Obesity and related disorders are on the increase. According to a report by WHO, around one billion human individuals were classified as overweight worldwide in 2003 and 300 million were obese1. Obesity affects populations increasingly earlier in life with around 22 million children under the age of 5 years being classed as overweight. Men and women appear to be differently affected, with the obesity rate being greater in women1. Obesity and overweight are associated with a range of disorders such as type 2 diabetesReference Kahn, Hull and Utzschneider2 and CVDReference Van Gaal, Mertens and De Block3 and are causing increasing concerns in both the Western and developing worlds, essentially because of their impact on the economy and welfare of populations. The cause of the widespread increase in obesity and overweight is generally attributed to overeating and, thereby, the difficulties that some people experience in controlling their appetite, combined with a lack of exercise. Appetite regulation can be even more challenging by the abundance and easy availability of so-called ‘junk foods’, which are defined as heavily processed, highly palatable and hyper-energetic and are often deprived of the vitamins and essential nutrients found in whole unprocessed foods.
Because over-eating and lack of exercise constitute growing threats to health and consequent economic repercussions, there is an increasing effort from governments worldwide to encourage healthier eating habits not only in adults but also in childrenReference Butler and Pearson4, Reference Butler and Schneider5. However, there is also accumulating evidence that appetite and activity levels can be influenced or ‘programmed’ by maternal nutrition during the fetal and suckling life of an individualReference Breier, Vickers, Ikenasio, Chan and Wong6–Reference Muhlhausler, Adam, Findlay, Duffield and McMillen11. Most studies on appetite programming have, however, predominantly been focused on maternal under-nutrition, and the influence of a maternal junk food diet on the feeding behaviour, body weight and activity levels of the offspring remains largely uncharacterised. In the present study, we have therefore given rats ad libitum access to a selection of palatable junk foods designed for human consumption, as previously describedReference Bayol, Simbi and Stickland12, in order to examine the feeding behaviour and food preference of pregnant and lactating rats and determine the influence of such a maternal junk food diet on appetite regulation, food preference, body weight gain and activity levels in the offspring up to 10 weeks of age. The aim of this study is therefore to examine whether exposure to a maternal junk food diet during pregnancy and lactation can be a contributing factor in the development of obesity by influencing the feeding behaviour, growth rate and activity levels in offspring.
Experimental methods
Animals
All animal work was carried out under UK Home Office licence to comply with the Animals (Scientific Procedures) Act 1986. All animals used were purchased from Charles River, Margate, Kent, UK. They had free access to water and were kept in a light-, temperature- and humidity-controlled environment throughout the experiment (14–10 h light–dark cycles, 20 ± 2°C, 45 % relative humidity). The animals were fed two types of diet throughout the study. They were fed either RM3 rodent chow alone ad libitum (SDS Ltd, Betchworth, Surrey, UK) or with a junk food diet, also known as cafeteria dietReference Bayol, Simbi and Stickland12, which consisted of eight different types of palatable foods, purchased from a British supermarket. The palatable food included biscuits, marshmallows, cheese, jam doughnuts, chocolate chip muffins, butter flapjacks, potato crisps and caramel/chocolate bars; a description of the nutritional value and ingredients is given in the supplementary data file. Each of the eight palatable foods as well as the chow was weighed before and 1 d after it was given to the rats, such that daily food intakes could be calculated following correction for humidity gain or loss. The animals on the junk food diet received excess quantities of each foodstuff including the chow, such that their intake was ad libitum.
Forty-two virgin female Wistar rats aged between 12 to 14 weeks were randomly mated with Wistar males in wire-bottomed cages. On the day a copulation plug was found, the females were isolated, fourteen females were given the chow diet and twenty-eight were given the junk food diet as illustrated in Fig. 1. From the day of parturition and throughout lactation (21 d), the fourteen females from the chow group and their offspring were maintained on the chow diet (CC group), fourteen litters from the junk food diet group were switched from the junk food to the chow diet (JC group) and the remaining fourteen were maintained on the junk food diet (JJ group). On the twenty-first day of lactation, the pups were weaned. Three males and three females from six litters in each nutritional group (216 pups in total) were kept and housed in groups of three siblings per cage such that the males were separated from the females. From weaning up to 10 weeks of age, the pups were either given the chow or the junk food diet, such that there was a total of six different dietary regimes as illustrated in Fig. 1. Therefore, thirty-six weanling pups from each of the CC, JC and JJ groups were weaned on the chow diet; these groups were named CCC, JCC and JJC respectively. Another thirty-six pups from each of the CC, JC and JJ groups were weaned on the junk food diet and were named CCJ, JCJ and JJJ respectively.

Fig. 1 Experimental design. Rats were either fed rodent chow alone (C) or with the junk food diet (J) during pregnancy, lactation and post-weaning up to 10 weeks of age. The numbers in parentheses indicate the number of dams (gestation), litters (lactation) and offspring (post-weaning) in each nutritional group. For details of animals and procedures, see Experimental methods.
Litter sizes were standardised by selecting those that contained between ten and sixteen pups while outsized litters were discarded from the study. Litter sizes at birth were therefore comparable and not statistically different among the six nutritional groups (one-way ANOVA). This method of standardising litter sizes was favoured over reducing litters at birth because it enabled a better standardisation of the number of fetuses during gestation, as previously describedReference Bayol, Simbi and Stickland12. Therefore, maternal feeding behaviour during gestation was studied in a population of dams that were expected to carry comparable numbers of fetuses.
Mothers and offspring were weighed daily throughout the study except for the day of parturition to avoid causing unnecessary distress. The body lengths of the offspring were measured at the end of the experiment (10 weeks from birth), after killing, and were taken from the tip of the nose to the base of the tail. These were used to calculate the BMI, i.e. kg/m2. The daily post-weaning growth rates were calculated according to the following formula: ((body mass at day 70) – (body mass at day 21) / 49 d).
Activity monitoring
In order to determine whether differences in body weights could be directly related to varying levels of activity and voluntary exercise, unstimulated activity levels were measured. The measurements were made in some pregnant mothers at gestation day 20 (G20), (ten for the chow group and sixteen for the junk food group) as well as in two male and two female offspring per litter at postnatal weeks 4, 6, 8 and 10 (twenty-four animals per group, i.e. 144 animals in total; the same animals were used throughout the study). Activity was measured using the Linton AM1053 activity monitor and associated Amonlite software (Linton Instrumentation, Palgrave, Diss, Norfolk, UK). The monitor consisted of two levels of IR light beams (forty-eight in total), which measured activity in X, Y, Z direction and was set up such that the lower set of beams measured the activity of rats walking at the bottom of the tank, while the higher set measured the activity of rats standing on their back legs, namely, ‘rearing’ activity. When the IR light beams were broken by the animals' movement, total activity counts were recorded by the Amonlite software. Activity measurements were performed during the light phase and the animals were left alone in the room while measurements were taken in order to minimise external visual, auditory or scented stimuli that might have interfered with their normal activity. The experiments were set up such that the animals were allowed to settle in the tank for 2 min before measurements were recorded. The measure of activity was recorded every 30 s for a total of 15 min, such that thirty individual measurements were taken from each animal in each experiment. The sum of the ‘total’ as well as ‘rearing’ activity counts from those thirty measurements was then calculated and analysed statistically.
Statistical analyses
Statistical analyses were performed using the SPSS 14·0 for Windows software (SPSS Inc., Chicago, IL, USA).
Pregnancy
Appropriate randomisation of the animals into the six nutritional groups at the start of the experiment was checked by examining the body mass averages and standard deviations. During pregnancy, there were only two types of dietary regimens, namely, chow and junk food diet. The variables (food, energy, macronutrient intakes, body weights and activity levels) were graphically tested for normal distribution using the ‘explore’ function of the SPSS software and non-normal adjustments were not necessary. Differences between these two groups were analysed using an unpaired (or ‘independent samples’) two-tailed Student's t test together with the Levene's test for equality of variances to determine if equal variances should be assumed. Results were considered statistically significant when P < 0·05.
Lactation
During lactation, there were three types of dietary regimens, namely, CC, JC and JJ. The variables (food, energy, macronutrient intakes and body weights) were also graphically tested for normal distribution and non-normal adjustments were not necessary. Differences among these three groups were then analysed by one-way ANOVA. When the one-way ANOVA indicated differences among the three dietary regimens (P < 0·05), post-hoc analyses were performed to determine more specific differences among the three groups. The variables were either tested by the Tukey honestly significantly different (HSD) or the Games-Howell test if the Levene's test was P>0·05 or P < 0·05 respectively. Results were considered statistically significant when P < 0·05 and as trends when 0·05 < P < 0·1.
Post weaning
After weaning, there were six different groups, namely, CCC, CCJ, JCC, JCJ, JJC and JJJ (Fig. 1). The variables (food, energy, macronutrient intakes, body weight, body length and related parameters) from the six groups were tested using a hierarchical two-way (also known as ‘nested’) ANOVA. The fixed factors were defined as ‘group’ and ‘sex’ while ‘mother’ was defined as random factor. The models were designed as ‘group’, ‘sex’, ‘mother(group)’ and ‘group × sex’. The residuals from these analyses were graphically tested for normal distribution and the residuals were plotted against the predicted values for each parameter to verify homogeneity of variances. Non-normal modifications were not necessary. For the food intake analyses, the hierarchical two-way ANOVA showed no significant interaction between group × sex; therefore, results were analysed for the entire rat population combining males and females. When the hierarchical two-way ANOVA indicated statistical differences between ‘group’ (P < 0·05), Tukey HSD post-hoc analyses were performed to determine more specific differences among the six nutritional groups.
For the results on body mass, length, BMI and growth rates, the hierarchical two-way ANOVA indicated interactions between group × sex (P < 0·05); therefore, the data file was split into sexes and differences among the six nutritional groups were analysed independently for males and females by one-way ANOVA followed by post-hoc analyses when P < 0·05. The Games-Howell post-hoc test was used in all cases as the Levene's test for equality of variances indicated that unequal variances should be assumed.
All results were considered statistically significant when P < 0·05 and as trends when 0·05 < P < 0·1. Each post-weaning growth stage was statistically analysed independently from the others and the P values and standard errors of the means for the food analyses are only represented on the graphs for week 10 for clarity. Because the food intakes are not always clearly differentiated on the graphs, the mean values and standard errors of the means for the total energy and energy from the junk food source consumed by the animals at postnatal weeks 4 and 10 are presented in Table 1.
Table 1 Total energy and energy from the junk food source consumed daily by the offspring during post-natal weeks 4 and 10* (Values are means with their standard errors of the mean)
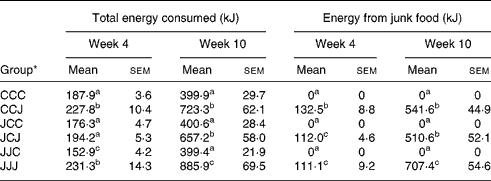
a,b,c Mean values with different superscript letters were statistically different between the six nutritional groups (P < 0·05).
* For details of animals and procedures, see Experimental methods.
Results and discussion
Pregnant dams exhibit hyperphagia and a marked preference for junk food over chow, which is associated with body weight gain and decreased activity levels at gestation day20
Fig. 2(A) shows that pregnant dams given free access to a selection of palatable junk foods together with their normal balanced chow ate approximately 40 % more food (g) and 56 % more energy on average every day compared with those given rodent chow alone (P < 0·001 in both cases). Pregnant rats fed the junk food diet exhibited a marked preference for junk food over chow with only 20 % of the total energy consumed throughout pregnancy originating from the rodent chow. Fig. 2(B) shows that the pregnant rats fed the junk food diet ate more total fat, including saturated fat, more carbohydrates, including sucrose, as well as more salt; however, they reduced their protein and dietary fibre intake compared with rats fed chow alone (P < 0·001 in all cases). These results clearly show that pregnant rats, given ad libitum access to junk food, exhibited hyperphagia characterised by a marked preference for foods rich in fat, sucrose and salt at the expense of protein-rich foods, when compared with rats that only had access to rodent chow. Although the body mass of dams was comparable among all groups at the start of the experiment, the increased energy intake in the junk food group throughout gestation was accompanied by an increase in body mass at G20 with the junk food-fed dams (438·5 (sem 5·2) g) being 13 % heavier than those fed chow alone (386·6 (sem 5·4) g, P < 0·001, supplementary Table 2, available online). The dams included in the study gave birth to statistically comparable numbers of pups, which indicates that the increase in body mass was probably not caused by an increased number of fetuses. At G20, the mothers in the junk food group also exhibited a 27 % (P = 0·038) and 37 % (P = 0·013) reduction in total and ‘rearing’ activity level respectively. Therefore, increased energy intake combined with reduced activity may explain the increased body mass observed at G20 in the cafeteria-fed dams.

Fig. 2 Average daily dietary intake during pregnancy. Pregnant rats fed the junk food diet (J:![]() ) exhibit an increased energy intake (A) as well as a preference for foods rich in fat, carbohydrates and salt at the expense of protein-rich foods (B) compared with those fed rodent chow alone (C:□). Results are means with their standard errors of the mean, n 13 for C and n 28 for J. ***P < 0·001 by unpaired two-tailed Student's t test. For details of animals and procedures, see Experimental methods.
) exhibit an increased energy intake (A) as well as a preference for foods rich in fat, carbohydrates and salt at the expense of protein-rich foods (B) compared with those fed rodent chow alone (C:□). Results are means with their standard errors of the mean, n 13 for C and n 28 for J. ***P < 0·001 by unpaired two-tailed Student's t test. For details of animals and procedures, see Experimental methods.
Lactating dams exhibit hyperphagia and a preference for junk food over chow but this is not accompanied by increased body mass
Fig. 3(A) shows that the hyperphagia and increased intake of foods rich in fat, sucrose and salt observed during pregnancy continued into lactation in rats kept on the junk food diet (JJ group). During lactation, 30 % of the total energy consumed by the JJ group originated from chow. Interestingly, the daily energy intake of rats that were switched from the junk food diet to chow alone from birth (JC group) was reduced by approximately 12 % (P = 0·024) compared with those fed rodent chow throughout (CC group). Consequently, the total fat (including saturated fat), sucrose, protein, fibre and salt intakes were also reduced by approximately 12 % in the JC group compared with the CC group (Fig. 3(B)).

Fig. 3 Average daily dietary intake during lactation. Lactating rat dams fed the junk food diet (JJ:![]() ) exhibit an increased energy intake (A) as well as a preference for foods rich in fat, sucrose and salt at the expense of protein-rich foods (B), while rats rehabilitated to a chow diet from a junk food diet during gestation (JC:
) exhibit an increased energy intake (A) as well as a preference for foods rich in fat, sucrose and salt at the expense of protein-rich foods (B), while rats rehabilitated to a chow diet from a junk food diet during gestation (JC:![]() ) exhibit a reduced energy and nutrient intake (A and B) compared with rats fed rodent chow throughout (CC:□). Results are means with their standard errors of the mean, n 14 for each group. a,b Mean values with different superscript letters were statistically different among the six nutritional groups (P < 0·05). *Indicates trends, i.e. 0·05 < P < 0·1 by one-way ANOVA and post-hoc analyses. For details of animals and procedures, see Experimental methods.
) exhibit a reduced energy and nutrient intake (A and B) compared with rats fed rodent chow throughout (CC:□). Results are means with their standard errors of the mean, n 14 for each group. a,b Mean values with different superscript letters were statistically different among the six nutritional groups (P < 0·05). *Indicates trends, i.e. 0·05 < P < 0·1 by one-way ANOVA and post-hoc analyses. For details of animals and procedures, see Experimental methods.
Despite consuming more energy throughout lactation, the body mass of mothers in the JJ group was comparable with those in the CC and JC groups at the end of lactation, namely, 21 d post-partum (CC 360·4 (sem 5·8) g; JC 363·9 (sem 5·7) g; JJ 349·4 (sem 6·1) g; P = 0·514; supplementary Table 2, available online). The activity levels were not measured in lactating dams but the lack of body mass increase, despite consuming more energy, suggests that the mothers fed the junk food diet during lactation may have invested more energy in milk production and therefore their milk may have been richer than that produced by the chow-fed lactating rats. In the present study, we did not measure milk composition; however, another study using a variation of the junk food diet model showed that cafeteria-fed dams produced milk that was richer in energy and fatReference Rolls, Gurr, van Duijvenvoorde, Rolls and Rowe13. Therefore, it is likely that in our model, too, the dams fed the junk food diet might also have produced richer milk and we propose that this might partly explain the lack of body weight gain in the JJ group despite increased energy consumption throughout lactation.
The influence of a maternal junk food diet on the body weight of offspring at birth and at weaning: similarities with the maternal low protein diet model
Despite increased maternal energy intake during pregnancy and comparable litter sizes, the offspring born to mothers fed the junk food diet (6·26 (sem 0·03) g) exhibited a marginal (4 %) but significant reduction in birth weight compared with those fed chow alone (6·55 (sem 0·06) g, P < 0·001). Similarly, at weaning, the body mass of pups from the JJ group (49·39 (sem 0·57) g) remained lower compared with those fed rodent chow alone throughout, namely, the CC group (52·85 (sem 0·40) g, P < 0·001). Switching from the junk food diet during gestation to rodent chow alone during lactation was even more detrimental for the growth of the offspring, as weanling pups from the JC groups were lighter in weight (43·92 (sem 0·57) g) compared with both the CC and JJ groups (P < 0·001 in both cases; supplementary Table 3). These results on offspring's body weights at birth and weaning are in line with previous observations made in our laboratory using the same cafeteria diet modelReference Bayol, Simbi and Stickland12. However, in the previous study, body mass results did not reach statistical significance due to much smaller numbers of animals being used.
Another study using a variation of the cafeteria diet modelReference Holemans, Caluwaerts, Poston and Van Assche14 showed that litter sizes were increased, whereas birth weights were not affected. However, in Holemans et al's study, the cafeteria diet was given to the animals 4 weeks before mating, while in the present study it was given on the first day of gestation. The reasons why the birth and weaning weights of offspring exposed to the junk food diet were reduced in the present study are unclear but one explanation might be maternal protein intake. Despite increased total energy intake, the cafeteria-fed dams decreased their protein intake by approximately 37 % and 34 % during gestation and lactation respectively. Previous reports have shown that isoenergetic but 50 % and 60 % protein-restricted diets throughout pregnancy induced a reduction in the offspring's birth weightsReference Langley-Evans and Nwagwu15, Reference Bieswal, Ahn, Reusens, Holvoet, Raes, Rees and Remacle16. In the current study, the voluntary protein restriction (37 %) during gestation was not as severe as 50 % and 60 % and the reduction in birth weight observed (4 %) was also less severe than the reported 9·5 % and 17 % reduction associated with 50 % and 60 % protein reduction respectivelyReference Langley-Evans and Nwagwu15, Reference Bieswal, Ahn, Reusens, Holvoet, Raes, Rees and Remacle16. Similarly, a 60 % protein restriction during both gestation and lactation led to a 25 % reduction in body weight at weaningReference Bieswal, Ahn, Reusens, Holvoet, Raes, Rees and Remacle16, which is also consistent with the present results, showing a 7 % reduction in body mass at weaning in pups from the JJ group compared with the CC group. In light of this, it thus appears that the voluntary reduction in protein intake during gestation and lactation in cafeteria-fed dams may be a key factor in explaining the reduced birth and weaning weights observed and that maternal protein intake rather than overall energy intake may play a major role in regulating the offspring's body mass at birth and at weaning. However, protein intake alone does not explain why the weaning weight of pups from the JC groups was lower compared with both the CC and JJ groups, as the protein intake during lactation in this group was greater than in the JJ group. It thus appears that increasing protein intake during lactation was not sufficient to restore normal body mass in weanling pups exposed to the junk food diet during their fetal life.
A maternal junk food diet before weaning promotes an exacerbated preference for junk food and leads to a greater propensity for obesity in the offspring
The main focus of the present study was to examine whether a maternal junk food diet during pregnancy and lactation could influence the long-term feeding behaviour, growth rate and activity levels of offspring. Results in Fig. 4(A) and Table 1 show that at postnatal week 10, the rats weaned on junk food, namely, the CCJ, JCJ and JJJ groups, increased their energy intake compared with those weaned on rodent chow alone, namely, the CCC, JCC and JJC groups. However, rats exposed to the junk food diet during gestation and lactation (JJJ group) exhibited an approximately 18 % and 26 % daily increase in energy intake compared with other offspring weaned on junk food, but which were fed rodent chow alone during both gestation and lactation (CCJ group) or during lactation alone (JCJ group), (P < 0·001 in both cases).We further characterised the source of energy intake in junk food diet-fed rats and results in Fig. 4(B) show that the energy intake from chow was comparable in all offspring weaned on the junk food diet, namely, CCJ, JCJ and JJJ groups. However, Fig. 4(C) shows that the energy intake from the junk food source was increased in the JJJ group compared with the CCJ and JCJ groups (P < 0·001 for both cases). Further analyses showed that the exacerbated intake of junk food in the JJJ group was characterised by a selective preference for foods rich in fat, sugar and salt but not in proteins and fibres (supplementary Fig. 2). Therefore, although all young rat offspring ‘enjoyed’ eating junk food and favoured it over chow, particularly during the latest stages of growth examined (Fig. 4(D)), the animals that were exposed to the junk food diet during pregnancy and lactation (JJJ group) exhibited exacerbated hyperphagia and a greater preference for junk food compared with offspring fed a balanced chow diet prior to weaning (CCJ group) or during lactation (JCJ group). It is important to note that offspring exposed to the junk food diet during gestation alone (JCC group) or during gestation and lactation (JJC group) and were then weaned on chow did not exhibit hyperphagia (Fig. 4(A)). Pups from the JJC group exhibited a reduction in energy intake for the first 2 weeks from weaning compared with both CCC and JJJ groups (Fig. 4, Table 1), indicating that removing access to the palatable food at weaning induced a temporary reduction in energy intake, which then returned to control levels by week 10.

Fig. 4 Average daily energy intake for postnatal weeks 4 to 10. Offspring exposed to the junk food diet (JJJ) throughout the study exhibit exacerbated hyperphagia (A) and an increased taste for junk food (B, C and D). Open symbols indicate animals weaned on chow alone while filled symbols indicate those weaned on junk food: CCC ∘; CCJ ●; JCC Δ; JCJ ▲; JJC □; JJJ ■; energy from chow ![]() ; energy from CD ■. a,b,c Different letters at week 10 indicate statistical differences among the six nutritional groups (P < 0·05) by hierarchical two-way ANOVA followed by Tukey honestly significantly different (HSD) post-hoc analyses. For details of animals and procedures, see Experimental methods.
; energy from CD ■. a,b,c Different letters at week 10 indicate statistical differences among the six nutritional groups (P < 0·05) by hierarchical two-way ANOVA followed by Tukey honestly significantly different (HSD) post-hoc analyses. For details of animals and procedures, see Experimental methods.
To examine whether energy intake in offspring could be directly influenced by variation in activity level, we measured activity levels during the light phase over a 15 min period at postnatal weeks 4, 6, 8 and 10. Results showed that the six dietary regimens examined did not influence the total and ‘rearing’ activity levels in the offspring, whether males or females (data not shown). Therefore, we found no evidence that the six dietary regimens influenced activity levels such that it might be the predominant explanation for the increased energy intake in cafeteria-fed rats. However, a full characterisation of the animals' activity including nocturnal activity, which is when rats are normally more active, over a longer period, is required to fully eliminate activity as a significant factor explaining the increased energy intake. We also noted large variations in activity levels in female offspring and feel that this might be caused by differences in ovulation status. Female rats appeared to shiver and be hyperactive when ovulating. Therefore, for future activity monitoring experiments, it might be beneficial to take into account the ovulation status of female rats to avoid such variability, which may mask the true effects of the treatment.
The increased energy intake in the JJJ group over all other groups was accompanied by an increase in body mass, BMI and post-weaning growth rates in both male and female offspring at week 10 (Fig. 5(A),(B),(D)), even though JJ offspring exhibited lower body mass at weaning compared with CC offspring. The increased body mass and BMI in the JJJ group compared with the CCC group were greater in females (32 % and 21 % respectively) than in males (22 % and 18 % respectively). This indicates that females were more prone to weight gain than males when exposed to a junk food diet throughout the study. Fig. 5(C) also shows that the dietary regimens examined did not influence the body length of male offspring. However, a junk food diet throughout the study (JJJ group) or after weaning (CCJ group) induced an increase in body length in female offspring (Fig. 5(C)).

Fig. 5 The influence of a maternal junk food diet on body mass, length and associated parameters in offspring. Male ![]() and
and ![]() female offspring fed the junk food diet throughout the study exhibit increased body mass (A), BMI (B) and post-weaning growth rates (D) while body length is only affected in female offspring (C). Different capital and lower case letters indicate statistical differences among the six nutritional groups in male and female offspring respectively (P < 0·05) by one-way ANOVA and Games-Howell post-hoc analyses. For details of animals and procedures, see Experimental methods.
female offspring fed the junk food diet throughout the study exhibit increased body mass (A), BMI (B) and post-weaning growth rates (D) while body length is only affected in female offspring (C). Different capital and lower case letters indicate statistical differences among the six nutritional groups in male and female offspring respectively (P < 0·05) by one-way ANOVA and Games-Howell post-hoc analyses. For details of animals and procedures, see Experimental methods.
Results in Fig. 5(A) also show that despite being heavier at weaning, pups in the CCJ group were 15 % and 18 % lighter than males and females from the JJJ group respectively at week 10 (P = 0·012 and P = 0·019 for males and females respectively). Surprisingly, males from the CCJ group were not statistically heavier than the males from the CCC group; however, their BMI was greater (Fig. 5(A),(B)). On the contrary, the females from the CCJ group were heavier than those in the CCC group but their BMI were comparable (Fig. 5(A),(B)). These results therefore show that a balanced diet during gestation and lactation can provide some protection over a junk food diet-induced obesity in offspring.
Taken together the results show that exposure to a maternal junk food diet during gestation and lactation promotes exacerbated hyperphagia with a selective preference for junk foods rich in fat, sugar and salt, as well as overweight gain when compared with offspring also given free access to junk food but which were exclusively fed a balanced chow diet before weaning or during lactation alone.
Is palatability the main driving stimulus for the exacerbated hyperphagia?
The present study shows that, like man, rats, whether pregnant and lactating dams or young offspring, exhibited a preference for palatable foods rich in fat, sugar and salt over plain yet nutritionally balanced rodent chow. In addition, when the palatable food was removed and the rats were given access to rodent chow alone (JC mothers and JJC pups), they reduced their energy intake compared with rats that had not been exposed to the junk food diet. This further emphasises that palatability plays a major role in appetite regulation and energy intake as previously reviewedReference Erlanson-Albertsson17. However, it is important to note that a similar reduction in food intake has been reported in non-pregnant obese female mice, which were switched from a high-fat to standard chow dietReference Gallou-Kabani, Vige, Gross, Boileau, Rabes, Fruchart-Najib, Jais and Junien18, indicating that variations in fat content alone can cause a reduction in food intake. Nevertheless, fat content alone may not explain the increased energy intake observed in the junk food-fed rats, as it has been reported previously that pregnant rats fed a high-fat chow diet reduced their food intake (g) such that their gross energy intake was comparable with a control group fed a standard chow dietReference Khan, Dekou, Douglas, Jensen, Hanson, Poston and Taylor19.
The mechanisms that regulate appetite are complex and not yet fully elucidated. Appetite regulation involves cross talk between appetite centres in the brain and peripheral factors, such as leptin, insulin and ghrelin, to regulate energy balance, satiety and hungerReference Broberger20. Nevertheless, feeding is not only a matter of regulating energy balance; it is also a pleasurable experience that involves ‘reward centres’ in the brain, such that the combination of pleasure with feeding may occasionally override the normal regulation of satietyReference Broberger20. More specifically, palatable foods rich in fat and sugar have been shown to inhibit the satiety signals while promoting hunger and stimulating the reward centresReference Erlanson-Albertsson17. One key question in the present study is what are the underlying mechanisms driving the JJJ group to overeat compared with all other groups? Factors such as activity, body composition and body weight maintenance as well as a varying sensitivity to food palatability might all contribute to this. More active rats would require more energy intake but activity measurements showed no conclusive evidence that the diets significantly influenced the rats' activity. We have shown previously that our junk food diet model could influence body composition in weanling rats, leading to muscle atrophy and increased adiposityReference Bayol, Simbi and Stickland12. Different body composition may influence energy expenditure and thereby energy intake. Heavier bodies might also require more energy to sustain a steady weight and, indeed, the rats from the JJJ group were overweight at week 10 compared with those in all other groups. Furthermore, examination of energy intake per g body weight at week 10 showed no differences among the three groups weaned on the junk food diet (supplementary Fig. 3(A)), suggesting that sustaining body mass might also influence the animals' food intake, particularly during the later stages of growth examined. However, sustaining body weight is unlikely to be the main driving factor explaining the exacerbated hyperphagia observed in the JJJ group, at least immediately after weaning, because at weaning these offspring were significantly lighter than those in the CCJ group and yet the energy intake between these two groups during the first week from weaning were comparable. Furthermore, pups in the JJC group, which were equally underweight as those in the JJJ group at weaning, did not increase their energy intake when weaned on chow alone. Therefore, if sustaining body mass or ‘catch-up growth’ were the major driving stimuli to explain the exacerbated hyperphagia observed in the JJJ group, then the JJC pups would also overeat regardless of their diet.
Further evidence that food palatability may be the main driving stimulus for the exacerbated hyperphagia observed in the JJJ group comes from the study of the source of energy consumed. All rats fed the junk food diet after weaning (CCJ, JCJ and JJJ groups) ate comparable quantities of rodent chow and the excess energy intake in rats from the JJJ group exclusively originated from the junk food source. In addition, these rats preferentially selected foods rich in fat, sugar and salt but not in protein, therefore arguably more palatable. This indicates that palatability as well as energy, fat, sugar and salt content might be a major driving stimulus for the excessive food intake observed in the JJJ group. This is further supported by the feeding behaviour of animals in the JJC group, which were also exposed to the junk food diet before weaning. In this group, when the palatable food, i.e. the factor that we suspect is promoting the exacerbated overeating, was removed and the rats were only given exclusive access to rodent chow, their energy intake was not only reduced compared with the JJJ group but was also reduced compared with the CCC group for 2 weeks from weaning, before reaching CCC levels by week 10. In our view, this indicates that food palatability was therefore a major driving stimulus for the exacerbated hyperphagia observed in the JJJ group.
In light of these, it thus appears that exposure to a maternal junk food diet during gestation and lactation promotes an exacerbated taste for palatable foods rich in fat, sugar and salt in the offspring. The present study therefore suggests that exposure to a maternal junk food diet during the fetal and suckling life of an individual might be a contributing factor as to why some individuals might find it easier than others to control their junk food intake when given free access to a cafeteria-style diet.
Lactation: an important period for the ‘programming’ of an exacerbated intake of junk food
It appears that post-weaning hyperphagia can be programmed during the fetal life of an individual through maternal under-nutritionReference Vickers, Breier, Cutfield, Hofman and Gluckman21 as well as during the suckling period by increased intake of milkReference Oscai and McGarr22, Reference Erlanson-Albertsson and Zetterstrom23. The present study further emphasises that maternal nutrition during lactation might play a key role in influencing the long-term appetite of the offspring given free access to junk food. Although groups CJC and CJJ were not included in the study, the importance of lactation is illustrated in rats exposed to the junk food diet during gestation and after weaning but not during lactation. Offspring from the JCJ group did not exhibit the long-term increased energy intake that was observed in the JJJ group (Fig. 4) and their body mass, BMI, body length and post-weaning growth rates were comparable with the CCC group. It thus appears that switching to a rodent chow diet during lactation and the associated 12 % voluntary reduction in energy intake (Fig. 2) prevented the exacerbated hyperphagia and overweight gain observed in offspring fed the junk food diet throughout the study (JJJ). The importance of the suckling period for the programming of hyperphagia in offspring has been previously described in the litter size reduction modelReference Oscai and McGarr22 and it was suggested that overall milk intake as well as milk composition might be key regulators of the development and maturation of the central and peripheral control of appetite. In the present study, we did not measure milk composition but it appears that switching from a junk food diet to chow at birth might have influenced milk production, composition and/or the lactating behaviour of the dams leading to some protection against the exacerbated hyperphagia and overweight gain observed in the JJJ group.
Conclusions
The present study shows that rats given free access to junk food increase their energy intake and spontaneously exhibit a preference for fatty, sugary and salty foods. The long-term preference and intake of junk food is further exacerbated when offspring have been exposed to the junk food diet during pregnancy and lactation leading to a greater propensity for obesity. However, a combination of mild energy restriction (12 %) and a balanced diet during lactation can prevent the over-excessive consumption of junk food and associated overweight gain. It also appears that food palatability might be an important driving stimulus for the exacerbated hyperphagia observed.
This study therefore emphasises that healthy eating habits should be encouraged, not only in young children but also in pregnant and breastfeeding women, to help combat the obesity epidemic. The message to instil in populations is that women may not consider pregnancy and breastfeeding as an opportunity to overindulge on fatty, sugary and salty foods, on the misguided assumption that they are ‘eating for two’. Indeed, we show evidence that a maternal junk food diet might promote an exacerbated taste for junk food and a greater propensity for obesity in offspring, which might in turn make it more difficult to encourage healthy eating habits and thereby control obesity and related problems.
Acknowledgements
The authors thank staff of the Biological Services Unit at the RVC, as well as Julie Dupau, Natalia Perez and the Muscle Unit for their help with the animal work. The authors also thank Aviva Petrie for her help and advice with the statistical analyses. This work was funded by the Wellcome Trust.
Note
Supplementary information accompanies this paper on the Journal's website (http://journals.cambridge.org).








