INTRODUCTION
Acute gastroenteritis (AG) is an important public health concern worldwide [Reference Kosek, Bern and Guerrant1]. It is a common but largely preventable illness. The symptoms of AG include diarrhoea and/or vomiting, occasionally abdominal pain, cramps, and fever [Reference Kuusi2]. Modes of transmission for AG can be through food, water, person-to-person, as well as animal contacts [Reference Mitakakis3–Reference Caprioli7]. Although AG is usually self-limiting, it can have severe consequences, particularly in the vulnerable groups such as children and the elderly. The medical and social costs associated with AG can be extensive [Reference Smith8]. In the USA, 211 million episodes of AG occur each year, resulting in >0·9 million hospitalizations and >6000 deaths [Reference Mead9]. Investigation of disease burden and risk factors have been extensively studied, based on laboratory confirmation and surveillance information [Reference Majowicz10, Reference Thomas11]. Several population-based studies of AG have been conducted in Western countries [Reference Flint12] such as UK [Reference Wheeler13], The Netherlands [Reference de Wit14], Norway [Reference Kuusi2], Ireland [Reference Scallan15], Australia [Reference Hall16], Malta [Reference Gauci17], and USA [Reference Jones18]. However, studies on burden of AG in Asia have mainly been hospital-based [Reference Nakagomi19], pathogen-specific [Reference Lee20], targeting children [Reference Lee, Poo and Nagaraj21], or on clinical characteristics, disease trends or outbreak cases [Reference Tsang22, Reference Cheng23]. Based on extrapolations from laboratory data and physician consultation, a recent study in Japan has estimated the incidences of foodborne infections from Campylobacter, Salmonella and V. parahaemolyticus to be 237, 32 and 15/100 000 per year, respectively [Reference Kubota24]. In most localities, disease reporting is mainly based on statutory notifications or laboratory reporting. As only a small proportion of AG illness comes into contact with the formal health service [Reference Scallan25], under-reporting of enterics based on this information is a recognized problem [Reference Thomas11, Reference Voetsch26]. We conducted the first population-based study in Hong Kong to investigate the disease burden of AG and also the types of risky behaviour more prevalent in those affected with AG. This is also the first such study conducted in Asia.
METHODS
Study design and population
The study was a territory-wide population-based retrospective telephone survey conducted over one year. A pilot study conducted in June 2006 consisting of 200 interviews provided an estimate of 6·6% AG prevalence [Reference Ho27]. A target sample size of 3700 subjects was planned according to the formula suggested by Gauci et al. [Reference Gauci17]. The study proper was conducted from 1 August 2006 to 31 July 2007. Subjects were recruited through random digit dialling with an even distribution of about 70 interviews conducted per week over the study period.
A modified random digit-dialling method was adopted. Telephone numbers were first selected by systematic sampling from the 2005 residential telephone directories. As some telephone numbers might not be listed in the directories, an expanded list was created. First, the last digit of the selected numbers were discarded, the numbers were appended with 0 to 9 to form the expanded list. A sample of numbers was selected from this expanded list by means of systematic sampling, and the final list contained 8335 listed numbers and 14 590 unlisted numbers.
At least five attempts were made at different times (between 09:00 and 22:00 hours) of the day and on different days of the week (two weekdays and one weekend) before a phone number was considered as non-contactable. One member of the household, selected by the last birthday method [Reference Watson28], was interviewed over the telephone. Proxy (guidance) response was adopted if the selected subject was aged <15 years or physically and/or mentally unfit to respond to the questionnaire interview. The study was approved by the Survey and Behavioural Research Ethics Committee of The Chinese University of Hong Kong.
Data collection
The questionnaire was based on the validated questionnaire developed and used by members of the International Collaboration on Enteric Disease ‘Burden of Illness’ Studies [Reference Hall16, Reference Jones18, Reference Majowicz29] but modified to suit the local situation. The questionnaire was first developed in English and then translated into Cantonese and Mandarin. It was then pre-tested and further refined using 120 respondents randomly selected from the telephone directory.
The interviews were conducted by telephone by trained interviewers in Cantonese, Mandarin or English as appropriate. The structured questionnaire collected information on (i) the occurrence of diarrhoea or vomiting during the 4 weeks prior to interview; the presence of other household members with AG; the duration of illness, the maximum number of diarrhoea and vomiting episodes during a 24-h period. The respondents were also asked to respond to a list of other symptoms which were AG-related including respiratory symptoms, coughing, sneezing, runny nose, sputum and nasal congestion. A subject would only be considered as having an AG-related respiratory symptom if they confirmed this in a follow-up question: self-interpretation on the cause of illness and, if food related, the food believed to have caused the illness, (ii) the use of medication, medical consultation sought, admission to hospital and whether a stool sample was sent for diagnostic purposes; (iii) practice of hand washing before meals, and questions which aimed to examine ‘risky’ food behaviours in the previous 4 weeks; meal types or food habits (takeaway foods, leftover foods, buffet meal, hot pot, self-grilled foods, poon choi Footnote †, snacks bought at the roadside); consumption of food items (ice-cream, salad, sushi, sashimi, oyster) and other selected food items and their degree of doneness when consumed (fish, egg, beef, chicken, oyster, shrimp, crab, clam, etc.; and (iv) demographic characteristics.
Definition of AG
The symptom-based definition of AG, as suggested by Majowicz et al. [Reference Majowicz30], was adopted for this study. AG was defined as ⩾3 loose stools or any vomiting in any 24-h period. Vomiting or diarrhoea due to non-infectious causes such as Crohn's disease, ulcerative colitis, irritable bowel syndrome, excess alcohol, pregnancy, menstruation or medication was excluded. AG respondents with chronic gastroenteritis who experienced infectious AG over the previous 4 weeks were included as AG cases [Reference Majowicz29]. Respondents with conditions not meeting the definition of AG were considered as non-cases. Only the last episode of AG was considered [Reference Majowicz29], using the most conservative approach for the estimation of disease burden.
Data analysis
The prevalence of AG in the 4 weeks prior to interview was calculated based on the defined AG cases (n=262) and the total number of respondents (n=3743); while the incidence per person-year was calculated by multiplying the prevalence rate by 13, as the number of 4-week periods in a year is 13·03 (365/28). The prevalence of AG in the Hong Kong population was estimated with adjustment made for age, sex and residential district according to the 2006 Population by-census (Table 1) [31]. χ2 and t tests were used for testing the association between socio-demographic factors and the occurrence of AG. Logistic regression analysis was used for the estimation of risky behaviours associated with AG. Data were analysed using SPSS 13.0 (SPSS Inc., USA).
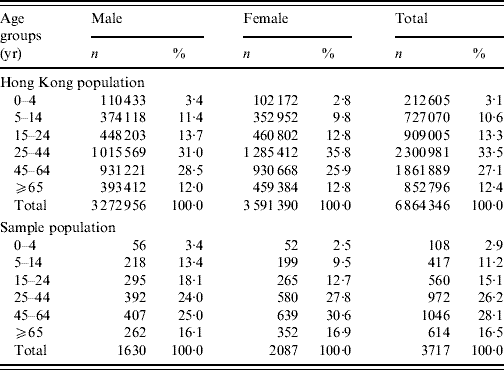
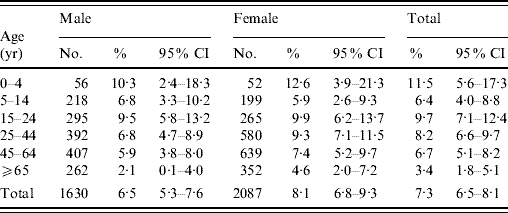
Table 1. Comparison of the distribution of the studied sample with the Hong Kong populationFootnote *, by sex and age groups
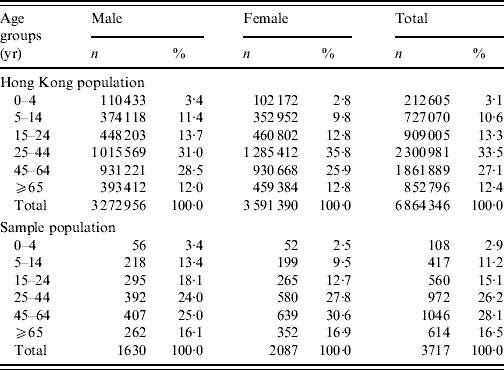
No significant differences were found between the sample and the Hong Kong population for both sexes, and overall population.
* Source: 2006 by-census, Census & Statistics Department, Government of HKSAR [32].
RESULTS
Response rate and study sample
The overall response rate was 41% with 3743 completed and 237 partially completed interviews. The incomplete questionnaires were excluded from analysis. The age distribution of the study participants and the general population was similar. Although the study sample had a slightly higher prevalence of females, the gender distribution was not significantly different from that of the general population (Table 1).
Of the completed interviews, 673 were conducted by proxy: 586 for those aged ⩽15 years and 87 for those with physical, mental or language difficulties in responding to the interview. In total, 482 experienced AG in the 4 weeks prior to interview. Of these, 44 experienced chronic gastroenteritis due to medication or medically confirmed chronic illnesses and two had pregnancy-induced vomiting. A total of 174 had ⩾2 episodes of diarrhoea within a 24-h period and were not considered as AG cases. Respondents (n=262) with episodes meeting the case definition were considered as having AG in the reporting period.
Prevalence of AG
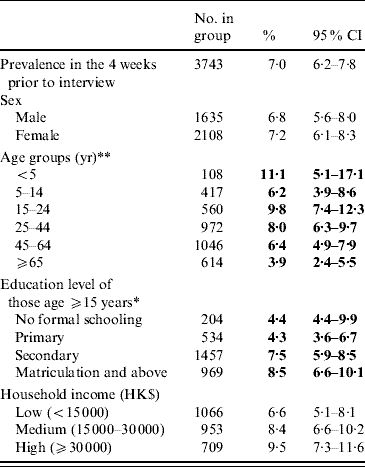
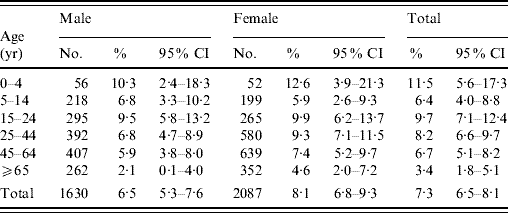
An overall prevalence of 7·0% of AG was found, with women having a higher prevalence than men (Table 2). Children aged ⩽5 years had the highest prevalence of AG while the older age group (⩾65 years) had the lowest prevalence. Respondents with higher education also tended to have a higher reporting of AG. Table 3 shows the distribution of AG by sex and age groups. An overall adjusted prevalence of 7·3% was observed when weighting for age, sex and residential district according to 2006 Hong Kong by-census data was applied [32]. Age standardization to the 2005 world population [31] resulted in a slightly higher prevalence of 7·7%.
Table 2. Prevalence of reporting acute gastroenteritis (AG) in the 4 weeks prior to interview in the different socio-demographic groups of the study population
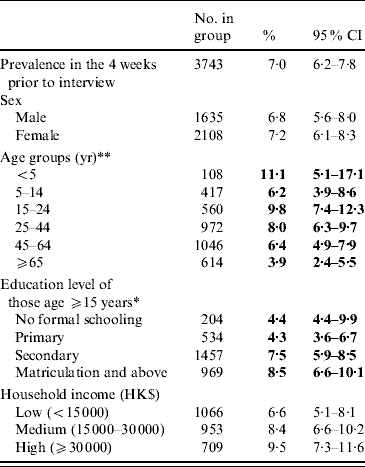
CI, Confidence interval.
* p<0·01, ** p<0·001 by χ2 test comparing AG prevalences in different categories.
Figures in bold denote statistical significance.
Table 3. Distribution of respondents and prevalence of acute gastrointestinal illness by sex and age groups in a 4-week period in a sample of the Hong Kong population (n=3743), with age, sex and residential district weighted according to the 2006 Hong Kong populationFootnote *
CI, Confidence interval.
* Source: 2006 by-census, Census & Statistics Department, Government of HKSAR [32].
† Twenty-six respondents with age missing.
Disease burden
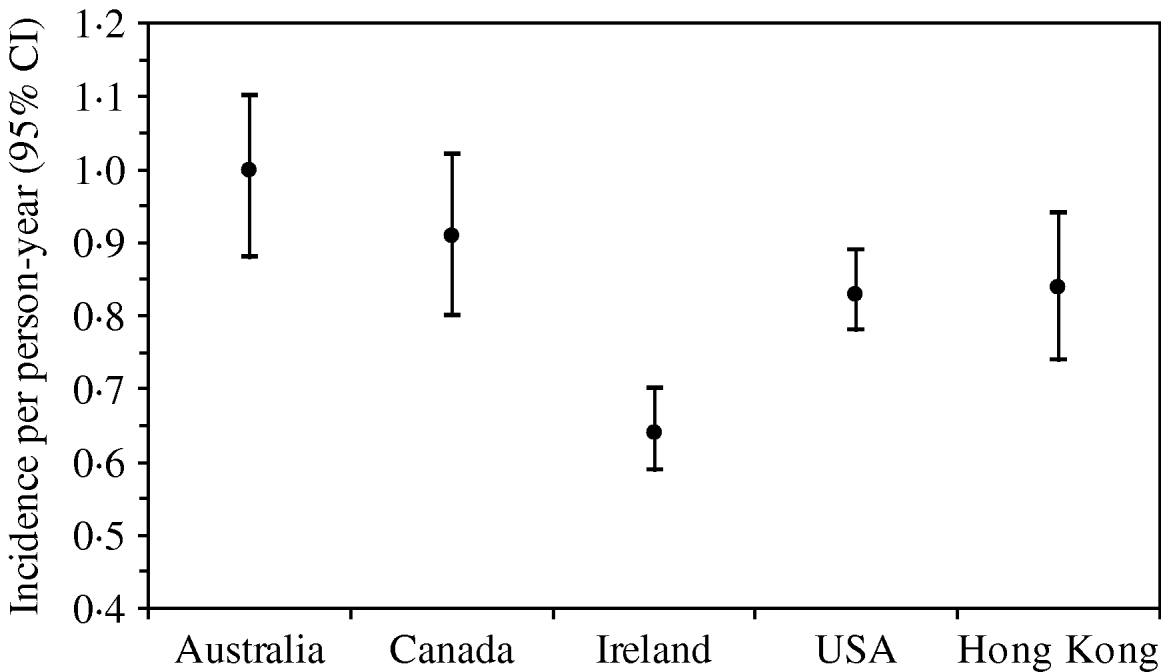
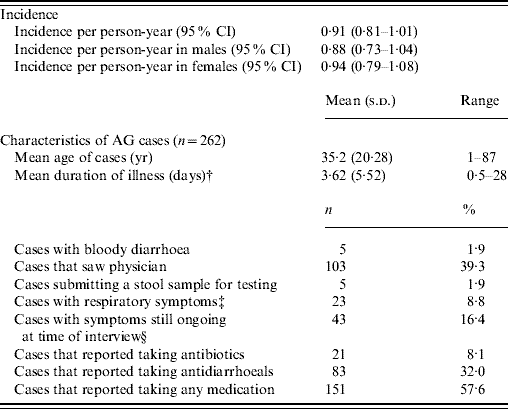
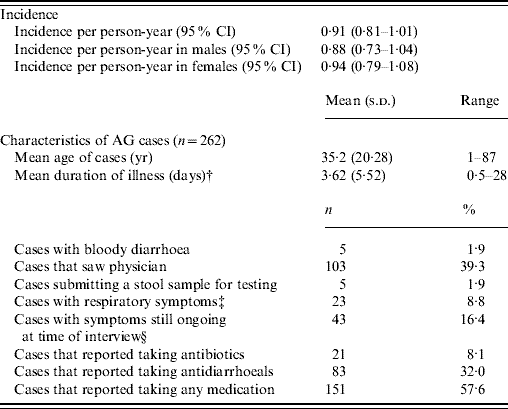
The rate of AG episodes per person-year was estimated as 0·91 (95% CI 0·81–1·01) (Table 4). Based on a similar AG case definition and method of estimation [Reference Majowicz30], the incidence of AG in Hong Kong (0·84) is similar to that of the USA, Canada and Australia while higher than that of Ireland (Fig. 1). The mean age of AG cases was 35·2 years (range 1–87), the mean duration of illness was 3·62 days (range 0·5–28), while 16% still had symptoms at the time of interview (Table 4). The report of bloody diarrhoea was rare (2%). Of AG cases, 8·8% also reported respiratory symptoms which included coughing, sneezing, sore throat, runny nose, sputum and nasal congestion. Thirty-nine percent consulted a physician but only 2% submitted a stool sample for testing. Of AG cases, 58% used medication, of which 14% took antibiotics and 55% antidiarrhoeal medicine; of the seven (2·6%) admitted to hospital, three were aged <10 years, one in the 15–19 years age group, and three were aged ⩾65 years.
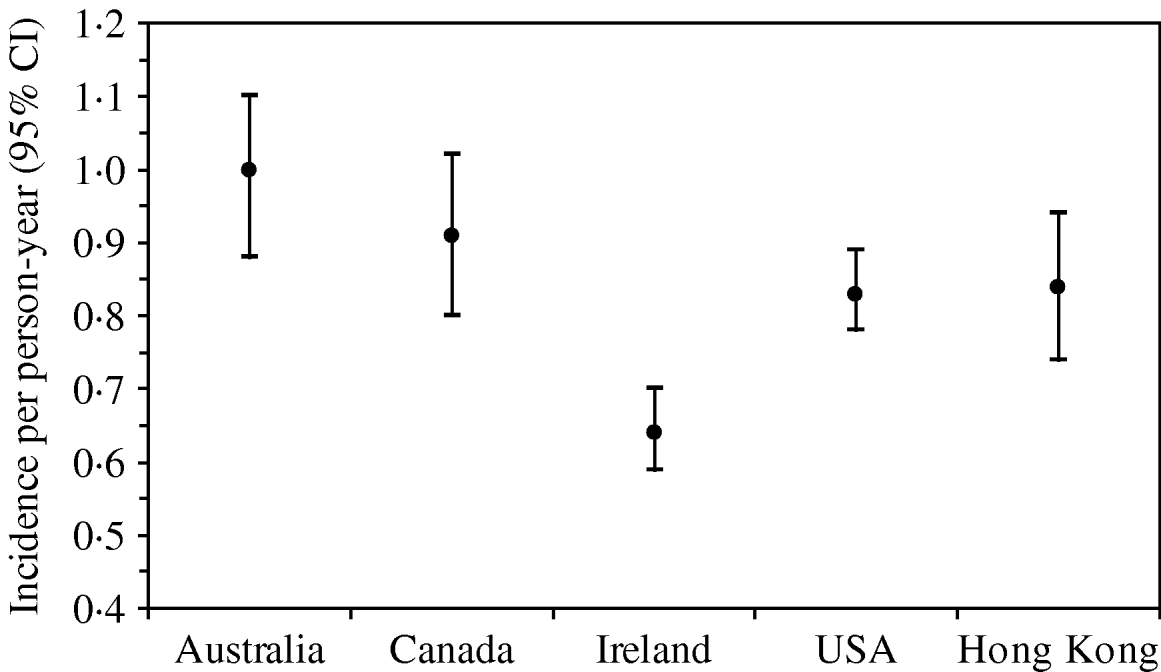
Fig. 1. Incidence and 95% confidence intervals of acute gastroenteritis using a similar standard case definition† in Australia, Canada, Ireland, USA and Hong Kong. († Standard case definition: ⩾3 loose stools, or any vomiting, in 24 h, excluding those (a) with cancer of the bowel, irritable bowel syndrome, Crohn's disease, ulcerative colitis, cystic fibriosis, coeliac disease, or another chronic illness with symptoms of diarrhoea or vomiting, or (b) who report their symptoms were due to drugs, alcohol, or pregnancy. Individuals meeting the exclusion criteria were retained in the non-case group [Reference Majowicz30].) (The incidence rate in Hong Kong was calculated by multiplying the 4-week prevalence by 12, as in other countries, for ease of comparison.)
Table 4. Epidemiology of acute gastroenteritis (AG) under the standard case definitionFootnote *
CI, Confidence interval.
Incidence rates were calculated by multiplying the 4-week prevalence by 13.
* Individuals not meeting the AG case definition were considered a non-case [case definition: ⩾3 loose stools, or any vomiting in 24 h, excluding those (a) with cancer of the bowel, irritable bowel syndrome, Crohn's disease, ulcerative colitis, cystic fibriosis, coeliac disease, or another chronic illness with symptoms of diarrhoea or vomiting, or (b) who report their symptoms were due to drugs, alcohol, or pregnancy].
† Mean duration calculated by averaging the duration of illness for all cases, regardless of whether they were still ongoing at the time of data collection.
‡ Coughing, sneezing, sore throat, running nose, sputum and nasal congestion.
§ For retrospective studies.
Eating practices and AG
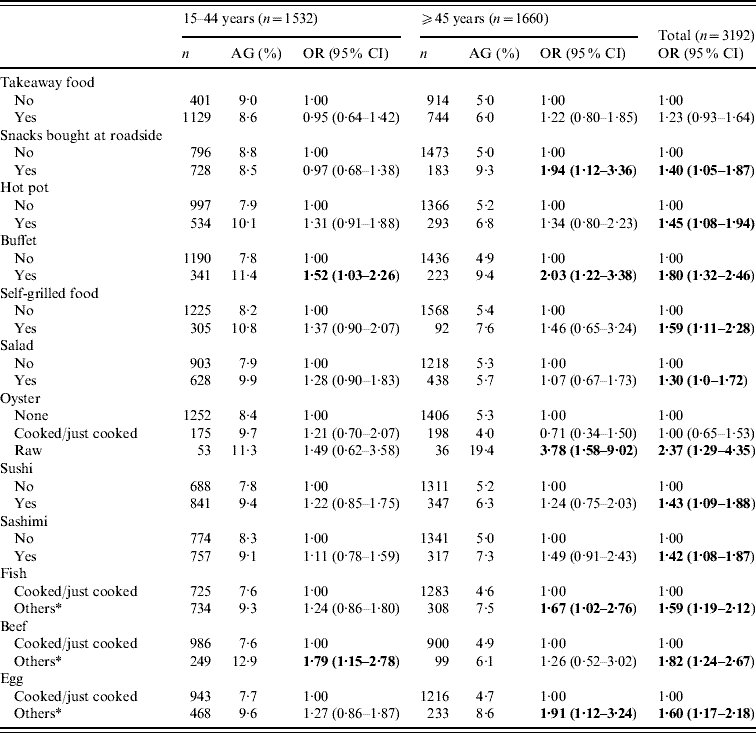
Of the AG cases, 45% (118/262) believed that their AG could be food related. Of the 77 subjects who could recall the specific problematic food items, 39% (30/77) thought it was due to seafood (oyster, shellfish, fish). The distribution of the other items was quite scattered, encompassing different types of meat, fruits and vegetables. Of the 77 cases, 45·5% believed that the contaminated food source was from dining out, 10·4% from takeaway foods, 9·1% from buffets, and 15·6% thought the source was from foods served at home.
A separate section of the structured questionnaire enquired about the food-eating practices and behaviour over the previous 4 weeks. Stratified analyses by age groups (<15, 15–44, ⩾45 years) showed that for those aged <15 years, only salad eating had a significant and inverse association with AG (OR 0·29, 95% CI 0·09–0·95). Table 5 shows the food or meal preferences found to be more prevalent in those with AG in the ⩾15 years age group. Some practices such as ‘snacks bought at roadside’, ‘eating raw oysters’, ‘partially cooked/raw egg or fish’ were more strongly and significantly associated with AG in those aged ⩾45 years than in the younger age groups (15–44 years). Overall, eating raw oysters (OR 2·37, 95% CI 1·29–4·35), partially cooked beef (OR 1·82, 95% CI 1·24–2·67) and buffet meals (OR 1·80, 95% CI 1·32–2·46) seemed to be the strongest risk behaviour found in those with AG; followed by partially cooked/raw egg, fish and self-grilled foods. Of those aged ⩾65 years, ‘snacks bought at roadside’ and ‘eating partially cooked/raw eggs’ were the only practices correlated with AG (data not shown). Only 5% reported ‘not washing hand before meals’ and such practice was not found to be associated with AG. The presence of ‘other family member(s) with AG’ was significantly associated with an increased risk of AG (OR 3·03, 95% CI 2·21–4·16).
Table 5. Association of eating practices in the previous 4 weeks of acute gastroenteritis (AG) reporting, by age groups for subjects aged ⩾15 years
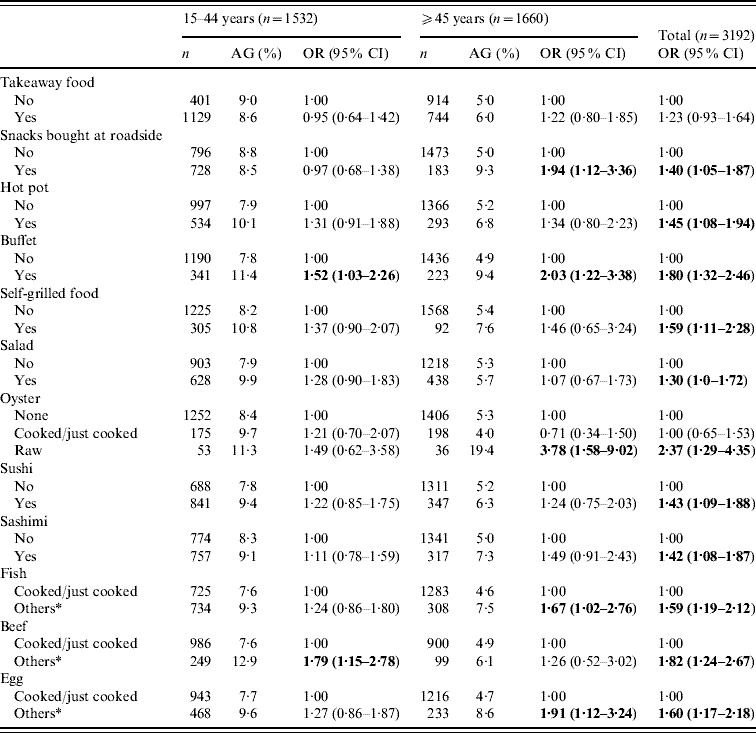
OR, Odds ratio; CI, confidence interval.
* Others included raw, half-cooked or a combination of both.
Figures in bold denote statistical significance.
DISCUSSION
A few population-based studies on the burden of AG have been conducted in different localities in the West [Reference Kuusi2, Reference Wheeler13–Reference Jones18, Reference Majowicz29] whereas this Hong Kong territory-wide survey is the first such study conducted in Asia. Using a similar symptom-based definition [Reference Scallan15–Reference Jones18, Reference Majowicz29, Reference Majowicz30], the rate of AG in Hong Kong was 0·91 episode per person-year, which is similar to that observed in Australia, Canada, and the USA, but higher than that in Ireland and Malta (0·37, 95% CI 0·36–1·89). The population-based study in Norway appears to indicate a higher AG rate (9·3% in the previous 4 weeks or 1·2 episodes per person-year), but it should be noted that the Norwegian study used a different case definition [Reference Kuusi2]. As in the other localities, women had a higher incidence of AG than men. The mean age (35·2 years) of subjects reporting AG and the mean duration of illness (3·62 days) were also comparable to that of other countries. However, the proportion of AG cases (39%) that consulted a physician was relatively high compared to the range of 13% (Norway) to 39% (Malta) in other developed countries. Most countries share a low rate of submitting a stool sample for testing, ranging from 1·8% in Ireland, 1·9% in Hong Kong to 8% in Norway. The percentage of AG cases with respiratory symptoms (8·8%) was lower than most other countries (19·2–47·83%) probably because a follow-up question re-confirmed if the symptoms were AG-related.
The highest prevalence of AG in children aged <5 years and the lowest in those aged ⩾65 years was consistent with reports from other population-based studies [Reference Kuusi2, Reference de Wit14, Reference Scallan15, Reference Jones18]. Rotavirus is a common cause of AG affecting mainly children, and globally almost every child has been infected with this pathogen at least once by age 5 years [Reference Velazquez33]. Although the elderly group has the lowest AG rate, they seem to share the largest disease burden in terms of hospitalization and mortality [Reference Herikstad34]. In the current study, three of the seven cases admitted to hospital for AG were in the elderly group. The lower AG rate observed in those aged ⩾65 years might be due to sampling bias as elderly individuals residing in nursing homes or hospitalized because of AG or other illnesses would not be captured by this survey [Reference Majowicz29]. The lower prevalence of risky behaviours might also partly explain the lower AG rates in the older population.
Eating seafood such as oysters or shellfish was the most commonly implicated cause of AG in those who believed their AG was caused by contaminated foods. About half also indicated a food source ‘away from home’. ‘Eating in restaurants’ has also been found to be a risk factor for foodborne disease outbreaks in the USA [Reference Jones and Angulo35]. However, information supplied in our study was based on subjective reporting and the AG pathogens have not been laboratory confirmed. Our study has also identified a higher prevalence of some ‘risky’ eating behaviours in subjects with AG compared to those not reporting AG in the previous 4 weeks, but a cause–effect relationship was not established. AG cases were more likely to have consumed raw oysters. Being a filter feeder, oysters can concentrate viruses from contaminated water [Reference Rippey36]. A local study found that 10·5% of the imported raw oyster samples were positive for norovirus [Reference Cheng23]. In an investigation of 13 outbreaks in which oyster was the implicated source of infection, norovirus was detected from at least one patient in all of the outbreaks and found in at least one oyster in six of these outbreaks [Reference Cheng23].
In our study, AG cases were twice as likely to have had buffet meals in the previous 4 weeks compared to non-AG subjects. Improper food storage, heating or handling can result in food contamination, with the proliferation of bacteria/viruses and accumulation of toxins causing AG [Reference Altekruse37]. The situation is particularly risky when large batches of foods like buffet meals are served. Buffet meals have become quite popular in Hong Kong in recent years, particularly during holidays and festivals. A number of food-poisoning outbreaks related to buffet meals have been reported [Reference Greig38, Reference de Wit39]. Even buffets served in prestigious hotels have been found to be related to food-poisoning outbreaks [Reference de Wit39, 40].
More of the AG cases reported having had self-grilled foods, hot pot, partially cooked or raw beef, fish and eggs in the previous 4 weeks compared to the non-AG subjects. Cross-contamination of raw and cooked foods and consumption of inadequately cooked foods are common during barbecues, or eating in restaurants where raw meats and foods are served in room temperature for self-grilling [41, Reference Mazick42]. Similarly, ‘hot pot’ is a traditional Chinese eating practice where mostly raw foods are served for self-cooking, with foods that are just cooked or partially cooked frequently preferred. Egg is well-known for Salmonella contamination [Reference Gantois43]. Raw or partially cooked egg is commonly served in some Hong Kong dishes such as beef rice, congee and hot pot. The situation can be risky in a temperate climate like Hong Kong, where pathogens can multiply quickly in foods.
The Japanese-style sashimi and sushi have become popular foods in Hong Kong, especially among the young. Inadequate hygienic preparation and storage of these foods are of concern. A recent survey by the Hong Kong Consumer Council revealed an excessive level of pathogens like Staphylococcus aureus and Escherichia coli in these foods served in some eating premises [44]. Salad has also been reported to cause large-scale outbreaks in other populations [Reference Schmid45]. Of those aged ⩾15 years, marginally more AG cases than non-AG cases reported ‘eating salad’ in the previous 4 weeks. As most of the ingredients in salad are eaten raw, cross-contamination of foods and being left out in room temperature for long periods could increase the risk of AG [Reference Gallimore46]. Roadside eating is relatively inexpensive and is still a common practice in Hong Kong, but the hygienic conditions of food preparation by the unlicensed hawkers can be questionable [47]. With the fast pace of life in Hong Kong, eating out is increasingly common, and certain popular foods can be risky. The higher prevalences of AG in those with higher education could also be related to their food or meal preferences.
Besides consumption of risky food items, personal hygiene is another concern regarding the cause of AG. A systematic review of intervention trials (2003) [Reference Curtis and Cairncross48] revealed ‘not washing hands with soap’ was associated with diarrhoeal diseases, with a pooled relative risk of 1·88 (95% CI 1·31–2·68). In our study, 95% reported ‘washing hands before meal’ but the quality of hand washing, such as the duration and use of disinfectant, was not investigated. The universal use of chopsticks by the Chinese when having meals may partly explain the lack of association between ‘hand washing’ and AG risk.
This 1-year telephone survey has estimated the AG rates in Hong Kong and revealed a number of risky behaviours in those affected by AG. The current study has several limitations. The AG cases were defined based on self-reported symptoms without laboratory confirmation but similar symptom-based case definition has been adopted in other population-based studies, so inter-population comparison is possible. The occurrence of AG was based on retrospective recall of events in the previous 4 weeks which is a rather long period. Bias due to the telescoping effect of some past illnesses may lead to an overestimation of the incidence of AG [Reference Majowicz29], although we tried to minimize such bias by referring to the exact calendar dates as the observation period. Our response rate of 41% is comparable to that obtained in previous telephone surveys with rates ranging from 27% to 71% [Reference Majowicz29]. Not possessing a telephone line by some households and a possible tendency for home-workers to respond to the interview (claiming to be the person with the last birthday) might affect the representativeness of the study population. Ninety-five percent of Hong Kong households have a telephone line [49], and every attempt was made to contact the selected respondent at a time that was convenient to them. The age and sex distribution in the study sample is quite comparable to that of the Hong Kong general population [32]. Because of the comprehensiveness of the questionnaire, each interview lasted about 15 min resulting in some incomplete questionnaires. ‘Being busy’ was the main cause of refusals although we offered to call back at a time more convenient to the respondent. The possibility of inaccurate answers provided by the proxy respondents for subjects aged ⩽15 years might be another drawback.
A number of eating or meal preferences were found to be more prevalent in subjects affected with AG. Because of the cross-sectional design and retrospective recall of events in the previous 4 weeks, no temporal or cause–effect relationships can be drawn from these associations. Although ‘risky’ foods and behaviours were enquired about in a separate section of the questionnaire (not immediately following the symptom questions), being aware of the ‘risky’ foods or eating practices possibly leading to AG might increase the respondents' tendency to report these items.
Despite these potential limitations, this is the first territory-wide study on the disease burden of AG in Asia. Based on the common, symptom-based case definition, our results reveal that the incidence and distribution pattern of AG in Hong Kong are similar to those observed in Western populations. This study has also highlighted some potential food or meal preferences associated with AG. These findings indicate a strong need for promotion of food hygiene and the exercise of caution in the consumption of some potentially risky foods or meal types.
ACKNOWLEDGEMENTS
This study was supported by Food and Health Bureau through the Research Fund for the Control of Infectious Diseases.
DECLARATION OF INTEREST
None.