 mg; CaH2PO4.H2O, 10 g; zeolite, 8·485.
Sample collection
At the end of the feeding trial, the fish were deprived of food for 24 h. Then, the fish in each cage were subjected to eugenol (1:10 000) and then weighed and counted. Five fish as a pool in each cage were chosen for whole-body lipid content analysis. Livers from five fish per cage were excised and pooled into 1·5-ml tubes, frozen in liquid N2 and stored at −20°C for the measurement of lipid content and TAG content. Livers from another five fish per cage were excised and pooled in to 1·5-ml RNAase-free tubes (Axygen), and then immediately transferred to liquid N2 and stored at −80°C for the assay of gene expression.
Phosphatidylcholine vesicle formation
PC vesicles were made as previously described with slight modification( Reference Yoon, Sun and Arauz 21 ). In brief, PC from soyabean (#P7443; Sigma) was dissolved in 5 ml of buffer consisting of 150 mm NaCl and 10 mm TRIS-Cl (pH=8·0) (Solarbio) and then vortexed to yield a final PC concentration of 5 mm. The PC suspension was then sonicated in a water bath sonicator (G112SPIT; Laboratory Supplies). The solution was sterilised by filtration through 0·22-μm filters (Pall). The aim of these procedures was to generate small unilamellar vesicles that could be easily assimilated by cells. PC vesicles were freshly made before experiments and added to the culture medium at the indicated concentrations.
Culture of primary hepatocytes isolated from large yellow croaker
Large yellow croaker livers were dissected out under sterile conditions and collected in cold sterile PBS (Gibco) supplemented with 200 U/ml penicillin and 200 μg/ml streptomycin (Gibco). After washing with Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium (Gibco), liver tissue was minced into 1 mm3 pieces and digested with 0·25 % Trypsin-EDTA (Gibco) at room temperature for 10 min. The reaction was terminated by adding DMEM/F12 medium containing 10 % fetal bovine serum (FBS) (Gibco) and then the cell suspension was purified through a cell strainer with 70 μm mesh size (BD Falcon). The isolated cells were collected in a 15-ml sterilised centrifuge tube and centrifuged at 500 g for 10 min at 4°C. Cell pellets were resuspended in complete medium (DMEM/F12 medium containing 10 % FBS, 100 U/ml penicillin and 100 μg/ml streptomycin). Primary hepatocytes were seeded into six-well culture dishes (2 ml/well) (Corning) at a density of 2×106 cells/well and incubated at 28°C in 5 % CO2.
Treatment of primary hepatocytes
Isolation and culture of primary hepatocytes were conducted according to the protocols mentioned above. During culture as above, the medium was replaced with new complete medium every 24 h. After 2 d of culture, primary hepatocytes were serum-starved overnight. For experiment one, primary hepatocytes were incubated with graded concentrations of PC (0 (control), 50, 100, 150, 200 and 250 μm, respectively) for 12 h. For experiment two, hepatocytes were transfected with small interfering RNA (siRNA) duplexes (5'-Chol, 2'-Ome) for CTP: choline phosphate cytidylyltranferase α (CCTα) (siRNA-CCTα) or negative control (NC) (siRNA-NC) (GenePharma), which were named CCTα group and control group, respectively. The sequences of CCTα siRNA duplexes were as follows: sense sequence, 5'-GGG UGU AUG CAG AUG GCA UTT-3'; anti-sense sequence, 5'-AUG CCA UCU GCA UAC ACC CTT-3'. The sequences of NC siRNA duplexes were as follows: sense sequence, 5'-UUC UCC GAA CGU GUC ACG UTT-3'; anti-sense sequence, 5'-ACG UGA CAC GUU CGG AGA ATT-3'. The delivery of siRNA duplexes was carried out using Lipofectamine® RNAiMAX Transfection Reagent (Invitrogen) according to the manufacturer’s instructions. Primary hepatocytes were incubated with siRNA–lipid complex for 48 h. The final concentration of siRNA used was 25 pmol/well. For in vitro study, each treatment was replicated four times (n 4). Cells were harvested after treatment for the indicated time.
Biochemical analysis
Crude protein of the experimental diets was determined according to the Kjeldahl method (Kjeltec 2300; Foss Tecator) and estimated by multiplying N by 6·25.
Crude lipid contents of the experimental diets and whole fish body were determined by diethyl ether extraction using the Soxhlet method (Soxhlet Extraction System B-811; Buchi). Hepatic lipid contents of fish were measured based on the described procedures( Reference Folch, Lees and Sloane Stanley 22 ). TAG contents in the primary hepatocytes were determined by TAG Assay Kit according to the manufacturer’s protocols (Polygen).
PL contents of the experimental diets were measured by determining P content (P×25) using molybdenum blue method( Reference Bao, Deng and Suo 23 ). In brief, about 100 mg of freeze-dried samples were digested with nitric acid and perchloric acid and added with molybdenum blue reagent. The P content can be calculated by assaying the absorbance at 830 nm with the UV-2401PC spectrophotometer (Shimadzu Corporation). PC content in primary hepatocytes was measured using the PC Assay Kit (Sigma) according to the manufacturer’s instructions.
Quantitative real-time PCR
Complementary DNA preparation and quantitative real-time PCR were conducted on the procedures described by Zuo et al.(
Reference Zuo, Ai and Mai
24
). Primers for each target gene were directly synthesised based on the corresponding sequences in published papers(
Reference Yan, Liao and Wang
20
,
Reference Cai, Xie and Mai
25
) (Table 2). The relative expression ratio was determined by the formula
![]() $$2^{{{\minus}\Delta \Delta C_{t} }} $$
(
Reference Livak and Schmittgen
26
). 18s rRNA, β-actin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), elongation factor 1α and ubiquitin were ranked according to their stability using geNorm (version 3.5) and NormFinder algorithms(
Reference Andersen, Jensen and Ørntoft
27
,
Reference Vandesompele, Preter and Pattyn
28
) and β-actin was used as the reference gene. For in vivo study, the relative mRNA expression of target genes in fish fed the LL-LP diet was selected as the calibrator; for in vitro study, the relative mRNA expression of target genes in primary hepatocytes incubated with 0 μM PC was selected as the calibrator.
$$2^{{{\minus}\Delta \Delta C_{t} }} $$
(
Reference Livak and Schmittgen
26
). 18s rRNA, β-actin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), elongation factor 1α and ubiquitin were ranked according to their stability using geNorm (version 3.5) and NormFinder algorithms(
Reference Andersen, Jensen and Ørntoft
27
,
Reference Vandesompele, Preter and Pattyn
28
) and β-actin was used as the reference gene. For in vivo study, the relative mRNA expression of target genes in fish fed the LL-LP diet was selected as the calibrator; for in vitro study, the relative mRNA expression of target genes in primary hepatocytes incubated with 0 μM PC was selected as the calibrator.
Table 2 Primer pair sequences for quantitative real-time-PCR

LPL, lipoprotein lipase; HL, hepatic lipase; CD36, cluster of differentiation 36; FATP1, fatty acid transport protein 1; FABP, fatty acid binding protein; FAS, fatty acid synthase; SCD1, stearoyl-CoA desaturase 1; DGAT2, acyl-CoA: diacylglycerol acyltransferase 2; SREBP1, sterol-regulatory element binding protein 1; CPT1: carnitine palmitoyltransferase 1; ACO, acyl-CoA oxidase; MTP, microsomal TAG transfer protein; APOB100, apo B100.
Western blotting
Total protein was extracted using Tissue or Cell Total Protein Extraction Kit (Sangon Biotech). Quantification of the concentrations was conducted by a Noninterference Protein Assay Kit (Sangon Biotech). Protein was separated by denaturing SDS-PAGE, and then transferred to a polyvinylidene difluoride membrane. Next, the membranes were incubated with CCTα or GAPDH antibody (#4454 and #2118; Cell Signaling Technology) before horseradish peroxidase-conjugated secondary antibodies (A0208; Beyotime Institute of Biotechnology) were added. Protein bands were visualised using ECL reagents (Beyotime Institute of Biotechnology).
Statistical analysis
Software SPSS 17.0 (SPSS Incorporation) was used for all statistical evaluations. The results are expressed as mean values with their standard errors. Data were analysed using two-tailed Student’s t test or one-way ANOVA, followed by Tukey’s test. Polynomial contrasts (linear, quadratic and cubic) were used to test the effects of PC concentrations on the various variables measured in the primary hepatocytes. The level of significance was set at P<0·05. Densitometry after western blotting was quantified by NIH Image 1.63 software (National Institutes of Health) and then normalised by GAPDH.
Results
Results for in vivo study
Survival and growth parameters for large yellow croaker fed the experimental diets
There were no significant differences in survival among dietary treatments (P>0·05). Fish in the HL-HP group had significantly higher weight gain and feed efficiency (FE) than in the LL-LP and HL-LP groups (P<0·05), whereas there were no significant differences between the LL-LP and HL-LP groups (P>0·05). Feed intake (FI) in the HL-HP group was comparable to that in the HL-LP group (P>0·05), but was significantly lower than that in the LL-LP group (P<0·05). In addition, there were no significant differences in weight gain, FI and FE between the LL-LP and LL-HP groups (Table 3).
Table 3 Survival and growth parameters for large yellow croaker fed the experimental diets (Mean values with their standard errors; n 3)
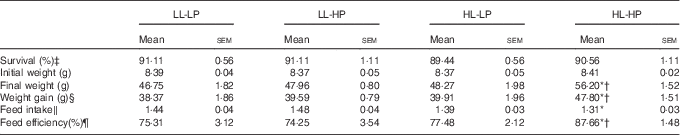
LL-LP, low lipid and low phospholipid; LL-HP, low lipid and high phospholipid; HL-LP, high lipid and low phospholipid; HL-HP, high lipid and phospholipid.
*Significant differences between the LL-LP and the other treated groups (P<0·05; two-tailed Student’s t test).
† Significant difference between the HL-LP and HL-HP groups (P<0·05; two-tailed Student’s t test).
‡ Survival (%)=100×final fish number/initial fish number.
§ Weight gain=final weight−initial weight.
‖ Feed intake (/day)=feed consumption (g)/(d×(final body weight+initial body weight)/2).
¶ Feed efficiency=wet weight gain (g)/feed consumption (g).
Lipid contents of the whole body and liver in large yellow croaker fed the experimental diets
No significant differences in the whole-body lipid contents were observed among the LL-LP, HL-LP and HL-HP groups (P>0·05). Compared with the LL-LP group, lipid levels in the livers were significantly higher in the HL-LP group (P<0·05), which was indicative of abnormal hepatic lipid accumulation. High dietary PL diminished the HL-LP diet-mediated up-regulation of hepatic lipid level (P<0·05). Although no significant differences were detected, the LL-HP diet tended to down-regulate lipid content of the liver compared with the LL-LP group (P>0·05) (Table 4).
Table 4 Lipid contents of the whole body and liver for large yellow croaker fed the experimental diets (%, wet weight) (Mean values with their standard errors; n 3)
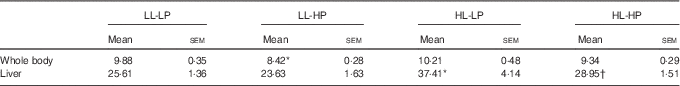
LL-LP, low lipid and low phospholipid; LL-HP, low lipid and high phospholipid; HL-LP, high lipid and low phospholipid; HL-HP, high lipid and phospholipid.
*Significant differences between the LL-LP and the other treated groups (P<0·05; two-tailed Student’s t test).
† Significant difference between the HL-LP and HL-HP groups (P<0·05; two-tailed Student’s t test).
Expression of genes related to fatty acid uptake in the liver of large yellow croaker fed the experimental diets
Compared with the LL-LP diet, the HL-LP diet significantly increased the mRNA expression levels of lipoprotein lipase (LPL), hepatic lipase (HL), cluster of differentiation 36 (CD36), fatty acid transport protein 1 (FATP1) and fatty acid binding protein 11 (FABP11) (P<0·05), which were reversed by the dietary inclusion of high PL (P<0·05). No significant differences were detected in FABP3 and FABP10 mRNA expression between the LL-LP and HL-LP groups, and similar results were observed between the HL-LP and HL-HP groups (P>0·05). Among all the seven proteins involved in fatty acid uptake in the liver, only HL and CD36 mRNA levels displayed a significant decrease in the LL-HP group than in the LL-LP group (P<0·05) (Fig. 1(a)).
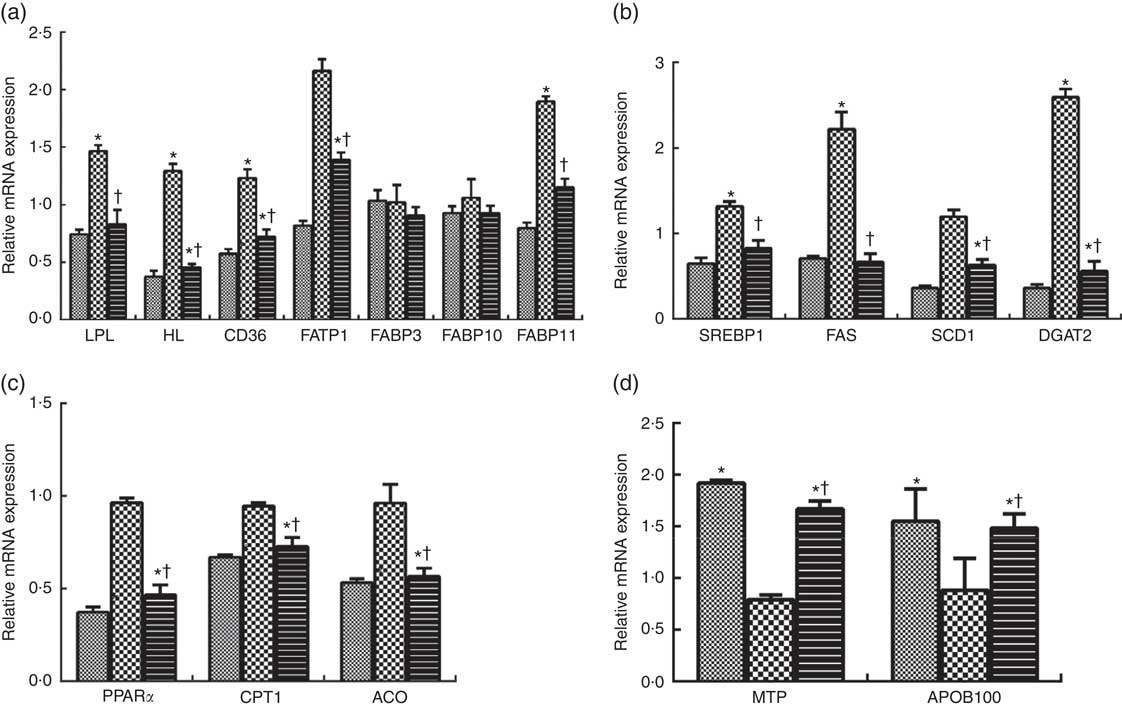
Fig. 1 Expression of genes related to fatty acid uptake (a), lipid synthesis (b), fatty acid oxidation (c) and VLDL assembly (d) in liver of large yellow croaker. Values are means (n 3), with their standard errors represented by vertical bars. The relative mRNA expression of target genes in the low lipid and low phospholipid (LL-LP) group was selected as the calibrator. ![]() , low lipid and high phospholipid (LL-HP);
, low lipid and high phospholipid (LL-HP); ![]() , high lipid and low phospholipid (HL-LP);
, high lipid and low phospholipid (HL-LP); ![]() , high lipid and phospholipid (HL-HP); LPL, lipoprotein lipase; HL, hepatic lipase; CD36, cluster of differentiation 36; FATP1, fatty acid transport protein 1; FABP, fatty acid binding protein; CPT1: carnitine palmitoyltransferase 1; ACO, acyl-CoA oxidase; APOB100, apo B100. Significance was evaluated by two-tailed Student’s t test. Significant differences between the LL-LP and the other treated groups: * P<0·05; significant difference between the HL-LP and HL-HP groups: † P<0·05.
, high lipid and phospholipid (HL-HP); LPL, lipoprotein lipase; HL, hepatic lipase; CD36, cluster of differentiation 36; FATP1, fatty acid transport protein 1; FABP, fatty acid binding protein; CPT1: carnitine palmitoyltransferase 1; ACO, acyl-CoA oxidase; APOB100, apo B100. Significance was evaluated by two-tailed Student’s t test. Significant differences between the LL-LP and the other treated groups: * P<0·05; significant difference between the HL-LP and HL-HP groups: † P<0·05.
Expression of genes related to lipid synthesis in the liver of large yellow croaker fed the experimental diets
Compared with the LL-LP group, the transcript levels of sterol-regulatory element binding protein 1 (SREBP1), fatty acid synthase (FAS) and acyl-CoA: diacylglycerol acyltransferase 2 (DGAT2) were significantly increased by about 0·36-fold, 1·22-fold and 1·59-fold in the HL-LP group, respectively (P<0·05), but there were no significant differences in stearoyl-CoA desaturase 1 (SCD1) mRNA expression between these two groups (P>0·05). Dietary incorporation of high PL significantly inhibited high lipid-induced up-regulation in the transcript levels of SREBP1, FAS and DGAT2 (P<0·05). In addition, fish in the HL-HP group displayed significantly lower mRNA expression level of SCD1 than in the HL-LP group (P<0·05). The mRNA expression levels of SCD1 and DGAT2 were significantly lower in fish fed the LL-HP diet than those fed the LL-LP diet (P<0·05) (Fig. 1(b)).
Expression of genes related to fatty acid oxidation in the liver of large yellow croaker fed the experimental diets
No significant differences were observed in key genes participating in fatty acid oxidation (PPAR α (PPARα), carnitine palmitoyltransferase 1 (CPT1) and acyl-CoA oxidase (ACO)) between the LL-LP and HL-LP groups (P>0·05). Fish in the HL-HP group had significantly lower mRNA expression levels of PPARα, CPT1 and ACO compared with the HL-LP group (P<0·05). The mRNA expression levels of PPARα, CPT1 and ACO of fish fed the LL-HP diet were significantly lower than those of fish fed the LL-LP diet (P<0·05) (Fig. 1(c)).
Expression of genes related to VLDL assembly in the liver of large yellow croaker fed the experimental diets
There were no significant differences in microsomal TAG transfer protein (MTP) and apo B100 (APOB100) mRNA expression levels between the LL-LP and HL-LP groups (P>0·05). Compared with the HL-LP group, the transcript levels of MTP and APOB100 were significantly higher in the HL-HP group (P<0·05). The mRNA expression levels of these two proteins were significantly higher in the LL-HP group compared with those in the LL-LP group (P<0·05) (Fig. 1(d)).
Results for in vitro study
Effects of graded phosphatidylcholine levels on TAG contents and mRNA expression of genes related to lipid metabolism in primary hepatocytes of large yellow croaker
TAG contents decreased with the increasing levels of PC from 0 to 150 μm; although it then increased a little with the increasing levels of PC from 150 to 250 μm, TAG contents in the 200 and 250 μm PC groups were still higher than in the 0 μm PC groups (Fig. 2).
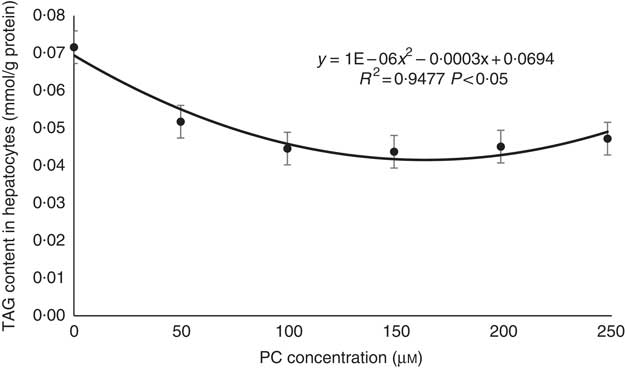
Fig. 2 Regression analyses about TAG contents in primary hepatocytes of large yellow croaker in response to graded concentrations of phosphatidylcholine (PC). Values are means (n 4), with their standard errors.
The mRNA expression levels of genes related to lipid synthesis, including SREBP1, FAS, SCD1 and DGAT2, increased with the increasing levels of PC (Fig. 3(a)).
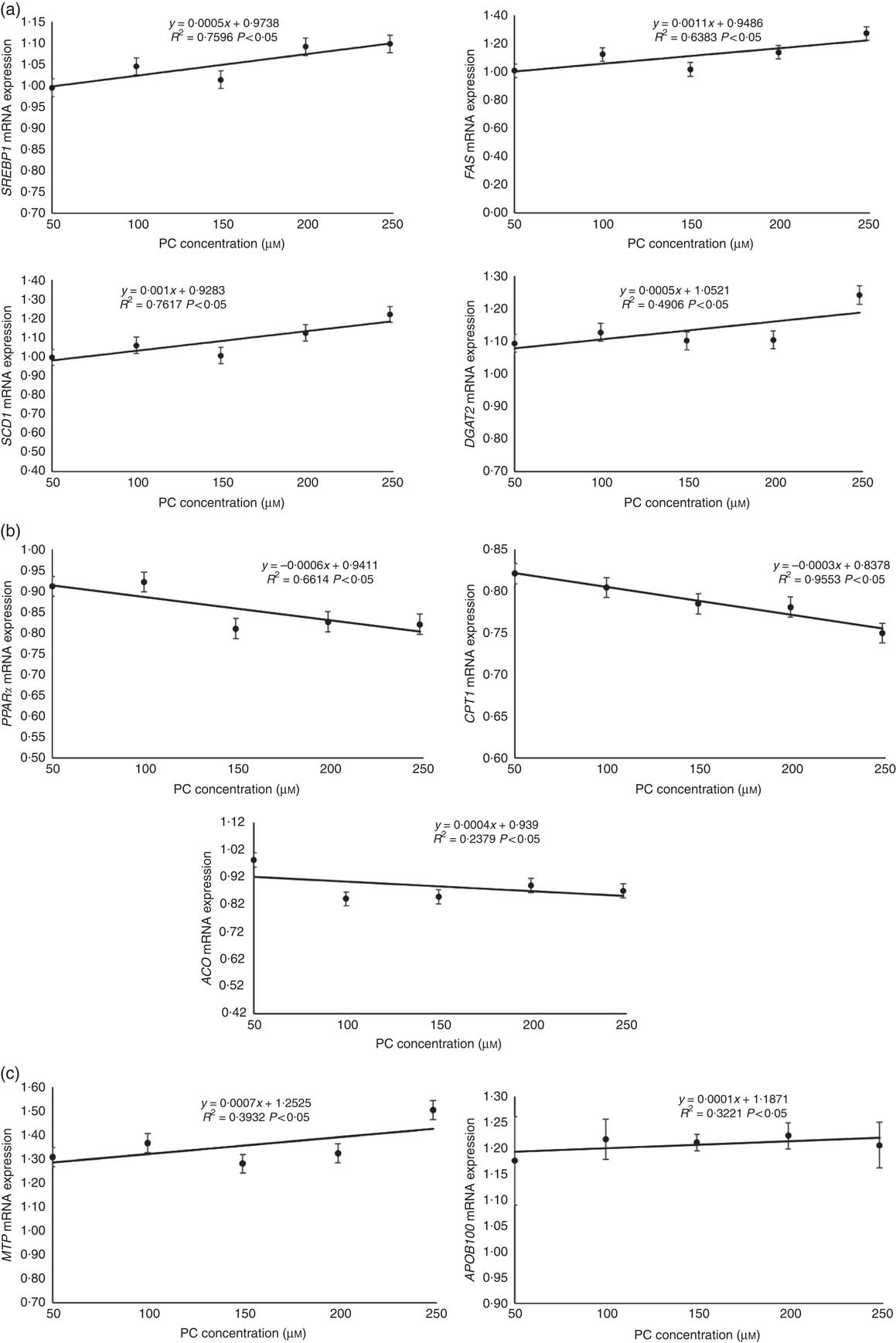
Fig. 3 Regression analyses about expression of genes related to lipid synthesis (a), fatty acid oxidation (b) and VLDL assembly (c) in primary hepatocytes of large yellow croaker in response to graded concentrations of phosphatidylcholine (PC). Values are means (n 4), with their standard errors. The relative mRNA expression of target genes in the control group was selected as the calibrator. SREBP1, sterol-regulatory element binding protein 1; FAS, fatty acid synthase; SCD1, stearoyl-CoA desaturase 1; DGAT2, acyl-CoA: diacylglycerol acyltransferase 2; CPT1, carnitine palmitoyltransferase 1; ACO, acyl-CoA oxidase; MTP, microsomal TAG transfer protein; APOB100, apo B100.
The mRNA expression levels of genes related to fatty acid oxidation, including PPARα, CPT1 and ACO, decreased with the increasing levels of PC (Fig. 3(b)).
The mRNA expression levels of genes related to VLDL assembly, including MTP and APOB100, increased with increasing levels of PC (Fig. 3(c)).
Effects of CTP: choline phosphate cytidylyltranferase α knockdown on CTP: choline phosphate cytidylyltranferase α mRNA expression, CTP: choline phosphate cytidylyltranferase α protein expression, PC and TAG contents and mRNA expression of genes related to lipid metabolism in primary hepatocytes of large yellow croaker
CCTα is the rate-limiting enzyme during de novo biosynthesis of PC in CDP-choline pathway in fish, as well as in mammals( Reference Tocher, Bendiksen and Campbell 29 ). Thus, in the present study, CCTα knockdown was applied for inhibition of endogenous PC production in primary hepatocytes. CCTα mRNA and protein levels were significantly reduced by about 50 % in primary hepatocytes transduced with siRNA-CCTα (P<0·05) (Fig. 4(a) and (b)). As a consequence, siRNA-CCTα significantly decreased PC content in primary hepatocytes, confirming the successful knockdown of CCTα in the present study (P<0·05) (Fig. 4(c)).
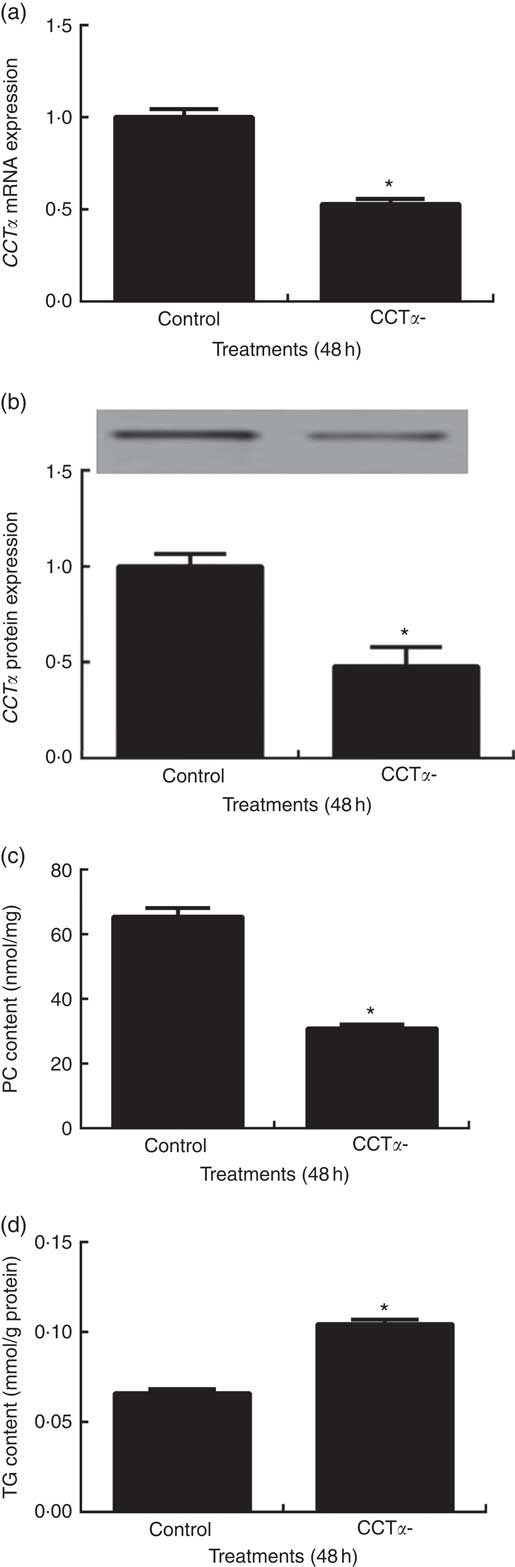
Fig. 4 Effects of CTP: choline phosphate cytidylyltranferase α (CCTα) knockdown on CCTα mRNA expression (a), CCTα protein expression (b), phosphatidylcholine (PC) (c) and TAG (d) contents in primary hepatocytes of large yellow croaker. Values are means (n 4), with their standard errors represented by vertical bars. CCTα, CTP: choline phosphate cytidylyltranferase α. Significance was evaluated by two-tailed Student’s t test. Significant difference compared with the control group: * P<0·05.
Primary hepatocytes transfected with siRNA-CCTα displayed significantly higher TAG content than those transfected with siRNA-NC (P<0·05) (Fig. 4(d)).
The mRNA levels of SREBP1, FAS, SCD1 and DGAT2 were reduced by about 0·36-fold, 0·28-fold, 0·39-fold and 0·34-fold, respectively, in primary hepatocytes transfected with siRNA-CCTα compared with those in the control group (P<0·05) (Fig. 5(a)).
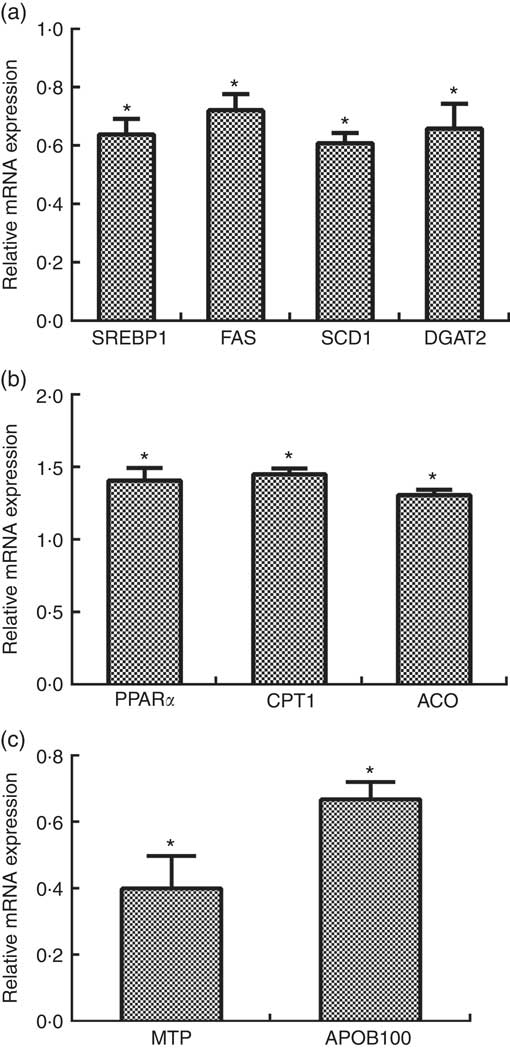
Fig. 5 Effects of CTP: choline phosphate cytidylyltranferase α (CCTα) knockdown on mRNA expression of key genes related with lipid synthesis (a), fatty acid oxidation (b) and VLDL assembly (c) in primary hepatocytes of large yellow croaker. Values are means (n 4), with their standard errors represented by vertical bars. The relative mRNA expression of target genes in the control group was selected as the calibrator. SREBP1, sterol-regulatory element binding protein 1; FAS, fatty acid synthase; SCD1, stearoyl-CoA desaturase 1; DGAT2, acyl-CoA: diacylglycerol acyltransferase 2; CPT1: carnitine palmitoyltransferase 1; ACO, acyl-CoA oxidase; MTP, microsomal TAG transfer protein; APOB100, apo B100. Significance was evaluated by two-tailed Student’s t test. Significant difference compared with the control group: * P<0·05.
The mRNA levels of PPARα, CPT1 and ACO were increased by about 0·41-fold, 0·45-fold and 0·30-fold, respectively, in the CCTαgroup compared with those in the control group (P<0·05) (Fig. 5(b)).
The mRNA levels of MTP and APOB100 were reduced by about 0·60-fold and 0·34-fold, respectively, in the CCTα group compared with those in the control group (P<0·05) (Fig. 5(c)).
Discussion
In the present study, no significant differences in survival and weight gain were observed between the LL-LP and LL-HP groups, which was consistent with studies conducted in juvenile large yellow croaker( Reference Feng, Cai and Zuo 1 ), Atlantic salmon( Reference Poston 30 ) and white sturgeon( Reference Hung and Lutes 31 ). In contrast, PL has been reported to exert beneficial effects on survival and growth performance in various fish species during their larval stage( Reference Feng, Cai and Zuo 1 , Reference Poston 30 – Reference Zhao, Ai and Mai 35 ). The possible reason for this discrepancy was that PL de novo synthesis was compromised in fish larvae, as many genes related with PL synthesis exhibited lower expression during the earlier developmental stage of fish( Reference Carmona-Antoñanzas, Taylor and Martinez-Rubio 36 ). There were also no significant differences in survival and weight gain between the LL-LP and HL-LP groups, which was supported by the findings of Yan et al. ( Reference Yan, Liao and Wang 20 ) and Wang et al. ( Reference Wang, Li and Hou 37 ) in the same species. Interestingly, in the present study, weight gain in the HL-HP group was significantly higher than in the LL-LP and HL-LP groups. An increase in FE in the HL-HP group might account for this observation. One explanation for these results was that high dose of PL could optimise lipid metabolism, and then facilitate lipid utilisation in fish fed the HL-LP diet, which in turn resulted in higher FE and better growth.
The important role of PL in alleviating abnormal lipid deposition has also been established in fish. Hepatic lipid contents markedly decreased from 21 to 16 % as dietary PL supplementation increased from 6 % to 18 % in large yellow croaker( Reference Feng, Cai and Zuo 1 ). The addition of PL to the diet caused a reduction in the number of lipid vacuoles in hepatocytes in catfish( Reference Lu, Zhao and Zhao 16 ). In this study, high dietary PL notably inhibited high lipid-induced increased hepatic lipid levels. Besides, 50 to 250 μm PC significantly reduced TG contents in primary hepatocytes and suppression in cellular PC production by CCTα knockdown resulted in an increase in TG content in primary hepatocytes. Taken together, these results from both in vivo and in vitro studies observed in the present study not only confirmed the lipid-reducing effects of PL as observed in the in vivo study but also further emphasised a key role of PC in reducing hepatic lipid accumulation. In agreement with these results in fish, in mammals, Liu et al. ( Reference Liu, Xue and Liu 10 ) found that PL significantly decreased hepatic TAG level in mice fed the high-fat diet. Similarly, the findings of Liu et al. ( Reference Liu, Shi and Liu 11 ) and Buang et al. ( Reference Buang, Wang and Cha 6 ) suggested that PC could markedly ameliorate hepatic TG accumulation in rats with non-alcohol fatty liver disease induced by orotic acid. In addition, Jacobs et al. ( Reference Jacobs, Lingrell and Zhao 15 ) reported an accumulation of intracellular TAG in CCTα-deficient hepatocytes isolated from mouse.
However, to our knowledge, the mechanisms underpinning the lipid-reducing effects of PL in fish are not fully understood. As PL has been shown to reduce hepatic lipid contents by decreasing lipid uptake and synthesis and increasing lipid catabolism and export in mammals( Reference Buang, Wang and Cha 6 , Reference Kabir and Ide 8 , Reference Liu, Xue and Liu 10 – Reference Jacobs, Lingrell and Zhao 15 ), it was postulated that for fish fed diets with PL or primary hepatocytes incubated with PC, the expression of genes related with fatty acid uptake and lipid synthesis would also be lower, and the expression of genes related with fatty acid oxidation and lipid export would also be higher, which together resulted into lower hepatic lipid contents. Thus, in the present study, the effects of PL on hepatic lipid metabolism, including fatty acid uptake, lipid synthesis, fatty acid oxidation and lipid export, were explored both in vivo and in vitro to verify our hypothesis. In the present study, compared with the LL-LP diet, the HL-LP diet significantly increased the mRNA levels of genes encoding key enzymes related with fatty acid uptake (LPL, HL, CD36, FATP1 and FABP11), which were reversed by dietary inclusion of high PL. The increased expression of these genes was assumed to enhance TAG hydrolysis in lipoproteins and fatty acid uptake( Reference Yan, Liao and Wang 20 ). Thus, these results indicated that high dietary PL might inhibit excessive fatty acid uptake in large yellow croaker liver caused by HL-LP diet at the transcriptional level, resulting in attenuated hepatic lipid accumulation. These results agreed well with the previous study in mammals demonstrating that PC reversed the increased expression of FATP and FABP in orotic acid-induced fatty liver( Reference Liu, Shi and Liu 11 ).
In the present study, high dietary PL significantly attenuated the increased SREBP1 transcript level induced by high lipid. SREBP1, as a transcription factor, can activate downstream genes dominating different steps of de novo fatty acid synthesis, such as FAS and SCD1 ( Reference Debose-Boyd and Brown 38 ). Together with the inhibition of SREBP1 mRNA abundance by high-dose PL, a concomitant marked reduction in the expression of FAS and SCD1 was observed in this study. Similar results were obtained in the studies of Buang et al. ( Reference Buang, Wang and Cha 6 ), Kabir & Ide( Reference Kabir and Ide 8 ), Liu et al. ( Reference Liu, Xue and Liu 10 ) and Liu et al. ( Reference Liu, Shi and Liu 11 ). In addition, as DGAT2 catalyses a reaction in which long-chain fatty acyl-CoA is covalently joined to diacylglycerol( Reference Cases, Stone and Zhou 39 ) to generate TAG, the suppression of fatty acid de novo synthesis by high-dose PL might reduce the substrates available for TAG production, partially characterised by decreased expression of DGAT2 caused by high-dose PL. In support of this, Ide et al. ( Reference Ide, Murata and Sunada 40 ) provided direct evidence that dietary soyabean PL appeared to reduce the availability of fatty acids for TAG synthesis in rat liver through a reduction in the rate of incorporation of [1-14C]acetate into fatty acids. Overall, these results indicated that PL might reduce hepatic lipid accumulation through inhibiting lipid synthesis at the transcriptional level. However, to our surprise, conflicting results have been observed for the expression of those genes involved in lipid synthesis in in vitro study. The mRNA expression levels of genes related to lipid synthesis, including SREBP1, FAS, SCD1 and DGAT2, increased with the increasing levels of PC. Being different from in vivo study, primary hepatocytes isolated from large yellow croaker were cultured in artificial medium relatively deficient in nutrients, such as TAG, which serves as highly reduced stores of oxidisable energy. Thus, one possible explanation for the discrepancies between in vitro and in vivo studies was that the up-regulation of lipogenic genes in primary hepatocytes might be driven by a compensation mechanism in response to low hepatic TAG contents. Similarly, the depression of lipogenic gene expression in primary hepatocytes by CCTα knockdown might be partially due to a feedback mechanism involving excessive lipid deposition. As comparable results in published articles on the manipulation of lipogenic genes in primary hepatocytes in response to PC were limited, more relevant works should be performed.
In the present study, no significant differences were detected in the mRNA expression of PPARα, CPT1 and ACO between the LL-LP and HL-LP groups. PPARα is an important transcription factor regulating the transcription of CPT1 and ACO, both of which were involved in fatty acid β-oxidation( Reference Goto, Lee and Teraminami 41 ). It was reported that PPARα deficiency led to massive hepatic lipid accumulation in response to short-term starvation or a high-fat diet( Reference Kersten, Seydoux and Peters 42 , Reference Leone, Weinheimer and Kelly 43 ). Thus, compared with the LL-LP group, lack of an increase in fatty acid oxidation in the HL-LP group might result in subsequent abnormal accumulation of TAG in the liver, which was confirmed by the findings of Wang et al. ( Reference Wang, Li and Hou 37 ) in the same fish species. The results obtained with the mRNA abundance of genes involved in fatty acid oxidation were contrary to our hypothesis. In this study, the mRNA levels of PPARα and its target genes CPT1 and ACO were markedly lower in fish fed diets with high levels of PL than those fed diets with low levels of PL regardless of dietary lipid levels. Similar to these results, the mRNA expression levels of genes related to fatty acid oxidation, including PPARα, CPT1 and ACO, decreased with the increasing levels of PC. However, previous research in mammals demonstrated that PL or PC derived from soyabean failed to affect β-oxidation-related gene expression( Reference Buang, Wang and Cha 6 , Reference Liu, Xue and Liu 10 ). These conflicting results might be attributed to different nutritional conditions and animal species. With respect to the present study, it was hypothesised that depression of PPARα, CPT1 and ACO expression might be secondary to lower lipid contents induced by high-dose PL as a feedback mechanism. Similarly, the up-regulation of fatty acid oxidation related genes caused by CCTα knockdown was probably owing to a feedback mechanism in response to massive lipid accumulation as well.
The export of lipids in liver to periphepatic tissues is mainly achieved by VLDL( Reference Sundaram and Yao 44 ). PL is a key component of VLDL in both mammals and fish( Reference Santulli, Messina and Modica 45 , Reference Sheridan 46 ). Furthermore, consistent with most vertebrates, PC appears to be the predominant PL class in fish lipoproteins( Reference Nelson and Shore 47 , Reference Chapman 48 ). Many studies in mammals have demonstrated that PL or PC play important roles in maintaining normal assembly and secretion of VLDL( Reference Fast and Vance 12 – Reference Jacobs, Lingrell and Zhao 15 ). A significant reduction in the appearance of lipoprotein particles in the lamina propria and in the size of such particles was observed in gilthead sea bream fed no soyabean PL-supplemented diets( Reference Liu, Caballero and Izquierdo 49 ). However, no study about the mRNA expression of key genes involved in VLDL assembly in response to PL or PC has been reported yet. Besides lipids, the assembly of VLDL requires APOB100 and MTP( Reference Hussain, Nijstad and Franceschini 50 ). MTP binds and chaperones lipids to the nascent APOB100 to prevent it from aberrant folding and degradation by proteasomes, which is directly related to the number of VLDL( Reference Olofsson, Boström and Andersson 51 – Reference Vergès 53 ). In the present study, the results demonstrated that the absence of enough PC could inhibit the expression of MTP and APOB100 at the transcriptional level. Moreover, additional PL or PC might facilitate VLDL assembly by increasing the expression of MTP and APOB100, which could eventually promote hepatic lipid exportation and subsequently attenuate abnormal hepatic lipid accumulation. The decreased lipid contents might subsequently regulate lipid synthesis and catabolism as a feedback mechanism as mentioned above in the in vitro study.
In summary, both in vivo and in vitro studies demonstrated that high levels of PL or PC could alleviate hepatic lipid accumulation in large yellow croaker. In addition, suppression of PC synthesis could lead to elevated lipid content in primary hepatocytes. High dietary PL might reverse the HL-LP diet-induced abnormal lipid accumulation in the liver through inhibiting fatty acid uptake and lipid synthesis, together with promoting lipid export at the transcriptional level. The in vitro study suggested that PC might affect the expression of lipid export-related genes (MTP and APOB100) to manipulate hepatic lipid deposition, which agreed well with the findings obtained in the in vivo study.
Acknowledgements
The authors are grateful to J. L. Du for his assistance in the feeding experiment.
This research was supported by the National Natural Science Foundation of China (31172425 and 31372541), the National Science Fund for Distinguished Young Scholars of China (31525024), AoShan Talents Program (2015ASTP), PhD Programs Foundation of the Ministry of Education of China (20120132110007).
Q. A. and K. M. designed the research; Z. C. conducted the research, analysed the data and wrote the paper. All authors have read and approved the final manuscript.
The authors declare that there are no conflicts of interest.












