Iodine is an essential micronutrient and lack of iodine in the diet may result in a spectrum of diseases known as iodine-deficiency diseases. Iodine-deficiency diseases are easy and cheap to prevent by the addition of a small amount of iodine to the salt that people consume daily. Salt iodisation is widespread, and iodine deficiency has been eliminated or decreased in many countries( Reference Andersson, de Benoist and Rogers 1 ). However, iodine deficiency has also reappeared in some countries that have been thought to have sufficient iodine intake( Reference Li, Eastman and Waite 2 – Reference Johner, Günther and Remer 4 ). Monitoring of the effect of iodine fortification is essential to ensure that it is functioning as planned and to provide the information needed to take corrective action if necessary( 5 ).
Before 1998, mild-to-moderate iodine deficiency existed in Denmark( Reference Rasmussen, Carlé and Jørgensen 6 ). A voluntary iodine fortification programme was introduced in Denmark in 1998, and in 2000, iodine fortification was changed to a mandatory fortification of household salt and bread salt at a level of 13 μg/g salt( 7 ). Before iodine fortification was introduced, and again in 2004–5, 4 to 5 years after the introduction of mandatory fortification, cross-sectional studies were carried out. The results of the cross-sectional study carried out in 2004–5 showed an increase in iodine urinary excretion to just above the recommended 100 μg/l( Reference Rasmussen, Carlé and Jørgensen 6 ). In 2008–10, a follow-up study including the participants from the first cross-sectional study (1997–8) was carried out.
Milk has been found to be the most important dietary source of iodine in Denmark( Reference Rasmussen, Carlé and Jørgensen 6 ). Likewise, milk is an important iodine source in other countries( Reference Johner, von Nida and Jahreis 8 – Reference Watutantrige Fernando, Barollo and Nacamulli 10 ). Therefore, possible changes in iodine content in milk can have a large effect on iodine intake, and should be assessed as part of a monitoring programme.
Results from the studies conducted in 2004–5 and in 2008–10, 4 to 5 years and about 9 years after the implementation of mandatory iodine fortification, respectively, have been published separately before( Reference Knudsen, Bülow and Jørgensen 11 – Reference Bjergved, Jørgensen and Perrild 13 ). In the present study, the results of the two studies are compared with regard to urinary iodine excretion and intake of iodine-rich foods. Thus, the aim of the present study was to describe possible differences in urinary iodine excretion from 2004–5 to 2008–10. Furthermore, iodine content in milk sampled in 2000–1 (not previously published) and in 2013 is presented, and possible reasons for the change in urinary iodine excretion are explored.
Methods
In the present study, results of urinary iodine excretion and intake of iodine-rich foods from two studies, both part of the Danish Investigation of Iodine Intake and Thyroid Diseases (DanThyr), were compared; a study carried out in 2004–5 and another study carried out in 2008–10. The study conducted in 2004–5 was a cross-sectional study and the study conducted in 2008–10 was a follow-up of a cross-sectional study carried out in 1997–8, before iodine fortification was implemented( Reference Knudsen, Bülow and Jørgensen 11 ).
The studies took place at two centres located in the cities of Copenhagen (situated in the eastern part of Denmark) and Aalborg (situated in the western part of Denmark). The two cities represented areas with mild and moderate iodine deficiency, respectively, before iodine fortification; the difference in iodine intake between the cities was mainly due to differences in iodine content in drinking water( Reference Pedersen, Laurberg and Nøhr 14 ). For the cross-sectional study (2004–5), a random sample was drawn from the Civil Registration System of all inhabitants in the two cities comprising the following groups: women aged 18–22, 25–30, 40–45 and 60–65 years, and men aged 60–65 years. These groups were chosen to represent women before the usual childbearing age, in the childbearing age and after the childbearing age, both pre-menopausal and postmenopausal. Primarily women were investigated as the occurrence of thyroid abnormalities is higher among women than among men. In total, 7658 subjects were invited, of which 3570 (46·6 %) participated in the 2004–5 cross-sectional study. For the second study (2008–10), participants from a cross-sectional study carried out in 1997–8 were invited. In the 1997–8 study, 9274 subjects were invited from the Civil Registration System in the same way and in the same age groups as described above, of which 4649 (50·1 %) participated. Of the 4649 participants, seventy-two had emigrated and 403 had died, leaving 4174 subjects to be invited for the follow-up study. From this sample, 2465 subjects (59·1 %) participated. The sample size for the baseline study in 1997–8 was determined to be able to identify a difference in thyroid volume of 3 ml and a difference in iodine concentration in the urine of 10 μg/d between the various age groups, with α = 0·05 and β = 20 %. For the study in 2004–5, the sample size was determined to be able to detect a difference in thyroid volume of 1 ml for the age group 18–30 years, 2 ml for the age group 40–45 years and 3–4 ml for the age group 60–65 years, and a difference in iodine concentration in the urine of at least 10 μg/d for each age group when compared with the study in 1997–8, with α = 0·05 and β = 20 %.
The two studies took place from 28 April 2004 to 14 July 2005 and from 22 February 2008 to 11 February 2010, respectively. All information gathered and procedures performed were standardised in both cities and were similar in both studies. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Danish Ethics Committee (no. 2-16-4-0001-97 and VN 96/208mch and N-VN-19960208MCH, the Northern Danish Region Committee). All participants gave written informed consent.
The studies have been described in more detail previously( Reference Knudsen, Bülow and Jørgensen 11 – Reference Bjergved, Jørgensen and Perrild 13 ).
Dietary supplements and FFQ
Participants were invited to one of the two centres, and asked to bring with them all dietary supplements taken and daily intake of iodine from supplements was recorded.
A FFQ covering iodine-rich foods was given to all participants in both studies during their visit. The FFQ was semi-quantitative and was similar in both studies, and included a list of sixty-three iodine-rich food items. The FFQ has been evaluated and described in more detail elsewhere( Reference Rasmussen, Ovesen and Bülow 15 ). In the present study, the FFQ was only used to explore changes in the intake of iodine-rich foods. The FFQ gave useful data for 3522 participants in the first study and 2424 participants in the second study.
Collection of urine samples
All participants were asked to give a single, random spot urine sample when they visited the centre. The visits took place between 08.00 and 17.30 hours.
Collection of milk samples
Milk was sampled in 2013, and the results on iodine content were compared with previously unpublished results on iodine content found in milk sampled and analysed in 2000–1. Denmark has only few dairies, which produce milk, and one of the dairies (Arla) has a market share of approximately 85 %.
2000–1 sampling
Milk samples were taken every month throughout a year at the three dairies that delivered virtually all milk to the Danish market and from the two largest organic dairies. Thus, the samples were representative of the Danish consumption. In total, thirty-six samples of non-organic and twenty-four samples of organic semi-skimmed milk (fat content approximately 1·5 %), and further thirty-six samples of non-organic whole milk (fat content approximately 3·5 %) were taken. Samples were taken throughout a whole year, from September 2000, thus taking the expected seasonal variation into account.
2013 collection
Milk was sampled from mainly the same dairies as in the 2000–1 sampling. In total, forty-seven samples of organic milk (whole milk and skimmed milk) and forty-two samples of non-organic milk (whole milk and skimmed milk) were collected. Milk was collected in April and in July/August 2013; in April, all cows are still kept in the stable, and therefore samples taken at that time represented winter milk.
Milk originated from mainly the same dairies in both collections; the non-organic milk from Arla's three dairies, two in Jutland and one in Zealand, and the organic milk from Arla's dairy in Zealand, from Naturmælk (in Jutland) and from Thiese (in Jutland). A few of the organic samples in the 2013 collection came from the dairy Øllingegård situated on Zealand. Furthermore, a few samples, which originated from Germany (n 4), were also collected in 2013, as German milk is now found in some supermarkets.
Iodine analyses
For the analyses of iodine content in urine samples from both population studies and in consumer milk collected in 2013, samples were first treated to remove organic materials as described in detail by Wilson & van Zyl( Reference Wilson and van Zyl 16 ). In brief, 1·25 ml of sample and 1·00 ml of 2 M-KOH and 0·5 % KClO3 were added to iodine-free thermo-resistant 16 × 100 mm Pyrex glasses, mixed and incubated in a furnace oven at 90–95°C until dryness (24 h). The oven temperature was then increased to 450°C for 60 min followed by 620°C for 180 min. After alkaline ashing, the remnants were resuspended in 1·25 ml distilled water and incubated for 5 min in a laboratory shaker, and subsequently centrifuged at 3000 rpm for 15 min. Inorganic iodine was measured in the supernatant by the Sandell–Kolthoff method, which is based on the catalytic effect of iodide on the reaction between Ce4+ and As3+, as described previously( Reference Laurberg 17 ).
Recovery of iodine during alkaline ashing was about 95 % in previous studies( Reference Laurberg 17 ), and it has been retested to be at this level for both urine and milk samples. In brief, fifteen urine samples with a median iodine content of 35 (range 15–80) μg/l were spiked with the analytical-grade KI solution used as the standard corresponding to an increase in iodine concentration of 32 μg/l. Recovery of iodine was 95·9 (sem 2·4) %. Similarly, the mean recovery of iodine added to milk was 94 (range 83–102) %, when measured on eight samples with a mean basic iodine concentration of 103 (range 91–116) μg/l and spiked with KI to contain an extra 100 μg/l. The final values were not corrected for percentage recovery.
The correctness of the level of iodine measured in inorganic supernatants was tested by participating in the Center for Disease Control (CDC, Atlanta, USA) Equip quality programme every 4 month. The received blind urine samples were treated as described above, and at the time of analyses in the present study, quality controls at three levels were measured to contain a mean of 59·2 μg/l (triplicate measurement, CV 1·7 %), 105·3 μg/l (CV 1·7 %) and 175·5 μg/l (CV 1·3 %), whereas average results from nine different measurements by the CDC ICP-dynamic reaction cell-MS reference method over at least three different days were 64·3, 110·9 and 184·3 μg/l, respectively. Serial dilutions of urine and milk samples gave curves parallel to the standard curve. Analytical precision was estimated from triplicate ashing and analysis of a urine sample containing 93·9 μg/l in eighteen assays, and the intra- and inter-assay CV for single determinations were 2·1 and 2·7 %, respectively. For the Sandell–Kolthoff analyses, the lowest standard above the zero blank contained 10 μg iodine/l. With this set-up, the limit of detection varied between 2 and 3 μg/l. The standard was prepared from KI for analysis (Merck).
Iodine concentrations in urine samples from the 2004–5 study and the 2008–10 study were measured in different runs. When thirty samples from the first study were measured again with regard to urine samples from the 2008–10 study, no significant differences (P>0·05) from the first measurement were observed.
Analyses of milk sampled in 2000–1 were performed by inductively coupled plasma MS (ELAN 6100DRC; Sciex/Perkin Elmer) following dilution of the milk samples (1+19) with 0·5 m aqueous NH3 (Merck)( Reference Fecher, Goldmann and Nagengast 18 ). Quantification was done using the 127I isotope by external calibration with internal standardisation using 125Te. The accuracy of the analysis was estimated by the analysis of a certified reference material BCR63R Skim milk powder (Institute of Reference Materials and Measurements). The results (812 (sd 62) μg/kg (n 11)) obtained are in good agreement with the certified target value at 810 (sd 50) μg/kg. Precision of the analysis was evaluated as satisfactory by duplicate analysis of selected samples (n 18) at CV = 8·5 %. The limit of detection was estimated at 0·4 (sd 3) μg/kg from the analysis of the blank samples.
Overall, five milk samples from 2013 were sent for analysis of iodine with inductively coupled plasma MS in the same laboratory as the samples from 2000–1 to compare the analytical methods. The mean value for the five samples analysed with inductively coupled plasma MS was 9·1 μg/100 g and for the Ce–As method was 8·2 μg/100 g (P>0·05).
Creatinine analyses
Urinary creatinine concentration was determined using Vitros creatinine slides and Vitros Chemistry Products Calibrator Kits on a Vitros 250 chemistry system (Ortho-Clinical Diagnostic System, Inc.). Intra- and inter-assay CV were below 5 %. Equipment was calibrated according to the manufacturer's instructions, and external standards included standards from the Danish Institute for External Quality Assurance for Laboratories in Health Care (DEKS). Dilution and recovery tests were satisfactory. In all assay runs, samples from the different subgroups of the study population were included in random order.
Expressions of iodine excretion
Urinary iodine excretion was expressed in two ways: as a concentration and as an estimated 24 h urinary iodine excretion. To calculate the estimated 24 h urinary iodine excretion, the iodine:creatinine ratio was multiplied by the calculated 24 h creatinine excretion. The calculation of the 24 h creatinine excretion was done by using a formula including weight, height and age for each sex, respectively( Reference Toft, Cerqueira and Andreasen 19 ). The formula was developed from data collected in Danish studies. Iodine concentration in the urine was available for 3554 participants in the 2004–5 study and for 2453 in the 2008–10 study.
Statistical analysis
Data were analysed with the Statistical Package for the Social Sciences statistical software (version 20.0; IBM SPSS, Inc.). Results for urine excretion are expressed in three different ways: as medians with 25th and 75th percentiles; as geometric means and standard deviations; as adjusted geometric means with 95 % CI.
Adjusted geometric mean values were calculated using mixed models with age at examination as the continuous variable, educational level (no further education, semi-skilled, short theoretical ( < 3 years) and theoretical ( ≥ 3 years)) and city as independent variables and logarithmically transformed iodine excretion as the dependent variable.
When comparing the two studies with regard to urinary iodine excretion or iodine-rich foods, the Mann–Whitney U test was used for median values and Student's t test for mean values. A χ2 test was used when comparing users of iodine-containing supplements in the two studies.
The relationships between urinary iodine excretion and the age group (18–22, 25–30, 40–45 and 60–65 years) were explored using the Kruskal–Wallis H test.
Iodine content in milk was normally distributed, and is expressed as means and standard deviations. Comparisons between iodine content in different milk samples were made by Student's t test.
Results
Urinary iodine excretion given as a concentration and as estimated 24 h iodine excretion in men and women, respectively, after approximately 4 and 9 years of mandatory iodine fortification is shown in Table 1. Both expressions of iodine excretion were significantly lower in women 9 years after (in 2008–10) than 4 years after (in 2004–5) the introduction of mandatory fortification, and also after adjustment for age, education and city. The percentage of participants who used dietary supplements with iodine was higher after 9 years of fortification than after 4 years of fortification for women, but did not change for men (Table 1).
Table 1 Urinary iodine excretion and users of iodine supplements in 2004–5 and in 2008–10, 4 and 9 years after the introduction of mandatory iodine fortification, respectively (Median values and 25–75 percentiles; geometric mean values and standard deviations; adjusted geometric mean values and 95 % confidence intervals)
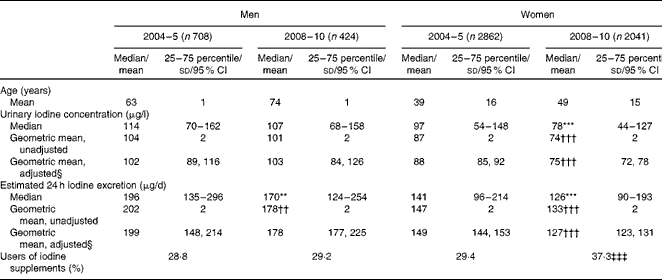
Median value was significantly different from that after 4 years of fortification (2004–5): **P= 0·001, ***P< 0·001 (Mann–Whitney test).
Mean value was significantly different from that after 4 years of fortification (2004–5): ††P= 0·001, †††P< 0·001 (t test).
‡‡‡ Value was significantly different from that after 4 years of fortification (2004–5) (P< 0·001; χ2 test).
§ Adjusted for age, education and city.
Estimated 24 h iodine excretion in women increased significantly with age both in 2004–5 (P <0·001) and in 2008–10 (P= 0·002). Likewise, iodine concentration in the urine increased with age in both studies (P <0·001; results not shown).
Geometric mean estimated 24 h iodine excretion was higher during winter (October to April) than during summer (May to September) in 2004–5 (160 (95 % CI 155, 166) μg/l v. 151 (95 % CI 145, 156) μg/l, P= 0·004), whereas no seasonal difference was found in 2008–10 (144 (95 % CI 138, 150) μg/l v. 142 (95 % CI 136, 149) μg/l, P= 0·744) when adjusted for city, age, sex and education.
Urinary iodine excretion in women for each city is shown in Table 2. Urinary iodine excretion decreased significantly in both cities from 2004–5 to 2008–10 both when expressed as a concentration and as estimated 24 h excretion.
Table 2 Urinary iodine excretion expressed as a concentration and as estimated 24 h iodine excretion in 2004–5 and 2008–10 (4 and 9 years after the introduction of mandatory iodine fortification, respectively) in the two investigated cities for women (Geometric means† and 95 % confidence intervals)
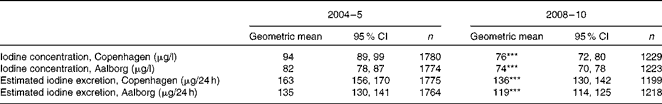
*** Mean value was significantly different from that after 4 years of fortification (2004–5) (P< 0·001).
† Adjusted for age and education.
Total calculated water intake (including water, coffee, tea, fruit juice and soft drinks) and calculated total fluid intake (including both water and milk) increased significantly in women, but decreased in men (Table 3). Total milk intake (including all kinds of milk and yogurt) and the intake of other iodine-rich foods (fish, bread and eggs) did not change significantly for either sex between the two studies.
Table 3 Intake of selected foods in 2004–5 and 2008–10, 4 and 9 years after the introduction of mandatory iodine fortification, respectively (Median values and 25–75 percentiles)
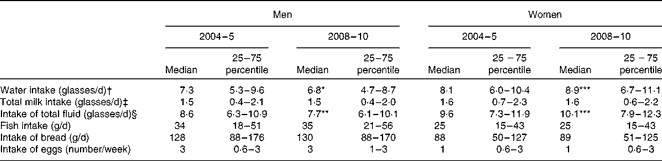
Median value was significantly different from that after 4 years of fortification (2004–5): *P= 0·014, **P= 0·001, ***P< 0·001.
† Includes all kinds of water, soft drinks, fruit juice, coffee, tea, etc.
‡ Includes all kinds of milk products and yogurt.
§ Includes water intake and drinking milk.
Fewer participants reported of buying mainly organic milk in the 2004–5 study (38 %) than in the 2008–10 study (50 %) (P< 0·001). Furthermore, fewer participants stated that they always or often made bread themselves in the 2004–5 study (24 %) than in the 2008–10 study (31 %) (P< 0·001), but the percentage of participants who always or often bought bread at a bakery store did not change (36 and 38 % in 2004–5 and 2008–10, respectively, P= 0·12).
In non-organic milk sampled in 2000–1, iodine content was significantly higher than in milk sampled in 2013, but iodine content in organic milk was highest in milk sampled in 2013 (Table 4). Iodine content was significantly higher in non-organic milk than in organic milk (P< 0·031). The mean iodine content in milk sampled in 2000–1 was 16 (sd 6) μg/100 g and in 2013, the mean iodine content weighted for the same distribution of organic/non-organic milk as in 2000–1 was 12 (sd 3) μg/100 g (P< 0·001). Iodine content in milk from dairies on Zealand was higher than that from dairies in Jutland (P= 0·001) in 2000–1, whereas in 2013, no difference was found in iodine content in milk from dairies in Jutland compared with that in dairies on Zealand. No difference in iodine content between semi-skimmed milk (21 (sd 4) μg/100 g) and whole milk (21 (sd 4) μg/100 g) was found in the 2000–1 study (P= 0·740). Likewise, iodine content in skimmed milk did not differ from that in whole milk 13 (sd 3) and 13 (sd 2) μg/100 g, respectively (P= 0·43) in 2013. The mean iodine content in milk that originated from Germany was 14 (sd 2) μg/100 g (n 4) in 2013 (none was sampled in the 2000–1 study).
Table 4 Iodine content in milk sampled in 2000–1 and 2013 (Mean values and standard deviations)
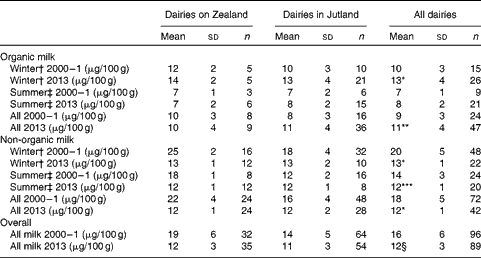
Mean value was significantly different from that of the 2000–1 sampling: * P< 0·01, ** P= 0·037, *** P= 0·015.
† Winter: October–April.
‡ Summer: May–September.
§ Weighted for the same distribution of organic/non-organic milk as in 2000–1. The unweighted iodine content is 11 μg/100 g.
Discussion
Iodine concentration in the urine showed a decrease in Danish women 9 years after mandatory iodine fortification compared with iodine concentration after 4 years, despite a higher intake of iodine-containing supplements, and the excretion was low compared with the recommended level( 5 ). The decrease in urinary iodine concentration could not be explained by a change in urinary dilution, as urinary iodine excretion also decreased when expressed as estimated 24 h urinary iodine excretion. Urinary iodine concentration did not change in men. However, only men aged 60–65 years in 2004–5 participated, and a lower urinary volume in 2008–10 could probably have counteracted a possible decrease in iodine excretion when expressed as a concentration in this age group as the estimated 24 h iodine excretion decreased.
The 24 h iodine excretion can be compared with the recommended intake of 150 μg/d( 20 ) with extraction of 10 μg/d excreted in the faeces; that is, the optimal daily urinary iodine excretion is approximately above 140 μg/d. According to this measure, the estimated 24 h iodine excretion was below the optimal level in women. The median urinary iodine concentration in women was below 100 μg/l, which is the level recommended by the WHO. It has been questioned whether this level is too high in adults, and a level of 60–70 μg/l has been suggested( Reference Zimmermann and Andersson 21 ). However, before iodine fortification was introduced in Denmark the median urinary iodine concentration was 68 μg/l in Eastern Denmark when 15 % had thyroid enlargement, and the occurrence of multinodular toxic goitre was 11·5 %( Reference Knudsen, Bülow and Jørgensen 11 ). Thus, iodine intake at that level does not seem to be optimal.
Iodine deficiency, defined as a median iodine concentration in the urine below 100 μg/l for a sufficiently large group, still exists in both children and adults in some European and other Western countries( Reference Andersson, de Benoist and Rogers 1 ). In addition, low iodine intake( Reference Brantsæter, Abel and Haugen 22 ) or low urinary iodine excretion( Reference Raverot, Bournaud and Sassolas 23 – Reference Andersen, Sørensen and Krejbjerg 25 ) have been found in pregnant women in a number of European countries. Moreover, iodine deficiency has re-emerged in some countries such as Australia( Reference Li, Eastman and Waite 2 ), Germany( Reference Johner, Günther and Remer 4 ) and the UK( Reference Vanderpump, Lazarus and Smyth 3 ). In Australia, decreased iodine intake is explained by chlorine-containing sanitisers replacing iodine-containing sanitisers in the dairy industry, with a lower iodine content in milk as a result, and by decreased consumption of iodised salt( Reference Li, Eastman and Waite 2 ). A possible reason for the negative trend in Germany is decreased use of iodised salt in industrial products( Reference Johner, Günther and Remer 4 ) and in the UK, the reason was proposed to be a decreased intake of milk( Reference Vanderpump, Lazarus and Smyth 3 ). In Iceland, which traditionally has a high intake of fish and thus a high iodine intake, intake of iodine has decreased during recent years, mainly as a result of decreased fish intake and perhaps because of lower iodine content in milk( Reference Gunnarsdottir, Gustavsdottir and Thorsdottir 26 ).
There could be several explanations for the lower urinary iodine excretion observed in Denmark, which occurs in spite of a higher use of iodine-containing supplements. First of all, participants in the 2008–10 study were older than those in the 2004–5 study. However, urinary iodine excretion increased with age within the two studies, and the decrease in urinary iodine excretion between the studies persisted after adjustment for age, making changes in age an unlikely explanation. More participants in the 2008–10 study, which was a follow-up study, had a long education and fewer had no education compared with participants in 2004–5. High educational level has been found to be associated with higher intake of fish in a Danish study( Reference Groth, Christensen and Knudsen 27 ). The intake of bread is probably not related to educational level( Reference Rasmussen, Andersen and Borodulin 28 ). A possible association of educational level with the intake of other iodine-rich food items such as milk and eggs has not been investigated, but seems unlikely due to the low cost of these foods. In a study from France, a high educational level was associated with a higher risk of having an iodine intake below 150 μg/d( Reference Valeix, Faure and Péneau 29 ). However, a study from Germany did not find a clear association between socio-economic status and urinary iodine excretion( Reference Völzke, Craesmeyer and Nauck 30 ). Although educational level could have a possible influence on urinary iodine excretion, adjustment for educational level did not change urinary iodine excretion significantly in the present study. Furthermore, the higher educational level in the 2008–10 study is expected to be associated with higher rather than lower urinary iodine excretion, and, thus, would have counteracted the decrease in urinary iodine excretion.
Dietary habits with regard to the intake of iodine-rich foods (milk, fish and bread) did not change significantly between 2004–5 and 2008–10 apart from the total fluid and water intake. This is in line with the results from the 2003–8 Danish National Survey of Dietary Habits and Physical Activity (S Fagt, unpublished results).
In Denmark, the main part of salt in the diet originates from industrial products, and the intake of household salt is approximately 1 g/d( Reference Andersen, Rasmussen and Larsen 31 ). The household salt and salt used in bread are iodised. An unpublished study based on the measurement of Na in spot urine samples found a decrease in salt intake of approximately 0·5 g/d in women from 2006 to 2010 (U Toft, unpublished results). Thus, this small decrease in total salt intake cannot explain the decrease in iodine intake.
The salt and iodine content in bread on the Danish market has not changed from 2001–2( Reference Rasmussen, Ovesen and Christensen 32 ) to 2009( Reference Mikkelsen, Rasmussen and Saxholt 33 ). However, the content of salt and iodine decreased non-significantly in industrially produced bread and increased significantly in breads bought in bakeries in that period (unpublished calculations based on Rasmussen et al. ( Reference Rasmussen, Ovesen and Christensen 32 ) and Mikkelsen et al. ( Reference Mikkelsen, Rasmussen and Saxholt 33 )). The percentage of participants who bought bread at bakeries did not change significantly, but a higher percentage claimed to bake their own bread in the 2008–10 study. This could have influenced iodine intake slightly dependent on the amount and type of salt used for baking. In addition, 7 % of the bread samples were not iodised in 2001–2( Reference Rasmussen, Ovesen and Christensen 32 ), whereas 13·5 % of the breads sampled in 2009( Reference Mikkelsen, Rasmussen and Saxholt 33 ) were not iodised. This fact can explain a minor part of the decrease in urinary iodine excretion.
We have earlier found a strong association between milk intake and urinary iodine excretion in both of the two cross-sectional studies( Reference Rasmussen, Carlé and Jørgensen 6 , Reference Rasmussen, Ovesen and Bülow 34 ) and in the follow-up study( Reference Rasmussen, Jørgensen and Perrild 35 ), and even a strong association between milk intake and thyroid volume before fortification was introduced( Reference Rasmussen, Ovesen and Bülow 36 ). A plausible explanation for the lower urinary iodine excretion could therefore be the decreased iodine content in milk. With a median intake of 1·6 glasses of milk, corresponding to 320 ml milk daily, the mean decrease in iodine content in milk from 16 to 12 μg/100 g would roughly account for a decrease of 13 μg iodine/d, and can explain a main part of the decrease in urinary iodine excretion.
The decrease in iodine content in milk is in contrast with the results from some other countries. In Germany, an increasing trend in iodine content in milk was found from 2004 to 2010( Reference Johner, von Nida and Jahreis 8 ), and, likewise, in Spain, iodine content has increased between 1991 and 2008( Reference Soriguer, Gutierrez-Repiso and Gonzalez-Romero 37 ). Furthermore, compared with newer data from other countries, the actual measured values were relatively low; in England, the median iodine content was 14·5 μg/100 g in organic milk (n 92) and 25·0 μg/100 g in non-organic milk( Reference Bath, Button and Rayman 9 ), data from Spain showed a mean content of 25·9 (sd 5·8) μg/100 ml in 2008( Reference Soriguer, Gutierrez-Repiso and Gonzalez-Romero 37 ), and in Italy, a median value of 26·4 μg/100 ml was found( Reference Watutantrige Fernando, Barollo and Nacamulli 10 ). On the other hand, data from Germany showed an iodine content of 11 μg/100 ml( Reference Johner, von Nida and Jahreis 8 ) and milk sampled during winter in Poland showed a content of 14·7 μg/100 ml( Reference Brzóska, Szybinski and Sliwinski 38 ), which is in line with the contents reported in the present study.
We did not find a difference in iodine content between skimmed milk and whole milk. This is in contrast to Soriguer et al. ( Reference Soriguer, Gutierrez-Repiso and Gonzalez-Romero 37 ) who found a slight, but significantly higher content in skimmed milk than in semi-skimmed and whole milk, but is in agreement with Norwegian data( Reference Dahl, Opsahl and Meltzer 39 ), data from England( 40 ) and older data from Finland( Reference Varo, Saari and Passo 41 ).
A limitation of the present results was that we compared a cross-sectional study (2004–5) with the results from a follow-up study (2008–10). The age groups differed between the studies, with the age being higher in the follow-up study and the participants in the follow-up study had a higher educational level than in the cross-sectional study. However, we adjusted the results for educational level and age, and these factors did not explain the decrease in urinary iodine excretion. Collection of milk samples was not part of the DanThyr studies. The milk samples presented here were sampled and analysed for iodine 3 to 4 years before the 2004–5 study and 3 to 4 years after the 2008–10 study, respectively. Thus, iodine content in milk might have been different at the time of collection of urine samples. The methods used to analyse iodine in milk were different in 2000–1 and in 2013, and this might explain a smaller part of the difference found in iodine content. However, the two iodine analysis methods gave similar results when adequately performed, and both procedures of sample preparation and subsequent analyses were tested against international iodine references( Reference Kathleen, Caldwell and Maxwell 42 ). Recovery of iodine during the alkaline ashing procedure was only about 95 %, and this loss of material can explain part of the slightly lower content found in 2013 samples compared with the samples from the 2000–1 study. An advantage of the study was that the number of participants was quite large. According to Andersen et al. ( Reference Andersen, Karmisholt and Pedersen 43 ) a sample size of at least 400 participants is needed to get a precision range of ± 5 % when using estimated 24 h iodine excretion in spot urine samples. The sample size was above this number in each sex group and in both regions. A further advantage was that the studies were carried out in exactly the same way including that the same FFQ and the same questions regarding the use of dietary supplements were asked in both studies.
In conclusion, urinary iodine excretion, expressed both as a concentration and as estimated 24 h iodine excretion, has decreased in women living in Denmark between 2004–5 and 2008–10. Iodine content in milk was lower in 2013 than in 2000–1, and this is probably the main explanation for the decrease in urinary iodine excretion. The median urinary iodine excretion in women is below the internationally recommended level, and it should therefore be considered to increase the general iodine intake in the Danish population slightly, for example by increasing the iodine content in salt.
Acknowledgements
The present study was supported by the Danish Medical Foundation, the North Jutland County Research Foundation, the Tømmerhandler Wilhelm Bangs Foundation, the Copenhagen Hospital Corporation Research Foundation, the Ministry of Food, Agriculture, and Fisheries, and the Danish Agency for Science, Technology, and Innovation.
The authors’ contributions are as follows: L. O., H. P., T. J. and P. L. formulated the research questions and designed the study; A. C., L. B. and A. K. carried out the data collection; P. K. designed the data collection of the milk samples; J. J. S. carried out the analytical measurement of the milk samples from the 2000–1 study; P. L. was responsible for the milk analyses in 2013; L. B. R. analysed the data and performed the statistical analysis, wrote most of the paper and had primary responsibility for the final content. All authors read and approved the final manuscript.
There are no conflicts of interest.






