Introduction
Members of the family Stomylotrematidae Poche, 1926 are endoparasites that are globally distributed and parasitize the digestive tract, caeca, bursa of Fabricii or cloaca of birds. Currently, the family includes 3 genera: Stomylotrema Loos, 1900, Laterotrema Semenov, 1928 and Srivastavatrema Singh, 1962 (Lotz and Font, Reference Lotz, Font, Bray, Gibson and Jones2008). The Stomylotrema genus represents the most diverse group within the family, with 17 known species. These species are morphologically characterized by the following features: broadly oval body; large, round, terminal oral sucker; well-developed, round ventral sucker; prepharynx short; well-developed pharynx; intestinal bifurcation in the middle third of the body; paired caeca extending near the posterior end of the body; paired, symmetrical testes; presence of a cirrus sac; marginal genital pore at the level of the ventral sucker; submedian, equatorial ovary; well-developed uterus; operculated eggs and presence of Laurer’s canal (Macko et al., Reference Macko, Špakulová and Casanova1999; Lotz and Font, Reference Lotz, Font, Bray, Gibson and Jones2008; Lunaschi and Drago, Reference Lunaschi and Drago2009; Pinto et al., Reference Pinto, Cantanhede, Thiengo, De Melo and Fernandez2015). In the Americas, 7 species of Stomylotrema have been recorded: S. bijugum Braun, 1901; S. fastosum Braun, 1901; S. gratiosus Travassos, 1922; S. perpastum Braun, 1902; S. tagax Braun, 1901; S. ucremium Brenes, Arroyo and Muñoz, 1966 and S. vicarium, Braun, 1901 (Szidat, Reference Szidat1964; Ostrowski, Reference Ostrowski1978; Macko et al., Reference Macko, Špakulová and Casanova1999; Pinto et al., Reference Pinto, Cantanhede, Thiengo, De Melo and Fernandez2015). Among these, S. vicarium has the widest distribution range in the Americas, extending from the central USA and Cuba to Brazil and Argentina. It has been reported as a parasite of birds from the families Accipitridae, Ardeidae, Ciconiidae, Podicipedidae, Laridae and Threskiornithidae (Macko et al., Reference Macko, Špakulová and Casanova1999; Lunaschi and Drago, Reference Lunaschi and Drago2009; Pinto et al., Reference Pinto, Cantanhede, Thiengo, De Melo and Fernandez2015). Macko et al. (Reference Macko, Špakulová and Casanova1999) performed a morphometric comparison between specimens of S. bijugum and S. vicarium recovered fromvarious definitive hosts and reported that both species exhibit significant phenotypic plasticity in all metric characteristics, such as the size of the suckers, pharynx, ovary, testes and cirrus sac. Additionally, Pinto et al. (Reference Pinto, Cantanhede, Thiengo, De Melo and Fernandez2015) mentioned the complexity of the species limits in Stomylotrema. This complexity is because most taxonomic descriptions are based on single adult specimens.
As part of our long-term studies on the biodiversity of helminth parasites of aquatic and passerine birds, digeneans belonging to Stomylotrema spp. were recovered from the intestines and cloaca of 9 bird species from 4 localities in the Mexican tropical lowlands. We performed extensive sampling, which allowed us to evaluate the morphology of 2 species, S. bijugum and S. vicarium. The objectives of the present study were as follows: (1) to provide a revised morphological description of S. bijugum and S. vicarium from new adult specimens collected from Mexico; (2) to compare morphological and molecular characteristics to investigate the phenotypic plasticity of S. bijugum and S. vicarium recovered from 9 host species; (3) to generate a haplotype network of the S. bijugum and S. vicarium specimens by using sequences of nicotinamide adenine dinucleotide dehydrogenase subunit 1 (Nad1) from mitochondrial DNA and (4) to test the phylogenetic affinities of S. bijugum and S. vicarium by using sequences of the D1–D3 domains of the large subunit (LSU) from nuclear ribosomal DNA (rDNA).
Materials and methods
Specimen collection and morphological analyses
Between 2011 and 2023, 9 bird species belonging to 4 orders from 6 families were collected in 4 localities from Mexican tropical lowlands: Himantopus mexicanus (Müller) (Charadriiformes: Recurvirostridae); Leucophaeus atricilla (L.) and L. pipixcan (Wagler) (Charadriiformes: Laridae); Mycteria americana (L.) (Ciconiiformes: Ciconiidae); Nyctanassa violacea (L.) (Pelecaniformes: Ardeidae); Eudocimus albus (L.) and Plegadis chihi (Vieillot) (Pelecaniformes: Threskiornithidae); and Pitangus sulphuratus (L.) and Tyrannus savana (Daudin) (Passeriformes: Tyrannidae) (Figure 1; Table 1). Birds were identified based on morphological characteristics using field guides for the region (Peterson and Chalif, Reference Peterson and Chalif1989; Howell and Webb, Reference Howell and Webb1995; Van Perlo, Reference Van Perlo2006), and the nomenclature follows the American Ornithologists’ Union (1996) until the 65th update (Chesser et al., Reference Chesser, Billerman, Burns, Cicero, Dunn, Hernández-Baños, Jiménez, Johnson, Kratter, Mason, Rasmussen and Remsen2024). Following the capture of the hosts, the digestive tract was removed from the body cavity of each bird and examined under a stereoscopic microscope. The digeneans were washed in 0.75% saline solution, relaxed with hot distilled water and preserved in 70% ethanol for the analyses.
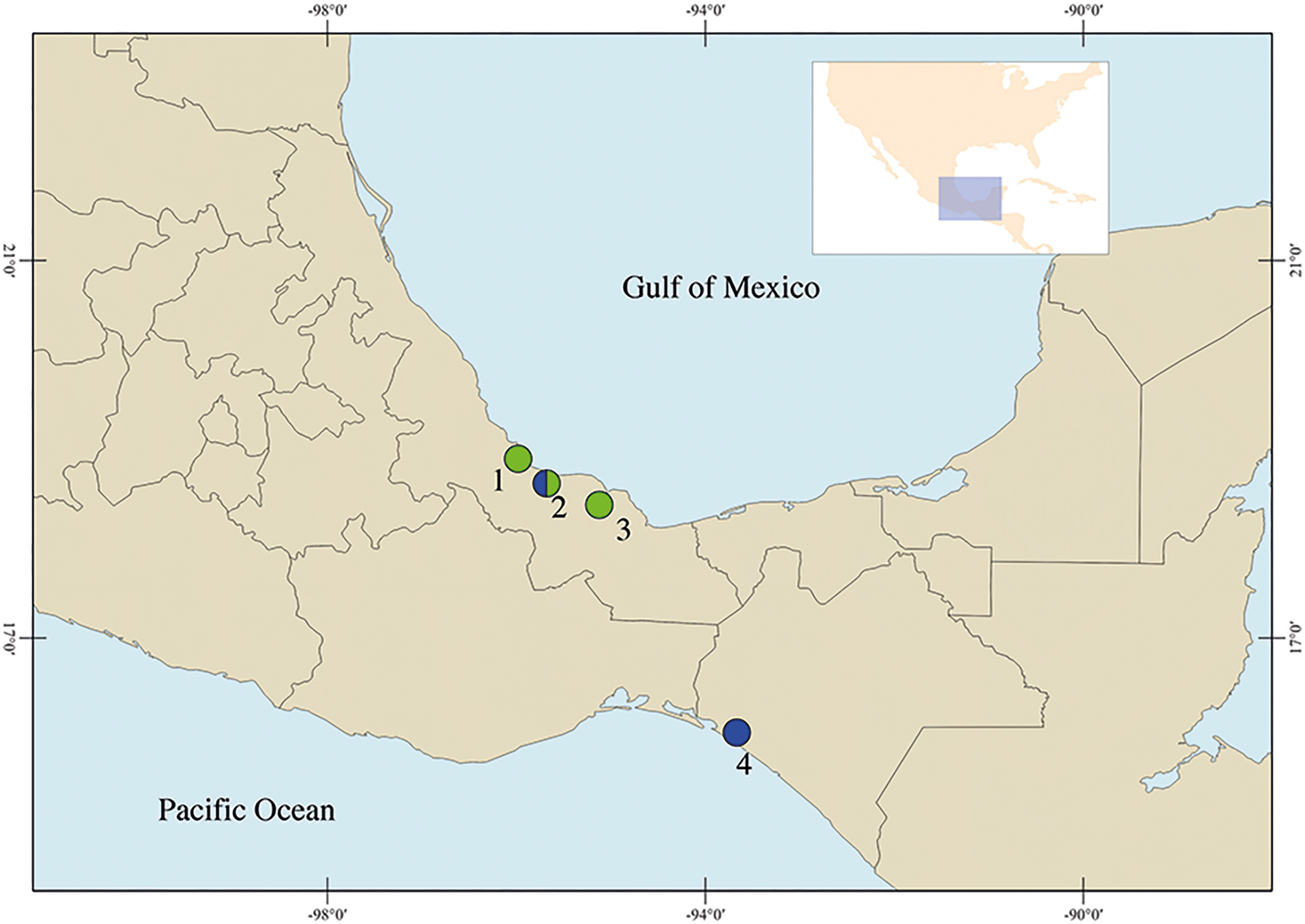
Figure 1. Sampling collection in Mexico. Veracruz: (1) Los Chivos; (2) Tlacotalpan; (3) Catemaco. Chiapas: (4) La Polka. The colours represent the species of Stomylotrema spp. recovered: in blue S. bijugum and in green S. vicarium.
Table 1. Taxa used in the present study
Localities: Veracruz: (1) Los Chivos. (2) Tlacotalpan. (3) Catemaco. Chiapas: (4) La Polka (localities in parentheses correspond with Figure 1), (M) metacercarie, (A) adult. Sequences in bold were generated in the current study.
The specimens were stained with Mayer’s paracarmine (Merck, Darmstadt, Germany) and mounted on permanent slides with Canada balsam. Digeneans were identified according to Macko et al. (Reference Macko, Špakulová and Casanova1999) and following the original descriptions. Specimens were photographed and measured using a Leica DM 1000 LED compound microscope (Leica Microsystems CMS GmbH, Wetzlar, Germany); measurements are reported in micrometres (μm). Internal morphological features were illustrated using a drawing tube attached to a Leica MC120HD microscope. Drawings were made using Adobe Illustrator 27.9 (Adobe, Inc., San Jose, CA, USA). The specimens were deposited in the Colección Nacional de Helmintos (CNHE), Instituto de Biología, Universidad Nacional Autónoma de México (UNAM), Mexico City.
Morphometric analyses
A total of 54 mature adult individuals, 36 of S. vicarium and 18 from S. bijugum, were analysed. We selected 32 morphological characters (BL, body length; BW, maximum body width; HB, hindbody; FB, forebody; OSL, oral sucker length; OSW, oral sucker width; VSL, ventral sucker length; VSW, ventral sucker width; PHL, pharynx length; PHW, pharynx width; CSL, cirrus sac length; CSW, cirrus sac width; PTL, poral testis length; PTW, poral testis width; ATL, aporal testis length; ATW, aporal testis width; OL, ovary length; OW, ovary width; FPV, field poral vitelline follicles; FAV, field aporal vitelline follicles; DPV, distance poral vitelline to anterior margin; DPP, distance poral vitelline to posterior margin; DAV, distance aporal vitelline to anterior margin; DAP, distance aporal vitelline to posterior margin; R(BL/VSL), ratio (BL/VSL); R(VSW/OSW), ratio (VSW/OSW); R(OSL/PHL), ratio (OSL/PHL); R(OSL/CSL), ratio (OSL/CSL); R(OSW/PHW), ratio (OSW/PHW); AVTW, average testes width; R(MTW/OW), ratio (MTW/OW); and R(CSW/OW), ratio (CSW/OW) (Figure 2). These measures were used in a linear discriminant analysis (LDA) by using a discrimination function that calculates the combination of a minimum number of characters necessary to separate both species sampled. In addition, a principal component analysis (PCA) was implemented to explore the morphological variation of each species analysed. These analyses were run using the ‘stats’ 3.6.2 library in R 4.1.2 (R Core Team, 2022).
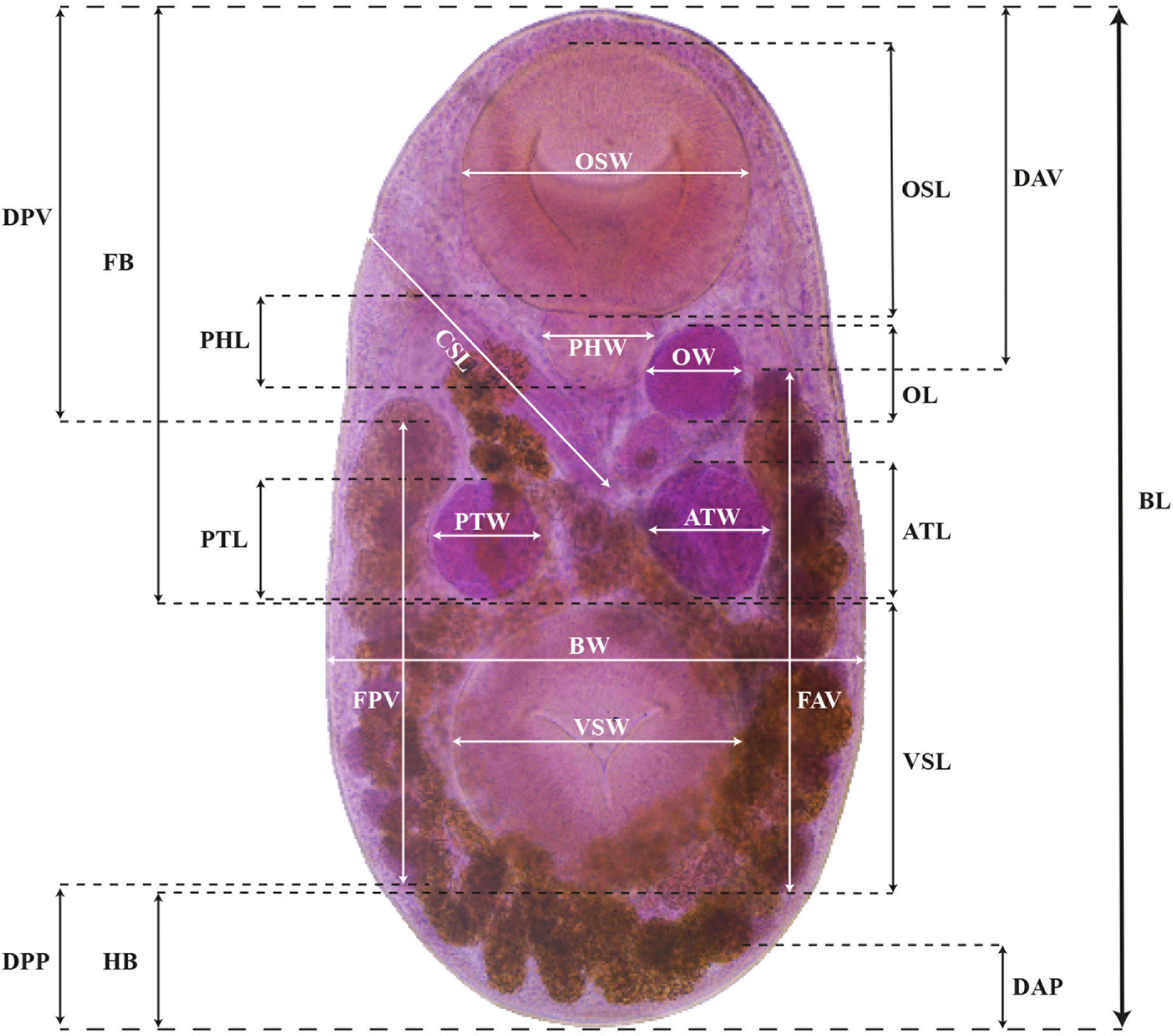
Figure 2. Photomicrograph of Stomylotrema bijugum, showing the morphological characters measured. Abbreviations as referred to in the text.
DNA extraction, amplification and sequencing
A total of 31 specimens of Stomylotrema spp. were analysed. The genomic DNA was isolated from each specimen, following the protocol described by González-García et al. (Reference González-García, Ortega-Olivares, Andrade-Gómez and García-Varela2020). The LSU of the nuclear rDNA and Nad1 were amplified using polymerase chain reaction (PCR). The LSU amplifications used forward primers 391 5’-AGCGGAGGAAAAGAAACTAA-3’ and reverse primers 536 (5’-CAGCTATCCTGAGGGAAAC-3’ (Stock et al., Reference Stock, Campbel, James and Nadler2001; García-Varela and Nadler, Reference García-Varela and Nadler2005). Additionally, the Nad1 was amplified using forward NDJ11F 5’-AGATTCGTAAGGGGCCTAATA-3’ (Morgan and Blair, Reference Morgan and Blair1998) and reverse NDJ2AR 5’-CTTCAGCCTCAGCATAAT-3’ primers (Kostadinova et al., Reference Kostadinova, Herniou, Barrett and Littlewood2003) PCR (final volume 25 μL) containing 2 μL of each primer (10 pmol μL−1), 2.5 μL of 10× buffer, 1.5 μL of 2 mM MgCl2, 2 μL of genomic DNA and 1 U of Taq DNA polymerase (Platinum Taq, Invitrogen Corporation, California, USA). PCR cycling parameters include denaturation at 94°C for 3 min, followed by 35 cycles of 94°C for 1 min; annealing at 50°C for LSU and 40°C for Nad1 for a min; and extension at 72°C for 1 min, followed by a post-amplification incubation at 72°C for 7 min. Sequencing reactions were performed with the initial primers plus 2 internal primers 503, 5’-CCTTGGTCCGTGTTTCAAGACG-3’, and 504, 5’-CGTCTTGAAACACGGACTAAGG-3’ for LSU (García-Varela and Nadler, Reference García-Varela and Nadler2005) using ABI Big Dye (Applied Biosystems, Boston, MA, USA) terminator sequencing chemistry. Reaction products were separated and detected using an ABI 3730 capillary DNA sequencer. Contigs were assembled and base-calling differences resolved using CodonCode Alligner version 12.0.1 (CodonCode Corporation, Dedham, MA, USA). Sequences were deposited in the GenBank database (Table 1).
Alignment, phylogenetic analyses, genetic divergence and haplotype network
Thirty-one new sequences of LSU were aligned with other sequences identified as Stomylotrema spp., 1 sequence of Stomylotrema pictum Creplin, 1837, 3 sequences identified as S. vicarium (KY982863, MW480895 and MF155659), plus 2 unidentified sequences of Stomylotrema sp. (MW988459 and MW988460), plus 10 sequences representing species from genera Maritrema, Microphallus and Levinseniella from the family Microphallidae and 3 sequences of Haematoloechidae, Plagiorchiidae and Telorchiidae were used as outgroups (Table 1). Sequences were aligned using the software SeaView version 4.0 (Gouy et al., Reference Gouy, Guindon and Gascuel2010) with default parameters and adjusted with the Mesquite program (Maddison and Maddison, Reference Maddison and Maddison2025). The alignment consisted of 50 sequences with 1338 nucleotides. The nucleotide substitution model was obtained using jModelTest v2.1.7 (Darriba et al., Reference Darriba, Taboada, Doallo and Posada2012), and the selection was based on the Akaike information criterion. The best model selected was GTR + I + G. Phylogenetic trees were constructed using maximum likelihood (ML) and Bayesian inference (BI) methods, using the online interface Cyberinfrastructure for Phylogenetic Research (CIPRES) Science Gateway version 3.3 (Miller et al., Reference Miller, Pfeiffer and Schwartz2010). The ML analysis was carried on with RAxML version 7.0.4 (Silvestro and Michalak, Reference Silvestro and Michalak2012) and was run with 1000 bootstrap replicates. BI analysis was inferred with MrBayes version 3.2.7 (Ronquist et al., Reference Ronquist, Teslenko, Van Der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012) and included 2 simultaneous runs of Markov Chain Monte Carlo for 10 million generations, sampling every 1000 generations, with a heating parameter value of 25% ‘burn-in’ %. Phylogenetic trees were drawn and edited using the FigTree program v. 1.4.2. (Rambaut, Reference Rambaut2012). The genetic divergences among taxa were estimated using p distances with the program MEGA version 6.0 (Tamura et al., Reference Tamura, Stecher, Peterson, Filipski and Kumar2013). To examine the haplotype frequency and relationships among the specimens of S. vicarium and S. bijugum recovered from 9 host species, a haplotype network was built with 25 Nad1 sequences by using the TCS algorithm (Clement et al., Reference Clement, Snell, Walker, Posada and Crandall2002) implemented in PopART software (Leigh and Bryant, Reference Leigh and Bryant2015).
Results
Morphological identification
Taxonomic summary
Class Trematoda Rudolphi, 1808
Order Plagiochiida La Rue, 1957
Family Stomylotrematidae Poche, 1926
Genus Stomylotrema Looss, 1900
Stomylotrema bijugum Braun, 1901
Site of infection: Cloaca
Type host: (1) Charadriiformes: Recurvirostridae: Himantopus mexicanus (Müller)
Type locality: (1) Brazil (unspecified locality).
Other localities: (2) Cuba; (3, 4) Mexico.
Records: Adult specimens, 1. Braun (1901); 2. Macko et al. (Reference Macko, Špakulová and Casanova1999) 3. CNHE 12081; 4. This study.
Other definitive hosts: Charadriiformes: Jacanidae: (3) Jacana spinosa (L.); Recurvirostridae: (4) Himantopus mexicanus (Müller); Laridae: (4) Leucophaeus atricilla (L.), Leucophaeus pipixcan (Wagler); Pelecaniformes: Ardeidae: (4) Nyctanassa violacea (L.); Threskiornithidae: (2) Platalea ajaja (L.); (4) Eudocimus albus (L.); (4) Plegadis chihi (Viellot). Passeriformes: Tyrannidae: (4) Pitangus sulphuratus (L.); (4) Tyrannus savana (Daudin).
Specimens deposited: CNHE 12336–12340
Representative DNA sequences: PV700558–PV700572 (LSU); PV738124–PV738139 (Nad1).
Redescription based on 21 specimens (Figure 3A–E). Comparative measurements from different hosts are provided in Table 2.
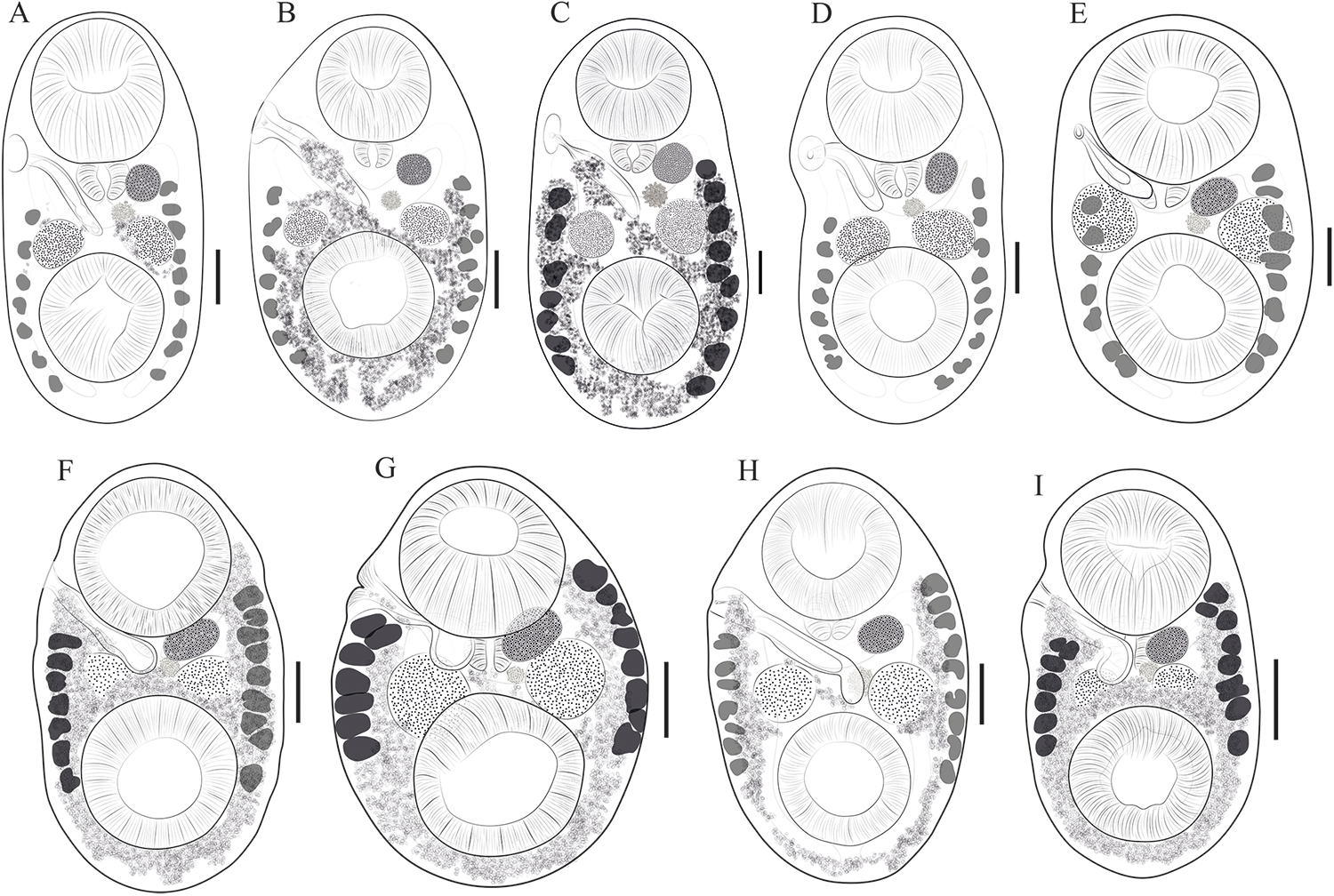
Figure 3. Drawings of Stomylotrema spp. from different hosts. (A–E) Stomylotrema bijugum; (A) Eudocimus albus from Tlacotalpan. (B) Himantopus mexicanus from Tlacotalpan. (C) Leucophaeus atricilla from La Polka. (D) Leucophaeus pipixcan from Tlacotalpan. (E) Plegadis chihi from Tlacotalpan. (F–I) Stomylotrema vicarium; (F) Eudocimus albus from Catemaco. (G) Eudocimus albus from Los Chivos. (H) Himantopus mexicanus from Tlacotalpan. (I) Plegadis chihi from Tlacotalpan. Scale bars A–I = 50 μm.
Table 2. Comparative measurements between adult specimens of Stomylotrema bijugum Braun, 1901 from different host species
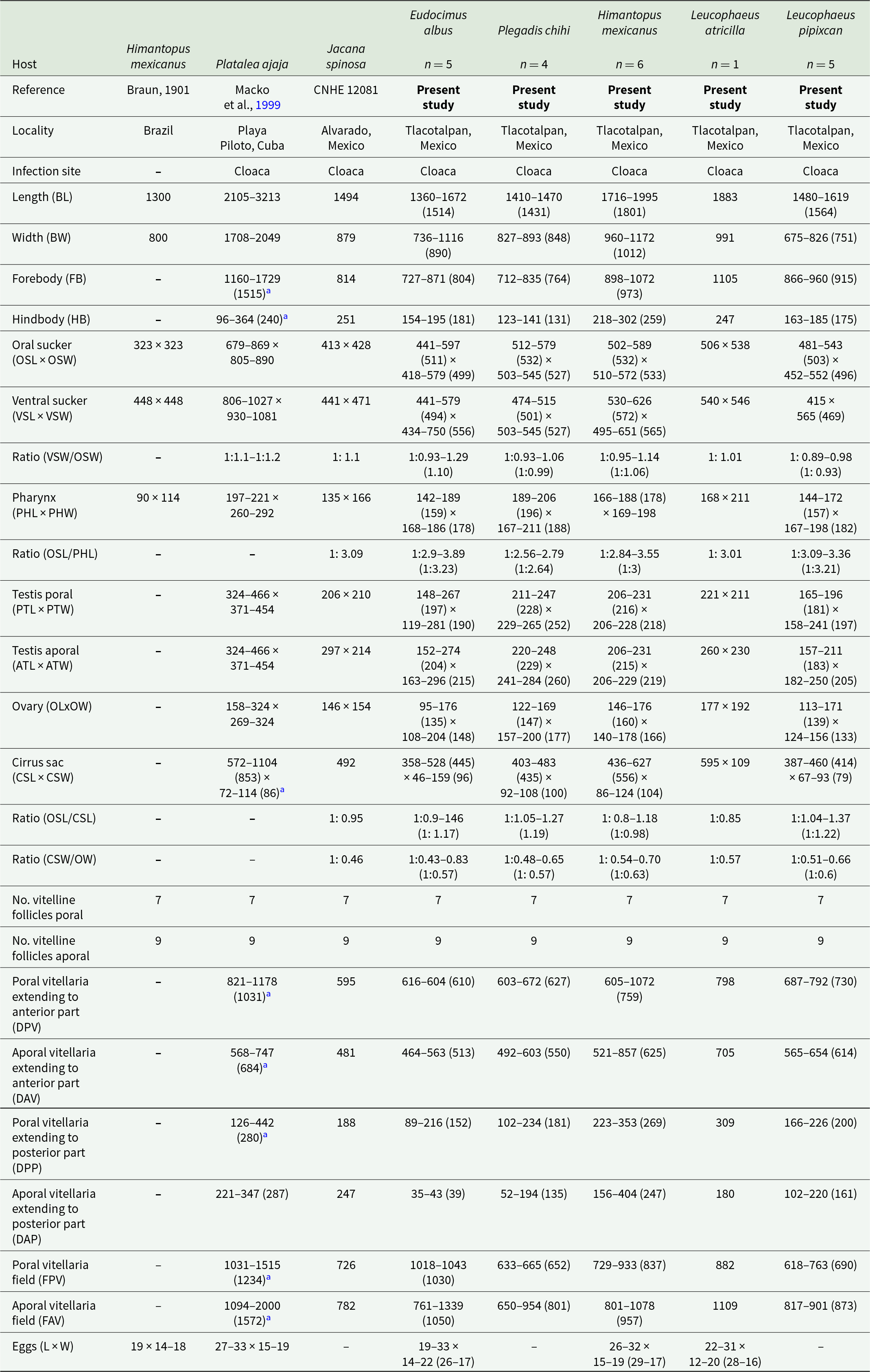
Measurements are reported in micrometres (μm).
a Measurements from the original drawing.
Morphological identification: Digeneans 1360–1995 (1638) length and 675–1172 (918) wide. Tegument unspined. Subterminal oral sucker 441–589 × 452–572 (520 × 525). Ventral sucker 415–626 × 434–750 (523 × 548). Length ratio of oral and ventral sucker 1:0.89 to 1:1.29 (1:1.01). Pharynx 142–206 (174) × 167–211 (185). Caeca sometimes overlaps the lateral part of testes, terminating blindly beyond ventral sucker, almost reaching the posterior region of the body (Figure 3A–E). Testes equatorial anterior to ventral sucker and symmetrical. Poral testis 148–267 × 119–281 (217 × 224), aporal testis 152–274 × 163–296 (219 × 233). Cirrus sac straight or j-shape, reaching mid-body between testes, containing tubular coiled internal seminal vesicle (Figure 3A–E). Genital pore on the right margin of body located anterior to pharynx. Round ovary 95–177 × 108–204 (155 × 164), situated anterior to aporal testis (Figure 3A–E). Ovary larger than pharynx and in some cases the pharynx is almost the same size of ovary. Mehlis’ gland posterior to ovary. Seven poral and 9 aporal vitelline follicles, vitellaria slightly separated, situated laterally and overlapping caeca. Poral vitelline field commencing posterior to pharynx 602–1043 (781). Aporal vitelline field commencing to level or posterior to pharynx 650–1339 (913). Distance of the first poral vitellaria to the anterior end of the body 563–1072 (700). Distance of the last poral vitellaria to the posterior end 89–353 (218). Distance of the first aporal vitellaria to the anterior end of the body 464–857 (590). Distance of the last aporal vitellaria to the posterior end 35–404 (174). Both vitelline fields terminate near to posterior margin of ventral sucker (Figure 3A–E), exceptionally extending beyond it (Figure 3C). Uterus filling body surrounded or partially overlapping reproductive organs and ventral sucker. Eggs yellow, small 19–33 × 12–22 (26 × 16).
Remarks
Our specimens, collected from E. albus, H. mexicanus, L. pipixcan and P. chihi from 2 localities in Veracruz, and L. atricilla from Chiapas, were identified as S. bijugum by having features consistent with the diagnosis of the original description of Braun (1901) and the descriptions by Macko et al. (Reference Macko, Špakulová and Casanova1999). The principal feature that distinguishes S. bijugum from other congeneric species is the horseshoe shape of the follicles and the termination of the vitelline fields near the posterior margin of ventral sucker. Additionally, our specimens showed great variability in features such as position, size and distribution of vitelline follicles, as well ovary and testes (Table 2).
Stomylotrema vicarium Braun, 1901
Site of infection: Intestine and cloaca.
Metacercaria in coelom and gizzard.
Cercariae in the digestive gland.
Type host: (1) Pelecaniformes: Threskiornithidae: Theristicus caerulenscens (Vieillot).
Type locality: (1) Brazil (unspecified locality).
Other localities: Adult specimens records: (2, 4, 5) USA; (3, 6, 8) Argentina; (7) Cuba; (9) Peru; (10, 11) Mexico. Metacercarie specimens records: (12) Argentina; (13) Brazil. Cercariae specimen record: (14) Argentina.
Records: Adult specimens, 1. Braun (1901); 2. Lumsden and Zischke (Reference Lumsden and Zischke1963); 3. Szidat (Reference Szidat1964); 4. Bush and Forrester (Reference Bush and Forrester1976); 5. Hon et al. (Reference Hon, Forrester and Williams1978); 6. Ostrowski (Reference Ostrowski1978); 7. Macko et al. (Reference Macko, Špakulová and Casanova1999); 8. Lunaschi and Drago (Reference Lunaschi and Drago2009); 9. Kanarek et al. (Reference Kanarek, Zaleśny, Sitko and Tkach2017); 10. Ramírez-Cañas et al. (Reference Ramírez-Cañas, George-Nascimento, García-Prieto and Mata-López2019); 11. This study. Metacercariae specimens, 12. Ostrowski (Reference Ostrowski1978); 13. Amato and Amato (Reference Amato and Amato2006). Cercariae, 14. Dellagnola et al. (Reference Dellagnola, Campoy-Diaz and Vega2022).
Other definitive hosts: Class: Mammalia. Didelphimorphia: Didelphidae: (10) Philander opossum (L.); Class: Aves. Galliformes: Phasianidae: (6) Gallus gallus domesticus (L.); (5) Meleagris gallopavo (L.); Podicipediformes: Podicipedidae: (7) Tachybaptus dominicus (L.); Accipitriformes: Accipitridae: (8) Busarellus nigricolis (Latham); (8) Buteogallus meridionalis (Latham); Charadriiformes: Recurvirostridae: (11) Himantopus mexicanus (Müller); Charadriidae: (6) Vanellus chilensis cayennensis (Gmelin); Laridae: (3) Larus dominicanus (Lichtenstein); Ciconiiformes: Ciconiidae: (11) Mycteria americana (L.); Pelecaniformes: Ardeidae: (7) Egretta caerulea (L.), (2) Nyctanassa violacea (L.); Threskiornithidae: (4, 11) Eudocimus albus (L.); (11) Plegadis chihi (Viellot); Passeriformes: Furnariidae: (9) Sclerurus mexicanus (Sclater).
Intermediate hosts: (12) Insecta: Coleoptera: Dytiscidae: Megadytes glaucus (Brullé); (13) Hemiptera: Belostomatidae: Belostoma dilatatum (Dufour); (14) Gastropoda: Architaenioglossa: Ampullariidae: Pomacea americanista (Ihering).
Specimens deposited: CNHE 12341–12345
Representative DNA sequences: PV700573–PV700587 (LSU); PV738140–PV738147 (Nad1).
Description based on 53 specimens (Figure 3F–I). Measurements are provided in Table 3.
Table 3. Comparative measurements between adult specimens of Stomylotrema vicarium Braun, 1901 from different host species
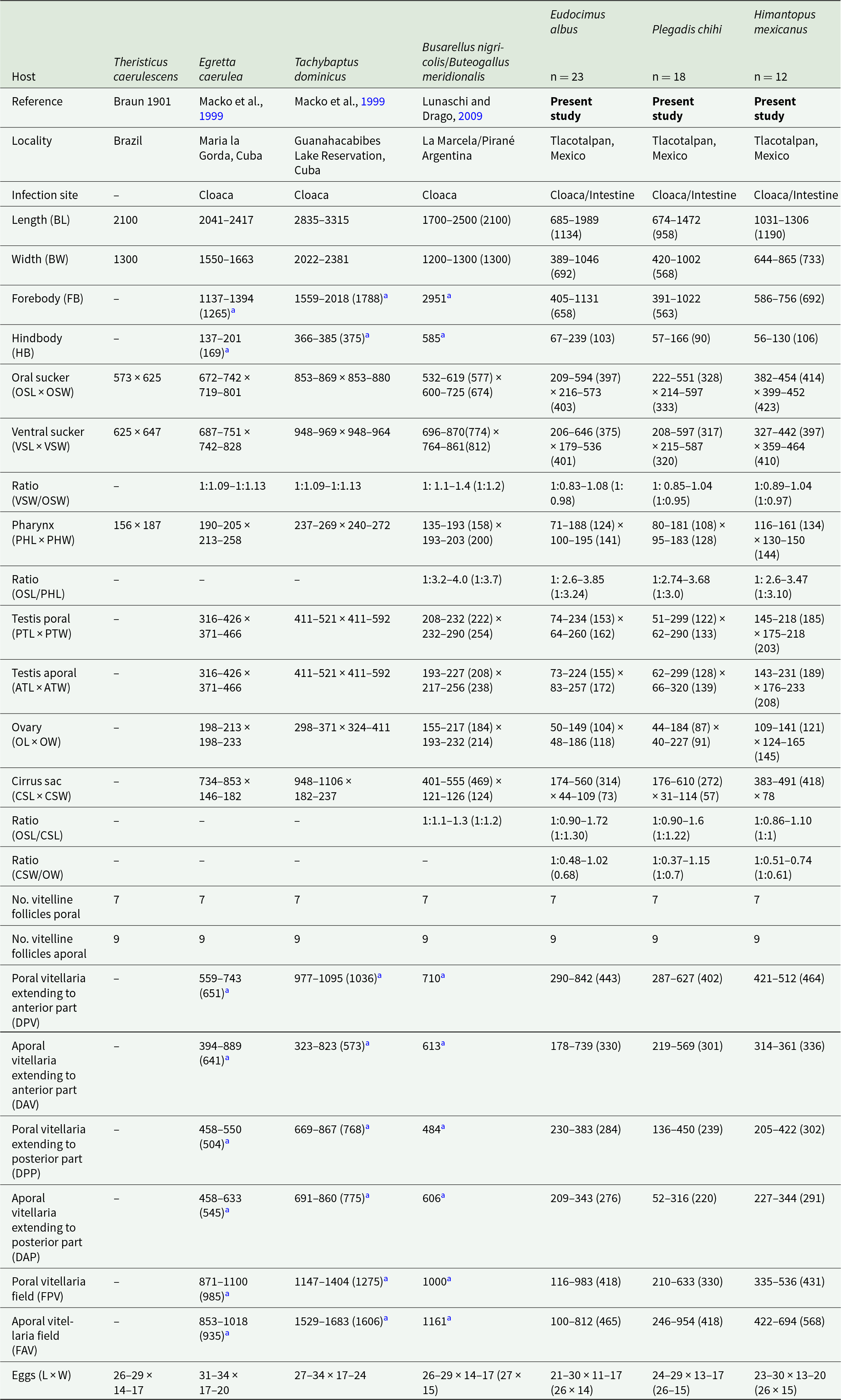
Measurements are reported in micrometres (μm).
a Measurements from the original drawing.
Morphological identification: Digeneans 862–1989 (1178) length and 534–1002 (726) wide. Tegument, unspined. Subterminal oral sucker 300–594 × 320–597 (419 × 430). Ventral sucker 288–646 × 303–587 (402 × 423). Length ratio of oral and ventral sucker 1:0.89–1.08 (1:0.98). Pharynx 83–181 × 115–195 (131 × 145). Caeca overlapping lateral part of testes, terminating posterior to ventral sucker (Figure 2F–I). Round or oval testes are symmetrical, anterior to ventral sucker. Poral testis 99–299 × 110–290 (175 × 188), aporal testis 107–299 × 116–320 (178 × 196). Cirrus sac straight or j-shape, reaching mid-body between testes and in some specimens, it ends before the poral testis, containing tubular coiled internal seminal vesicle (Figure 3F–I). Genital pore on right margin or submarginal of body located posterior to distal half of oral sucker. Round ovary 69–184 × 75–227 (116 × 136), situated anterior to aporal testis (Figure 3F–I). Mehlis’ gland posterior to ovary. Seven poral and 9 aporal vitelline follicles, vitellaria compact, situated laterally and marginal. Poral vitelline field commencing at level of the pharynx 116–983 (425). Aporal field commencing at mid-part of oral sucker 71–954 (533). Distance of the first poral vitellaria to the anterior end of the body 324–842 (459). Distance of the last poral vitellaria to the posterior end 205–450 (296). Distance of the first aporal vitellaria to the anterior end of the body 178–739 (336). Distance of the last aporal vitellaria to the posterior end 52–344 (280). Both vitelline fields terminate laterally to the mid-part of the ventral sucker (Figure 3F–I). Uterus filling body and winding around the ventral sucker. Eggs yellow, small and oval 21–30 × 11–20 (26 × 15).
Remarks
The specimens collected from E. albus, P. chihi and H. mexicanus from 3 localities in Veracruz were identified as S. vicarium by having features consistent with the original description by Braun (1901). The principal feature that distinguishes to S. vicarium from other congeneric species is the lateral and linear position of the vitelline fields.
Statistical analyses
Based on 32 morphological measurements, the LDA was performed to evaluate the morphological differentiation between Stomylotrema bijugum and S. vicarium. The coefficients of the discriminant functions indicate the contribution of each variable to the separation between the species. LD1 explains 100% of the variance, which indicates that all relevant and useful information to differentiate species is contained in a single dimension. Morphometric ratios, such as the relationship between R(OSW/PHW), R(MTW/OW), R(CSW/OW) and R(OSL/CSL), contributed the most to the separation between the species in LD1 (Figure 4A). In addition, the density distribution of LD1 values shows a clear separation between the 2 species, with S. bijugum (in red colour) clustering at negative values and S. vicarium (in blue colour) at positive values (Figure 4B), and the lack of significant overlap between the density distributions was significant, and confirms that LDA effectively differentiates these species based on morphological traits (Wilks’ Lambda = 0.045; F = 13.75, P < 0.0001).
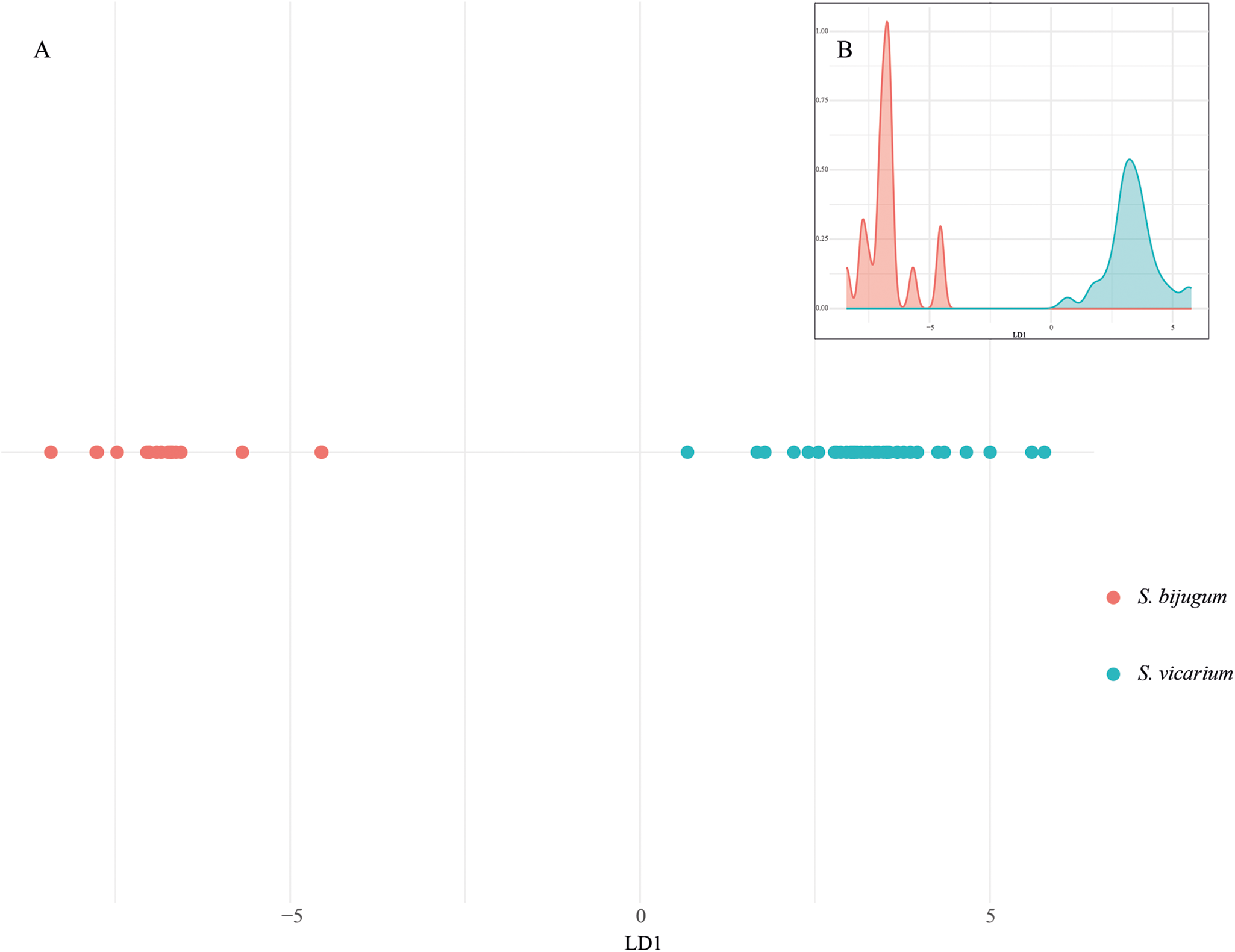
Figure 4. Statistical analyses. Discriminant analysis (A); linear discriminant analysis (B); density distribution of LD1. The colours represent the species of Stomylotrema spp. recovered: in red S. bijugum, in blue S. vicarium.
A PCA was conducted to explore the morphological differences among the isolates of S. bijugum and S. vicarium (Figure 5A and B). The measurements of specimens of S. bijugum from 6 different host species formed 3 not overlapping polygons corresponding to the 3 host species (P. chihi, H. mexicanus and L. pipixcan) addressed on this study. However, the specimens from the remaining 3 hosts (E. albus, J. spinosa and L. atricilla) did not form polygons due to the limited number of measurements (n < 3) available per host species. The first and second components explain 34.21% and 23.51% (57.72% accumulative) of the variance, respectively (Figure 5A). Conversely, the measurements of the specimens of S. vicarium from 3 different host species formed 3 polygons overlapped with each other. The first and second components explain 47.39% and 14.47% (61.86% accumulative) of the variance, respectively (Figure 5B).
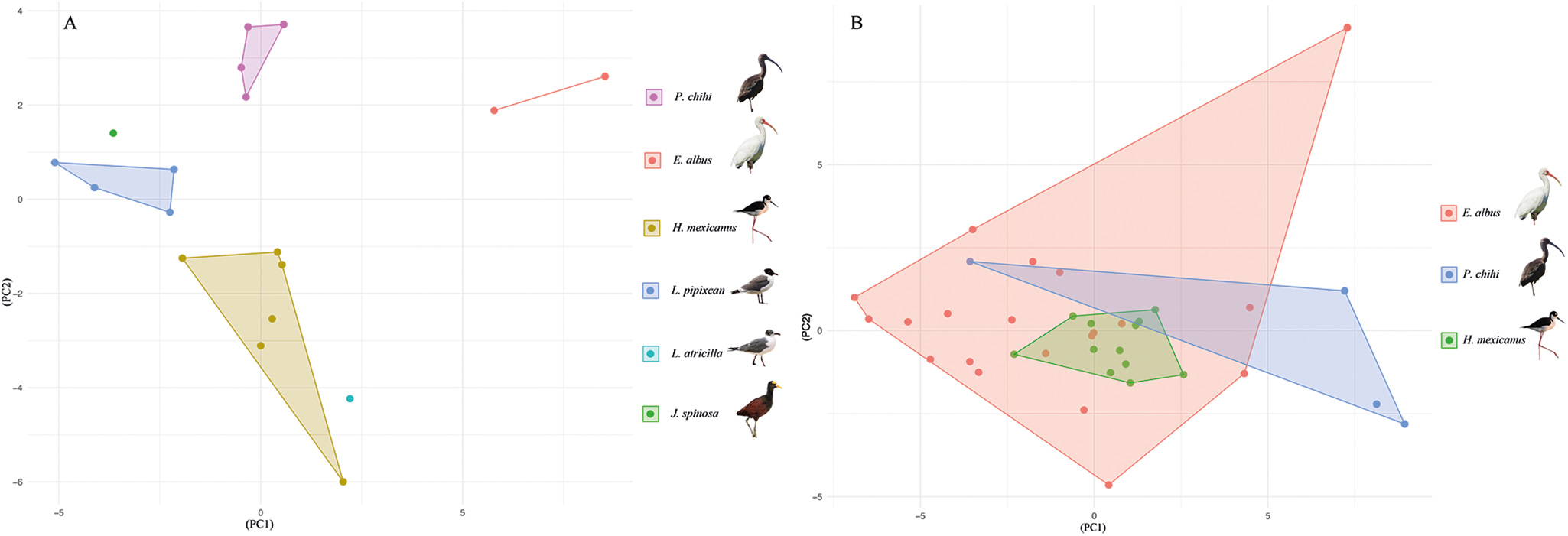
Figure 5. Principal component analysis conducted with 32 morphometric variabilities from 54 specimens of Stomylotrema spp., analysed by host species (A) S. bijugum and (B) S. vicarium.
Phylogenetic analysis and haplotype network
Phylogenetic analyses inferred with ML and IB showed that all sequences of Stomylotrema formed a clade with strong bootstrap support and Bayesian posterior probability values (Figure 6). All the sequences obtained in this study formed 2 independent subclades. The first one was formed with 15 newly sequences identified as S. bijugum, recovered from 8 host species (E. albus, H. mexicanus, L. atricilla, L. pipixcan, N. violacea, P. chihi, P. sulphuratus and T. savana from Tlacotalpan, Veracruz and La Polka, Chiapas, Mexico) and its sister species was S. vicarium (MF155659) from the grey 4-eyed opossum (Philander opossum L.) from Mexico with a weak support of bootstrap and Bayesian posterior probabilities.
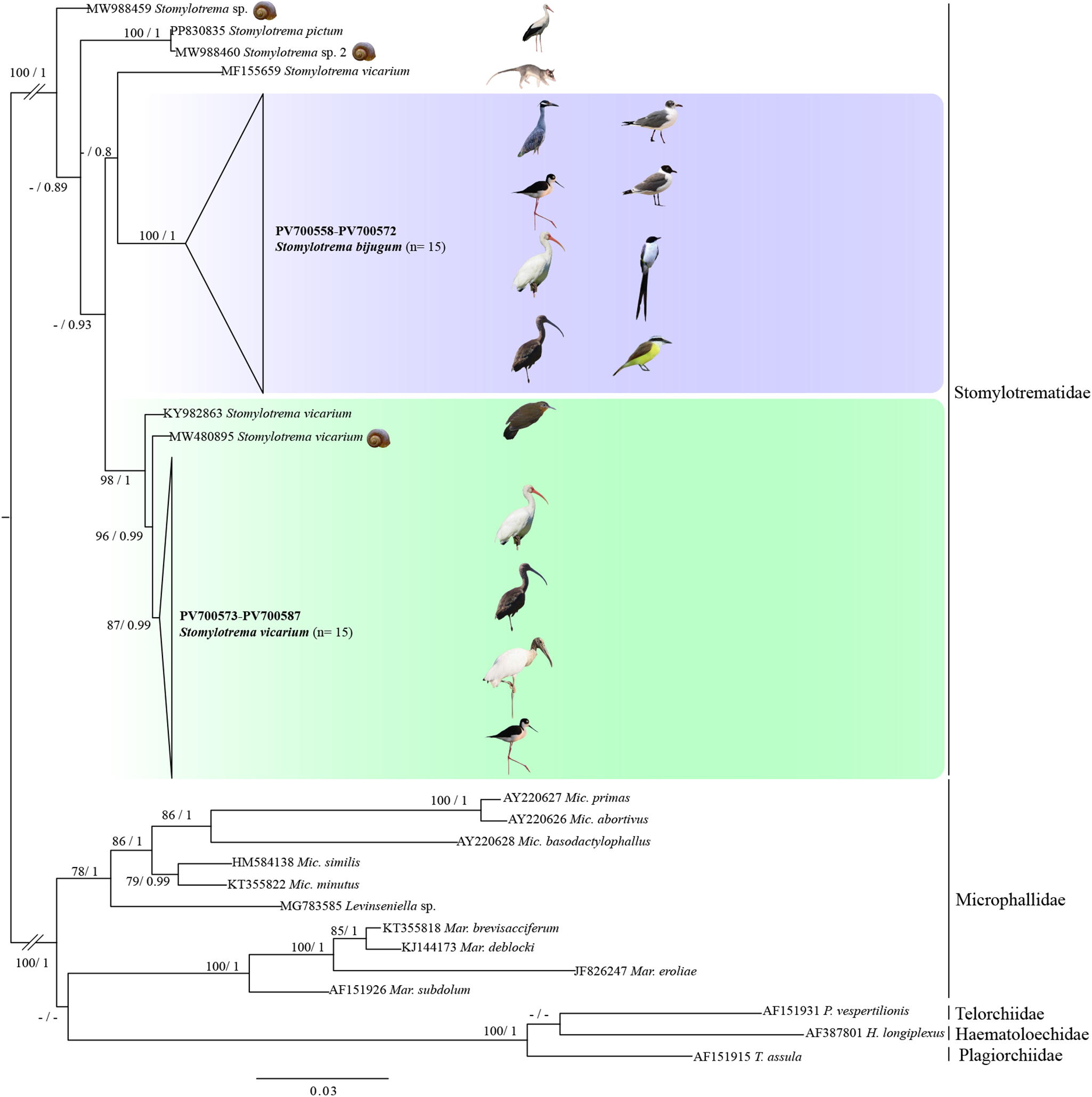
Figure 6. Phylogenetic trees inferred with maximum likelihood (ML) and consensus Bayesian inference (BI) of LSU from nuclear ribosomal DNA. Numbers near internal nodes show maximum likelihood bootstrap percentage values and Bayesian posterior probabilities. Sequences generated in this study are in bold.
The second subclade was formed with 16 newly sequences identified as S. vicarium from 4 host species (E. albus, H. mexicanus, M. americana and P. chihi from Tlacotalpan, Veracruz, Mexico), plus 2 sequences identified as S. vicarium from Peru (KY982863) and Argentina (MW480895). Two unidentified isolates of Stomylotrema sp. (MW988459-460) formed 2 independent subclades. An isolate identified as Stomylotrema sp. 2 (MW988460) from Egypt is sister to an isolate identified as S. pictum (PP830835) from Turkey (Figure 6). The genetic divergence estimated with the LSU among the species of Stomylotrema ranged from 2.2% to 4.8%, and among the newly sequences identified as S. bijugum and S. vicarium ranged from 2.6 to 4.2%. In addition, the genetic distances among our isolates of S. vicarium and S. vicarium available in GenBank (MW480895 and KY982863) ranged from 0.3% to 1.1% and between our isolates of S. vicarium and S. vicarium (MF155659) from the grey 4-eyed opossum (P. opossum) ranged 3.3% to 3.9%. Finally, among the newly sequences of S. bijugum and S. vicarium (MW480895 and KY982863) ranged from 2.7% to 3.2%, S. vicarium (MF155659) ranged from 3.6% to 4.8%. The intraspecific genetic divergence ranged 0% to 1.4% on S. vicarium and from 0% to 1.1% on S. bijugum (Table 4).
Table 4. Genetic divergence estimated among the species of Stomylotrema with the large subunit of the nuclear ribosomal DNA
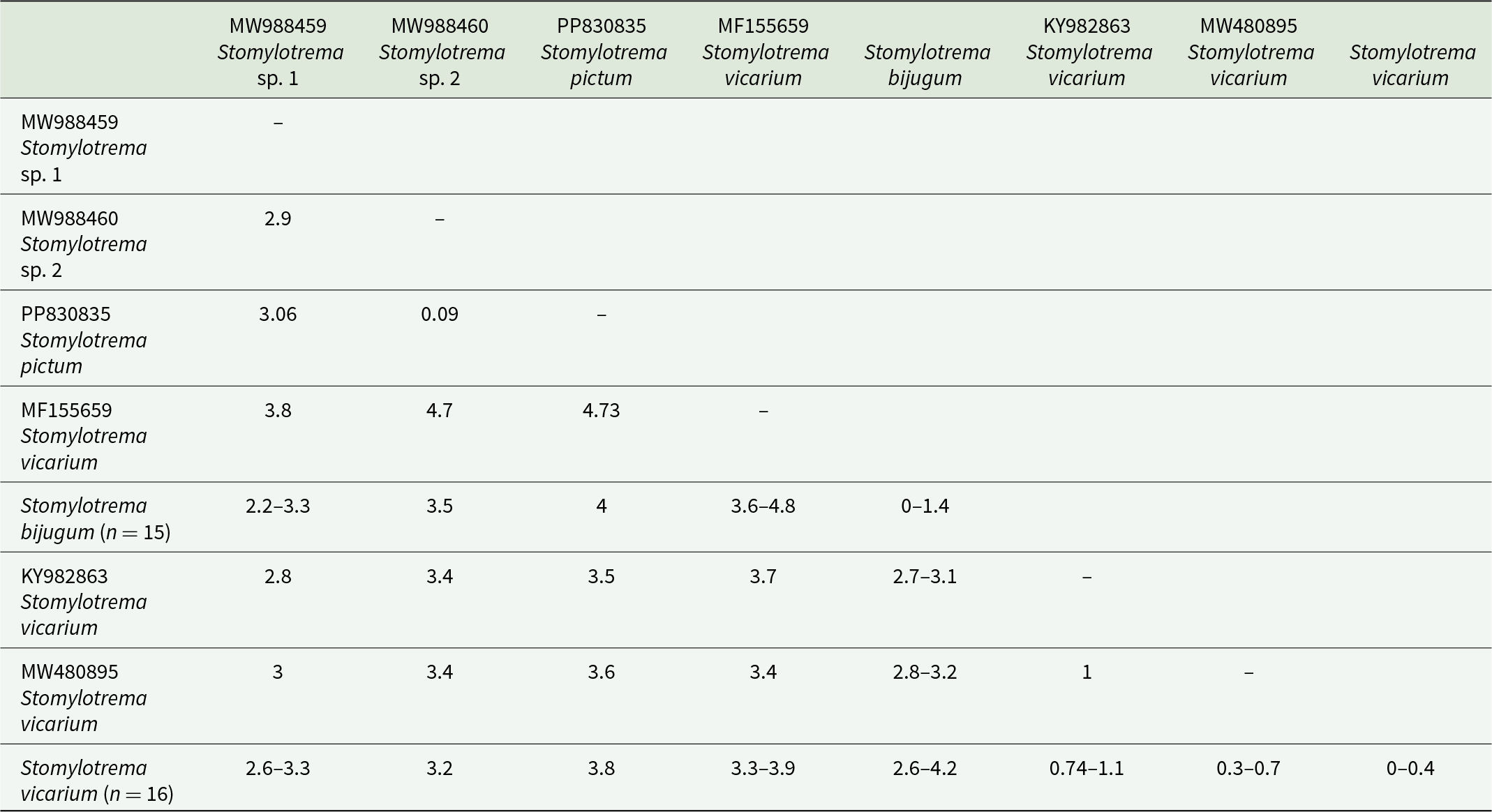
Uncorrected p distances are expressed as percentages.
The haplotype network built in this study was inferred with 24 sequences and 449 characters. The haplotype network yielded 2 subgroups representing S. vicarium and S. bijugum, clearly separated from each other by 98 substitutions. The first subgroup contained 8 specimens of S. vicarium with 4 haplotypes separated each other by a few substitutions and were shared among 3 definitive hosts sampled. The most frequent haplotype (H1, n = 4) corresponded to specimens from 2 hosts (P. chihi and H. mexicanus). The second subgroup contained 16 specimens of S. bijugum with 3 haplotypes separated each other by 1 or 2 substitutions. The most frequent haplotype (H1, n = 14) corresponded to specimens from 7 definitive hosts sampled (E. albus, H. mexicanus, N. violacea, P. chihi, P. sulphuratus, T. savana and L. pipixcan) (Figure 7).
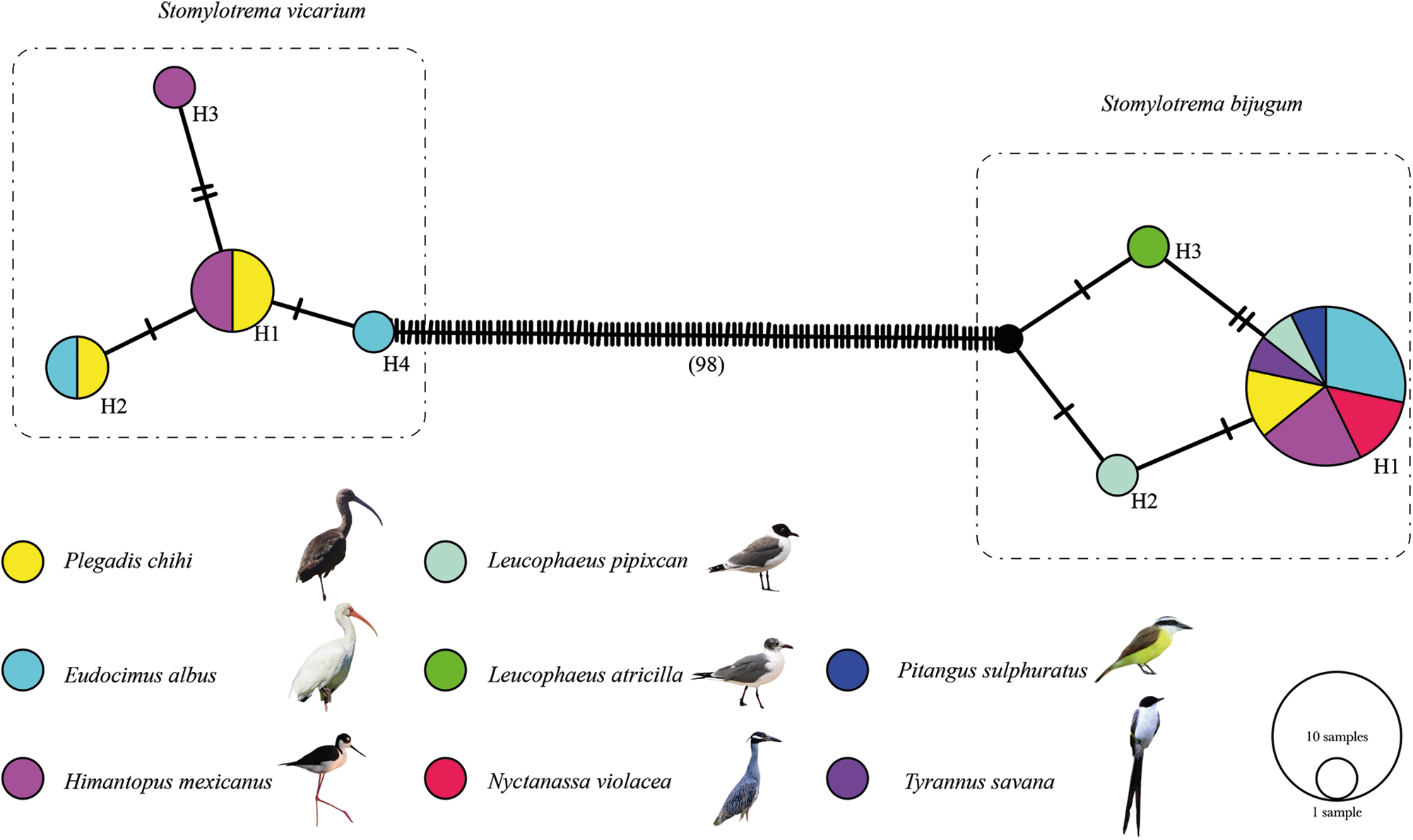
Figure 7. Haplotype network of Stomylotrema spp., built with the gene nicotinamide adenine dinucleotide dehydrogenase subunit 1 (Nad1). Each circle represents a haplotype, with size proportional to the haplotype´s frequency.
Discussion
The present study provides morphological, morphometric, molecular and ecological evidence of 2 congeneric Stomylotrema species. Adult specimens of S. bijugum and S. vicarium were recorded for the first time in aquatic and passerine birds from southeastern Mexico, revealing new hosts and geographical areas in the Americas. Our morphological observations revealed that S. bijugum can be distinguished from S. vicarium in terms of the position of the vitelline follicles. In S. bijugum, the vitellin follicles start posterior to the genital pore, reach the posterior part of the ventral sucker and end in the posterior end of the body, whereas vitellin follicles start anterior-posterior to the genital pore and end in the middle part of the ventral sucker in S. vicarium.
In addition, the LDA clearly revealed that 6 morphometric variables (the ratio between the suckers, pharynx, ovary and cirrus sac) were able to discriminate between the 2 species. The species S. bijugum was recorded from the Black Wing (Himantopus himantopus) from Brazil and later from the Roseate Spoonbill (Platalea ajaja) in Cuba (Macko et al., Reference Macko, Špakulová and Casanova1999). In the present study, S. bijugum was recorded from 5 host species in southeastern Mexico. Adult specimens were measured and compared with previous records, and the PCA clearly revealed 3 independent polygons corresponding to the specimens from the 3 host species from southeastern Mexico, suggesting host-induced phenotypic plasticity.
The species S. vicarium was recorded from plumbeous ibis (Theristicus caerulenscens) in Brazil and later from 5 host species in the USA, Argentina and Cuba (Macko et al., Reference Macko, Špakulová and Casanova1999). Ostrowski (Reference Ostrowski1978) and Macko et al. (Reference Macko, Špakulová and Casanova1999) noted that S. vicarium is a species with a high level of morphological variability caused by the age of the parasite. In the present study, adult specimens of S. vicarium from 3 host species were evaluated and compared with previous descriptions. Our observations and morphometric data revealed morphological differences among the specimens sampled from the 3 host species. The PCAs revealed 3 clustered together, suggesting that those specimens were morphometrically homogeneous.
The application of molecular analyses to distinguish species of the genus Stomylotrema has rarely been addressed, but it is key to the delineation of the species. Therefore, in this study, sequences of Nad1 from the mitochondrial gene were generated and analysed. The haplotype network analysis of Nad1 sequences predicted with 25 sequences revealed the presence of 2 clusters belonging to S. bijugum and S. vicarium, which were separated from each other by 98 substitutions, confirming that both clusters belong to 2 species. In addition, our phylogenetic analyses inferred with the LSU sequence from nuclear rDNA confirmed that our specimens identified as S. bijugum and S. vicarium formed 2 independent subclades. The phylogeny presented here has suggested that 2 isolates identified as S. vicarium whose sequences are available in GenBank (MW480895 and KY982863) from Argentina and Peru, respectively, were placed in a clade together with our S. vicarium specimens, confirming that all the specimens are conspecific. Another isolate from the grey 4-eyed opossum (Philander opossum L.) in Mexicoidentified as S. vicarium available in GenBank (MF155659) represents in this study a lineage independent of both S. vicarium and S. bijugum, suggesting that this isolate may correspond to a new species. However, this specimen was not deposited in any collection, and the report could not be verified.
The level of genetic divergence found among the Stomylotrema species could provide additional evidence for the delineation of these species. The intraspecific genetic divergence among 15 isolates of S. bijugum was very low, ranging from 0% to 1.4%; among the 16 isolates of S. vicarium, the intraspecific genetic divergence ranged from 0% to 0.4%; among our isolates of S. vicarium and S. vicarium available in GenBank (MW480895 and KY982863), the intraspecific genetic divergence ranged from 0.3% to 1.1%; between our isolates of S. vicarium and S. vicarium (MF155659) and the specimen isolated from the grey 4-eyed opossum (P. opossum), the intraspecific genetic divergence ranged from 3.3% to 3.9%; and between the new sequences of S. bijugum and S. vicarium, the intraspecific genetic divergence ranged from 2.6% to 4.2%. The percentage of interspecific genetic divergence found is similar to that reported in other species of the family Microphallidae (sister to Stomylotrematidae), ranging from 1.0% to 9.3%, from 5.25% to 7.92% and from 1.5% to 3.3% among species of Maritrema (Presswell et al., Reference Presswell, Blasco-Costa and Kostadinova2014; Hernández-Orts et al., Reference Hernández-Orts, Capasso, Pinacho-Pinacho and García-Varela2020; Aldama-Prieto et al., Reference Aldama-Prieto, González-García, Mendoza-Garfías, Pérez-Ponce and García-Varela2024), or among species of Microphallus, ranging from 1.1% to 7% (Galaktionov and Blasco-Costa, Reference Galaktionov and Blasco-Costa2018) and from 6.5% to 11 % (Kudlai et al., Reference Kudlai, Cutmore and Cribb2015).
Ecological evidence suggests that S. bijugum and S. vicarium have low host specificity and may have a wide range of definitive hosts, facilitating their dispersion and distribution in the Americas. The life cycle of S. bijugum is unknown. However, the life cycle of S. vicarium was recently characterized by combining morphological and molecular data. Dellagnola et al. (Reference Dellagnola, Campoy-Diaz and Vega2022) reported that the apple snail (Pamacea americanista) serves as the first intermediate host and that invertebrates, such as the coleopter (Megadytes glaucus) and hemipter (Belostoma dilatatum), serve as second intermediate hosts, which are eaten by several birds that serve as definitive hosts (Ostrowski, Reference Ostrowski1978; Amato and Amato, Reference Amato and Amato2006), and that mammals also serve as definitive hosts of species of Stomylotrema (Ramírez-Cañas et al., Reference Ramírez-Cañas, George-Nascimento, García-Prieto and Mata-López2019). In this study, birds from the families Ardeidae, Laridae, Threskiornithidae and Tyrannidae were recorded as definitive hosts to S. bijugum and S. vicarium, suggesting that the life cycle of both species addressed in this study can be completed in southeastern Mexico.
Acknowledgements
M.T.G.-G. thank the support of the Programa de Posgrado en Ciencias Biológicas, UNAM and CONACYT (CVU 956064). We thank Laura Márquez and Nelly López Ortiz from LaNabio for their help during the sequencing of the DNA fragments. We thank Luis García Prieto for the loan of material of CNHE and for providing bibliography.
Author contributions
M.T.G.-G., M.G.-V. and M.P.O.-O. conceived and designed the study. M.T.G.-G., V.P.-M. and M.P.O.-O. conducted data gathering. M.T.G.-G. and V.P.M. performed phylogenetic analyses. C.A.R.-M., A.L.S.-U. and M.P.O.-O. performed morphological analyses. M.T.G.-G., M.G.-V. and M.P.O.-O. wrote and edited the article.
Financial support
This research was supported by the Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT-UNAM) IN204425 to M.G.-V.
Competing interests
The authors declare there are no conflicts of interest.
Ethical standards
The sampling in this work complies with the current laws and animal ethics regulations of Mexico. Specimens were collected under the Cartilla Nacional de Colector Científico (FAUT 0202) issued by the Secretaría del Medio Ambiente y Recursos Naturales (SEMARNAT) to M.G.-V.















