Postprandial hyperglycaemia and high glycaemic index (GI) diets in humans play a major role in the development of type 2 diabetes( Reference Hodge, English and O’Dea 1 – Reference Steven, Ahn and Juhaer 4 ), and furthermore low GI diets show favourable changes in health markers such as plasminogen activator inhibitor-1( Reference Järvi, Karlström and Granfeldt 5 ), glycated proteins( Reference Brand-Miller, Hayne and Petocz 6 , Reference Livesey, Taylor and Hulshof 7 ) and fasting blood glucose, especially in those with already elevated values( Reference Livesey, Taylor and Hulshof 7 ). Recent meta-analyses report that low-carbohydrate and low-GI diets have promising effects in diabetes management( Reference Ajala, English and Pinkney 8 ). Strategies to reduce the GI of foods, even without altering the total carbohydrate content, are therefore of growing interest for reducing diabetes risk. The GI and response depend on several related factors including the nature and amount of carbohydrate, the rate of carbohydrate digestion in the gastrointestinal tract, the rate of absorption of the resulting glucose, the insulin response to the absorbed sugar and the intrinsic insulin sensitivity( Reference Augustin, Franceschi and Jenkins 9 ). The presence of naturally occurring polyphenols has been associated with low GI foods for many decades( Reference Thompson and Yoon 10 ). Fibre can also play a role in reducing hyperglycaemia by delaying glucose absorption, increasing insulin secretion and sensitivity, and binding of bile acids( Reference Scazzina, Siebenhandl-Ehn and Pellegrini 11 ). In addition, soluble fibre attenuates postprandial glucose by increasing the viscosity in the gastrointestinal tract, which disturbs carbohydrate breakdown and glucose absorption( Reference Dikeman and Fahey 12 ). Possible mechanisms by which polyphenols may affect postprandial glycaemia are the inhibition of carbohydrate digesting enzymes and glucose transporters, stimulation of pancreatic β-cells to secrete insulin, activation of insulin receptors, modulation of the release of glucose from the liver and effects on intracellular signalling pathways and gene expression( Reference Hanhineva, Torronen and Bondia-Pons 13 , Reference Williamson 14 ). The potential action of polyphenols can be compared with that of acarbose, an α-glucosidase and α-amylase inhibitor, which reduces diabetes risk( Reference Hanefeld, Schaper and Koehler 15 ). The Study To Prevent Non-Insulin dependent Diabetes Mellitus trial in impaired glucose tolerant (IGT) subjects showed a 36 % risk reduction in the progression to diabetes after treatment with acarbose( Reference Chiasson 16 ). The use of diet-related intervention either on its own or in combination with acarbose would be an alternative to the use of acarbose alone, which can lead to side-effects such as flatulence, nausea and diarrhoea.
Some polyphenols inhibit starch-digesting enzymes (α-amylase and α-glucosidase) in addition to the glucose transporters SGLT1 (SLC5A1) and GLUT2 (SLC2A2)( Reference Hanhineva, Torronen and Bondia-Pons 13 ). Most intervention studies carried out so far have focused on the effect of polyphenols with the endogenous carbohydrates already present in the food; however, addition of polyphenols and fibre to reduce the GI of that food has not been fully explored, but it is the normal way in which most foods are consumed – that is, as a total meal in combination with other foods. We therefore tested the hypothesis that a combination of components in the diet (polyphenols and fibre), capable of inhibiting the different stages of starch digestion, would reduce postprandial blood glucose and insulin using a randomised, controlled, single-blind, cross-over intervention. The test diet consisted of an α-glucosidase inhibitor (green tea), α-amylase inhibitors (green tea, blackberry, blackcurrant and strawberry) and glucose transport inhibitors (apple peel and strawberry), with all fruits providing fibre as well.
Methods
Subjects
The recruitment of subjects was carried out at the University of Leeds, School of Food Science and Nutrition, Leeds, UK. Poster advertisements around the University of Leeds notice boards were used to recruit interested potential volunteers, who were then screened for fasting blood glucose (required to be between 3·9 and 5·9 mmol/l). They were then asked to assess themselves using criteria to ensure that they could be classified as healthy and free of symptomatic disease. The eligibility criteria were as follows: aged 18–75 years, apparently healthy, not diabetic, not on long-term prescribed medications, not pregnant or lactating and not on a special diet (e.g. for losing weight or fruit supplements). The preferred order of consumption was reference meal at the first and last visit, with randomised consumption of the high and low dose on their second and third visits. However, because of unavailability of some volunteers, who started late and required a break of 3 weeks after two visits, the order was changed for them to reference, high/low dose, reference, then low/high dose, in order to start with the control meal after a break (Fig. 1). In total, sixteen healthy volunteers aged 26 (sd 4) years with BMI of 24 (sd 3) kg/m2 gave their written informed consent and completed the four study visits as shown in Fig. 1. The fasting plasma glucose and insulin concentrations were 4·8 (sd 0·4) mmol/l and 24 (sd 10) pmol/l, respectively.

Fig. 1 Participant flow diagram. Block randomisation was used to generate the allocated sequences, which were assigned to participant codes. Sequences were automatically allocated to participants according to the participant codes.
Study design
A randomised, controlled, single-blind, cross-over intervention was carried out on a total of sixteen healthy volunteers with the primary outcome of postprandial blood glucose AUC. Because of the nature of the test meals, it was impossible to blind participants. However, analysis of the plasma samples was blinded and was only unblinded after data analysis. Subjects were cannulated to ensure comfortable collection of blood samples. Each participant had four visits, two of which were reference meals and two visits were test meals (single and double dose of polyphenol- and fibre-rich food (PFRF) in a randomised pattern).
Test meals
All meals contained 109·0 (sd 1·2) g of white bread (50 g available carbohydrate as analysed by the method of Englyst( Reference Englyst, Kingman and Cummings 17 )). The higher dose consisted of 1 g green tea powder in 200 ml water, with 20 g each of apple peel, blackberry, blackcurrant and strawberry freeze-dried powders mixed with water to make a paste and spread on the bread. The reference meal included 0·8, 5·4 and 8·6 g of sucrose, glucose and fructose, respectively, dissolved in 200 ml water to standardise the amounts of sugars present in the extracts of the high dose. The volunteers consumed the reference meal on two of the visits to determine any variability in the measurements( Reference Brouns, Bjorck and Frayn 18 ). The lower dose of the test meal contained half the amount of fruits and green tea with half the amounts of balancing sugars dissolved in 200 ml water to equalise the amount of sugars present in all doses. A PFRF containing polyphenols that are effective inhibitors of different stages of starch digestion and absorption was used in this study. It comprised of an α-glucosidase inhibitor (green tea)( Reference Honda and Hara 19 – Reference Matsui, Tanaka and Tamura 21 ), α-amylase inhibitors (green tea, blackberry, blackcurrant and strawberry)( Reference Forester, Gu and Lambert 22 – Reference McDougall and Stewart 24 ) and glucose transport inhibitors (apple peel and strawberry)( Reference Hossain, Kato and Aoshima 25 – Reference Manzano and Williamson 27 ), with all fruits providing some fibre. The PFRF components were analysed for total polyphenol content, specific major polyphenols and for α-amylase and α-glucosidase inhibition in vitro.
Materials
Human salivary amylase, acetone extract of rat intestine, sucrose, maltose, glucose, fructose and glucose hexokinase reagent were from Sigma-Aldrich Co. Ltd. Freeze-dried fruit extracts were from Healthy supplies, UK, and green tea powder was from Nestlé Research Centre. The insulin immunoassay kit was from Mercodia AB.
Study protocol
The University of Leeds, Faculty of MaPS and Engineering Ethics Committee (MEEC) approved the study protocol (MEEC 12-037), and the protocol was registered with ClinicalTrials.gov, ID number NCT01994135. Each participant had one visit per week, and hence completed the study in four weeks, with body weight and height measurements taken on the first visit. On each visit, a cannula was inserted in the forearm of each subject. A fasting blood sample was collected, and after that the volunteer consumed the meal; the timer started upon first bite or sip. The volunteers consumed the whole meal, and blood samples were collected after 15, 30, 45, 60, 90, 120, 150 and 180 min. Neither harm nor side-effects occurred during the consumption of the meals. Blood samples were collected in fluoride/oxalate and EDTA tubes for glucose and insulin measurements, respectively, and immediately placed on ice. The tubes were then centrifuged within 15 min at 4000 g at 4°C for 15 min. Thereafter, plasma samples were placed in storage tubes and stored at −80°C. Plasma glucose concentrations were determined using hexokinase linked to NADH oxidation (Sigma-Aldrich) and insulin concentrations by immunoassay.
Statistical analysis
The incremental areas under the glucose curves (IAUC) were calculated for each subject for each visit using the trapezoidal rule. Data were analysed using the two-tailed paired t test analysis, and the results were confirmed using the one-factor repeated-measures ANOVA between the two references, reference and dose 1, reference and dose 2 and between lower and higher doses. Sample size was determined by designing the trial to have 90 % power to detect a clinical difference of 15 % IAUC between test and reference meals. The study required fifteen participants each for reference and test meals. With participants being controls of themselves, a minimum of fifteen participants was required.
Enzyme assays in vitro
Green tea and fruit extracts were tested separately in vitro to determine the inhibition of starch-digesting enzymes. Measurement of human salivary α-amylase inhibition was carried out as described previously( Reference Nyambe-Silavwe, Villa-Rodriguez and Ifie 28 ). In brief, sugars were removed from the fruit extracts using oasis max 3-cc cartridges. The sugar-free extracts, in water, were used as the inhibitor stock for the experiments. The 500-µl assay volume consisted of 200 µl of amylose or amylopectin, 50 µl of PBS and 50 µl of the inhibitor, and the assay was started by adding 200 µl of 1·25 U/ml human salivary α-amylase (Sigma-Aldrich Co. Ltd). After 10 min of incubation at 37°C, the reaction was stopped by placing the tubes in a water bath at 100°C. The tubes were cooled to room temperature, and solid-phase extraction (SPE) was used to remove polyphenols from the assay contents before the addition of 1 ml 3,5-dinitrosalicylic acid (DNS), as some polyphenols can interact with DNS, and thus interfere with the reaction( Reference Nyambe-Silavwe, Villa-Rodriguez and Ifie 28 ). A plate reader was used to measure the absorbance at 540 nm, and inhibition was calculated as a percentage of the control.
The inhibition of rat α-glucosidase method was adapted from Adisakwattana et al.( Reference Adisakwattana, Charoenlertkul and Yibchok-anun 29 ). The apparent K m values for sucrose, iso-maltose and maltose were determined and calculated using the Lineweaver–Burk plot with a chosen enzyme concentration and incubation times giving linear rates of reaction. The K m values obtained were 16, 6 and 3 mm for sucrose, iso-maltose and maltose, respectively, and these were subsequently used as the substrate concentrations in the assays. The assay consisted of substrate (200 µl of sucrose, iso-maltose or maltose), sodium phosphate buffer (50 µl, 10 mm), inhibitor or extra buffer (50 µl), and the reaction was started by adding 200 µl of acetone-derived protein intestinal extract from rat intestine (20 mg solid/ml for sucrose and iso-maltose and 4 mg solid/ml for maltose). After incubation at 37°C for 20 min, the reaction was stopped by placing the tubes in a water bath at 100°C for 10 min. After cooling to room temperature, SPE was used to remove polyphenols, and the resulting solution was analysed for glucose at 340 nm in a plate reader using hexokinase, which catalyses NADH reduction. Inhibition was calculated as a percentage of the control.
Total polyphenols by Folin’s assay
Extracts from the fruits were analysed for total polyphenols using Folin’s assay( Reference Singleton, Orthofer and Lamuela-Raventos 30 ), including a control for each sample to account for any interference from, for example, ascorbic acid, and data were expressed as µg/mg gallic acid equivalents. To 15-ml falcon tubes, 1 ml of each solution (standards and samples) was added. The assay was conducted by adding 5 ml of the Folin–Ciocalteu reagent to all the samples and standards. The tubes were capped, votexed and 4 ml of sodium carbonate solution was added within 3–8 min from the addition of the Folin–Ciocalteu reagent. The tubes were capped, votexed quickly and then placed in the water bath and incubated at 26°C for 2 h. Absorbance readings at 765 nm were relative to a gallic acid standard curve.
Analysis of polyphenols by HPLC
The major polyphenols in the fruit samples and green tea were characterised using HPLC as described previously( Reference Manzano and Williamson 27 ). An Agilent 1200 SL system (Agilent Technologies) equipped with a diode array detector (DAD) was used. It comprised of a binary pump, degasser, column oven (35°C) and a well plate autosampler (5°C). A Zorbax Eclipse plus C18 column (1·8 µm, 100×2·1 mm) and Agilent-Zorbax eclipse XDB-C18 (1·8 µm, 50×4·6 mm), both from Agilent Technologies, were used for green tea and fruit extracts, respectively. Other parameters were 5-µl injection volume, at 0·5 ml/min flow rate with needle wash in the flush point for 3 s. For all analyses, ultrapure, nuclease-free water (≥18·2 MΩ cm at 25°C) from a Millipore Q water purifying system (Millipore) was used. For sample preparation, a Genevac (EZ-2 plus model, Fisher Scientific Ltd) was used for centrifugal evaporation. Polyphenols were identified by their retention times compared with authentic standards, and standard curves were used for quantification.
Sugar analysis by HPLC
Sugar quantification of the fruit extracts was conducted using a Shimadzu HPLC instrument equipped with a model DGU-20 A5 degasser, a LC-20 AD XR pump system, a SIL-20 AC XR auto sampler (Shimadzu), column oven, a DAD system (SPD-M20A; Shimadzu) and a Shimadzu ELSD-LTII low-temperature, evaporative, light-scattering detector. A sample volume of 10 µl was injected, and separations were achieved on a Prevail Carbohydrate ES 5-μm column (250×4·6 mm; Grace). The column was held at 20°C, and individual sugars were eluted isocratically using a 1 ml/min flow of 75 % acetonitrile. Solutions of standard sugars prepared in water (Millipore, HPLC grade) with concentrations between 0 and 10 mg/ml were used for the calibration curve. The sugars were identified by their retention time characteristics at 40°C. Quantification was achieved using standard calibration curves obtained by plotting area v. concentration (r 2>0·98). Data from the sugar analysis allowed balancing of glucose, fructose and sucrose in the fruit in the control samples as indicated above.
Fibre estimation
The AOAC (Association of Official Analytical Chemists) method( 31 ) was used for fibre determination by Healthy Supplies.
Results
Polyphenol and sugar analysis
Total polyphenol contents of the fruits and green tea are shown in Table 1, and all data fell within the normal range as recorded in phenol explorer( Reference Neveu, Perez-Jimenez and Vos 32 ). Sugar analysis of PFRF gave a total of 0·8, 5·4 and 8·6g/80g sucrose, glucose and fructose, respectively, and these values were used to balance the control meal. Fibre contents were 0·22, 0·53, 0·43 and 0·2 g/100 g dry weight in apple peel, blackberry, blackcurrant and strawberry, respectively.
Table 1 Polyphenol content of green tea and freeze-dried fruitsFootnote * (Mean values and standard deviations; n 3)
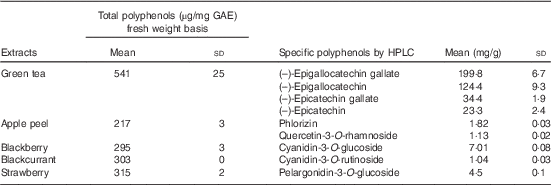
GAE, gallic acid equivalents.
* Total polyphenol contents and specific polyphenol contents of green tea and extracts from the tested fruit as analysed by Folin’s assay and HPLC.
Postprandial plasma glucose and insulin
Both the low-dose and the high-dose test meals containing PFRF showed a significant dose-dependent decrease in the mean glucose IAUC compared with the control meals (Fig. 2), −27·4 (sd 7·52) % (P<0·01) and −49·0 (sd 15·3) % (P<0·01), respectively, with no significant difference between the two reference (control) meals. The peak glucose concentration was also significantly lower in both PFRF test meals compared with the reference meals. There was a reduction in insulin IAUC for the PFRF meal compared with the reference meal of −46·9 (sd 13·4) % (P<0·01) (Fig. 3). The PFRF meal also attenuated the peak postprandial insulin concentration, but any differences had disappeared by about 120 min.

Fig. 2 Average glucose curves (a) after consumption of reference, test meal dose 1 and test meal dose 2 for sixteen volunteers. There is a significant difference between incremental areas under the glucose curves (IAUC) of reference meals and test meals (b) as well as between the peak rise in glucose concentration (c). **P<0·01, NS denotes no significant difference.

Fig. 3 Average insulin curves (a) after consumption of the reference meal and test meal dose 2 and for sixteen volunteers. There is a significance difference between insulin incremental areas under the glucose curves (IAUC) of the reference meal and the test meal (b) as well as between the peak rise in glucose concentration (c). ** P<0·01.
Inhibition of α-amylase and α-glucosidase activities
Green tea and extracts from blackberry, blackcurrant and strawberry inhibited human salivary α-amylase (IC50 values=0·009, 1·2, 1·5 and 2·5 mg dry powder/ml water (amylose as substrate); 0·025, 1·6, 1·7 and 3·9 mg/ml (amylopectin as substrate)) (Fig. 4 and Table 2), where IC50 is the concentration required for 50% inhibition. Green tea inhibited maltase, sucrase and iso-maltase in vitro with IC50 values of 0·02, 2·3 and 2·0 mg solid/ml water, respectively (Table 2 and Fig. 4(c)).

Fig. 4 Inhibition of human salivary α-amylase by green tea (▼), freeze-dried strawberry (■), blackcurrant (●) and blackberry (▲) using amylose as substrate (a) or amylopectin as substrate (b) and inhibition of maltase (■), sucrase (▲) and iso-maltase (●) by green tea (c).
Table 2 Inhibition of carbohydrate-digesting enzymes (Mean values and standard deviations)
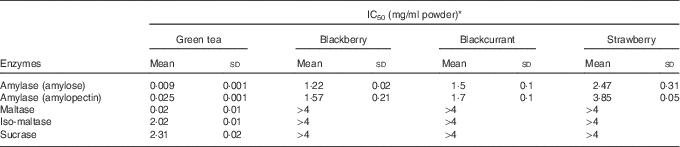
* Experimental IC50 values for human salivary α-amylase using amylose and amylopectin as substrates and α-glucosidase using maltose, sucrose and iso-maltose as substrates for green tea and freeze-dried fruits (n 3).
Discussion
Consumption of foods rich in polyphenols and fibre (PFRF) together with bread resulted in a highly significant dose-dependent lowering of the glucose AUC, as well as an associated attenuation of insulin. Although there was substantial inter-individual variation, the cross-over design minimised the consequences of this, and the two curves obtained for the control meals were not significantly different. We propose that the effects of the test meals are most likely due to the results observed in the in vitro inhibition of human salivary α-amylase (mainly green tea, blackberry, blackcurrant and strawberry), α-glucosidase (green tea) and glucose transport (green tea, apple and strawberry)( Reference Kobayashi, Suzuki and Satsu 26 , Reference Manzano and Williamson 27 ), as well as the additional effect of fibre( Reference Anderson, Baird and Davis 33 , Reference Weickert and Pfeiffer 34 ). Although it is not possible to define the exact contribution of inhibition of the different steps (inhibition of α-amylase, α-glucosidase or glucose transport) to the attenuation of blood glucose, we would speculate that partial inhibition of multiple steps is important for the observed effect on the glycaemic response. These reductions can play a major, long-term role in the management or risk reduction of diabetes type 2, comparable with the drug acarbose, an α-glucosidase inhibitor( Reference Hanefeld, Schaper and Koehler 15 ), as high concentrations of postprandial glucose lead to insulin resistance, pancreatic exhaustion, glucose intolerance and an increased insulin demand( Reference Willett, Manson and Liu 35 ). A highly significant effect on blood glucose was observed at both doses, with the higher dose (approximately 2-fold higher than the lower dose) leading to a doubling of the measured reduction in AUC. On the other hand, the peak glucose concentration was not further decreased by the higher dose.
A limited number of studies have reported the effects of isolated polyphenols or polyphenol-containing foods or extracts on postprandial glycaemia( Reference Bryans, Judd and Ellis 36 – Reference Tsujita, Takaku and Suzuki 49 ), but the results are mixed with only some studies reporting significant differences between test meals and reference meals, and sometimes only at one or two time points, possibly owing to the use of different sugar sources – for example, glucose( Reference Bryans, Judd and Ellis 36 , Reference Johnston, Clifford and Morgan 41 , Reference Johnston, Clifford and Morgan 42 , Reference Linderborg, Jarvinen and Lehtonen 44 – Reference Schulze, Bangert and Kottra 46 ) or sucrose( Reference Torronen, Sarkkinen and Niskanen 47 ). A limited number of starch-based interventions, using rice, pancakes and white bread, have shown mixed results( Reference Clegg, Pratt and Meade 38 – Reference Hlebowicz, Darwiche and Bjorgell 40 , Reference Josic, Olsson and Wickenberg 43 , Reference Tsujita and Takaku 48 ). All of these above-mentioned studies have not designed the study meal by considering the mechanism and including foods capable of attenuating the rate of each step of the digestive process. No significant difference was observed when a starch-based meal (pancakes) was used together with 100 g berries as the source of polyphenols( Reference Clegg, Pratt and Meade 38 ). When used alone, green tea as the sole source of polyphenols also did not give a significant difference in the IAUC( Reference Josic, Olsson and Wickenberg 43 ). There was a significant difference in the IAUC when apple juice was used as a polyphenol source, clearly attributed to the inhibition of glucose transport by polyphenols in apple, especially phlorizin( Reference Johnston, Clifford and Morgan 41 ). Polyphenols and fibre (14·7 g)( Reference Linderborg, Jarvinen and Lehtonen 44 ), present in lingonberries, nulled the glycaemic effect of the endogenous sugars present in the lingonberries. In vitro, polyphenols, phenolic acids and tannins in strawberry and apple reduced glucose transport using Caco-2 intestinal cell monolayers by inhibiting the glucose transporters SGLT1 and GLUT2. Phlorizin contributed 52 % (IC50=146 μm) and pelargonidin-3-O-glucoside (IC50=802 μm) 26 % to the total inhibition by apple and strawberry, respectively( Reference Manzano and Williamson 27 ). These concentrations, together with those obtained for α-amylase and α-glucosidase inhibition (Table 2), were theoretically obtained in the gut lumen (Table 1) after taking into consideration calculated 3-fold dilution of consumed substances( Reference Williamson 14 ). For example, the IC50 value for α-amylase inhibition by green tea was 0·009 mg/ml in vitro; 3-fold dilution in vivo would require 0·027 mg/ml in the original sample, and the test meals contained 2·5 and 5 mg/ml in the low and high dose, respectively. Therefore, we propose that polyphenols and fibre present in PFRF act together by inhibiting α-amylase, α-glucosidase and glucose transporters and leading to the observed reduced glycaemic response in vivo. The observed reduction in postprandial blood glucose and insulin can play a major role in the management and reducing the risk of type 2 diabetes, as hyperglycaemia is a risk factor for developing insulin resistance, IGT and consequently type 2 diabetes.
Conclusion
Polyphenols and fibre present in fruits, together with a cup of green tea, have a pronounced lowering effect on postprandial glucose and insulin when consumed together with a starchy food (bread), owing to the inhibition of different stages of starch digestion.
Acknowledgements
The authors thank the volunteers, Penny Rice (research nurse), David Tembo (assistance with sugar analysis) and the School of Food Science and Nutrition (University of Leeds) for meeting the research nurses’ costs.
H. N.-S. received a student grant from the Commonwealth Scholarship Commission UK (ZMSC-2012-593) and the National Institute for Scientific and Industrial Research, Zambia, for PhD funding. Neither body was involved in any way in the design, interpretation or writing of the article.
H. N.-S.: design of study, carried out study and in vitro work, data analysis, writing the paper. G. W.: design of study, data analysis, writing the paper.
H. N.-S. has no conflicts of interest. This work did not receive funding from a commercial organisation, but G. W. has recently, or currently, received other research funding from Nestlé and Florida Department of Citrus, and conducted consultancy for Nutrilite, USA, and Suntory, UK.









