Zn, an essential trace element and a component of many enzymes, is involved in the synthesis, storage and release of insulin(Reference Gómez-García, Hernández-Salazar and González-Ortiz1–Reference Jansen, Karges and Rink3). Specially, Zn plays a major role in the stabilisation of insulin hexamers and the pancreatic storage of the hormone(Reference Wiernsperger4) and is an efficient antioxidant(Reference Prasad5), while oxidative stress is considered to be a main component in the initiation and progression of insulin resistance (IR) and diabetes(Reference Kaneto, Katakami and Matsuhisa6).
Furthermore, Zn may have an indirect insulin-like effect, since genetic studies have identified the islet-restricted Zn transporter ZnT8 as a likely player in the control of insulin secretion(Reference Wiernsperger and Rapin7, Reference Rutter8). When the serum Zn concentration falls, there is a concomitant reduction in insulin secretion and peripheral insulin sensitivity(Reference Gómez-García, Hernández-Salazar and González-Ortiz1–Reference Jansen, Karges and Rink3) that may increase the risk of glucose intolerance, diabetes mellitus and IR(Reference Hashemipour, Kelishadi and Shapouri2, Reference Jansen, Karges and Rink3, Reference Rutter8, Reference Sun, van Dam and Willet9).
IR is an important public health problem in children and adolescents(Reference Artz and Freemark10, Reference Lee11). It can contribute to the onset of CVD and type 2 diabetes mellitus(Reference Hashemipour, Kelishadi and Shapouri2, Reference Sun, van Dam and Willet9); anything that could prevent IR would therefore be of great public health interest. Correcting situations of Zn deficiency can reduce the risk for type 2 diabetes(Reference Sun, van Dam and Willet9) and some authors report IR or insulin sensitivity to be improved by Zn supplements(Reference Hashemipour, Kelishadi and Shapouri2, Reference Jansen, Karges and Rink3, Reference Marreiro, Geloneze and Tambascia12, Reference Kelishadi, Hashemipour and Adeli13).
Specifically, insulin sensitivity was improved in non-diabetic obese women after a Zn supplementation for 4 weeks, while no changes were observed in the placebo group(Reference Marreiro, Geloneze and Tambascia12). Kelishadi et al. (Reference Kelishadi, Hashemipour and Adeli13) evaluated the effects of zinc sulphate v. placebo on markers of IR, oxidative stress and inflammation in a sample of obese prepubescent children. This randomised, placebo-controlled, crossover trial was conducted among sixty obese children who were randomly assigned to two groups: one received 20 mg elemental Zn and the other received a placebo on a regular daily basis for 8 weeks. After a 4-week washout period, the groups were crossed over. There was no significant carryover effect, and a significant decrease was documented for apoB:apoA-I ratio, oxidised LDL, leptin and malondialdehyde, and total and LDL-cholesterol after receiving Zn supplement, while there were no significant changes after receiving a placebo. Moreover, high-sensitivity C-reactive protein decreased and markers of IR improved significantly after receiving the supplement, while they increased or impaired after receiving the placebo. Also, the mean weight, BMI and BMI Z-score decreased significantly in the supplemented group, whereas these values increased after receiving the placebo. These results are particularly important in view of the adverse consequences of both childhood obesity and early changes in markers of oxidative stress and inflammation. The authors suggest the need for studies examining the clinical usefulness of Zn supplementation in childhood obesity.
Very few studies have examined the potential effects of micronutrients on IR, particularly with respect to the paediatric age group(Reference Hashemipour, Kelishadi and Shapouri2, Reference Wiernsperger and Rapin7). Therefore, the hypothesis of the present study is that poor Zn status affects appreciable percentage of Spanish children and is associated with increased risk of IR.
Experimental methods
The present study involved six primary schools in Madrid, Spain, randomly selected from a list of all such schools in the city.
Subjects
Sample recruitment
The study subjects were 357 schoolchildren aged between 8 and 13 years. The school principals were contacted by phone to arrange an interview. Permission was requested to meet with the parents of the children in the 8- to 13-year age group. Once permission was given, the parents were explained the details of the study and all questions were answered. Signed permission was then sought to include their children in the study. All the subjects took part voluntarily.
The exclusion criteria were:
(1) A lack of authorisation to take part or the non-acceptance of any of the conditions required for the study to proceed.
(2) Non-attendance on days when tests or interviews were performed.
(3) Having a pathology that might modify the results, that might alter food habits (and therefore nutrient intake) or that recommended non-inclusion.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Human Research Review Committee of the Pharmacy Faculty, Complutense University of Madrid. Written informed consent was obtained from all the parents of the children.
Methods
Anthropometric survey
All measurements were carried out at the schools in the morning and by following the norms set out by the World Health Organization(14).
Body weight and height were determined using a digital electronic balance (range 0·1–150 kg, precision 100 g; Seca Alpha, GmbH, Igni, France) and a Harpenden digital stadiometer (range 70–205 cm, precision 1 mm; Pfifter, Carlstadt, NJ, USA) respectively. For these measurements, subjects were barefoot and wore only underwear. Subject's BMI was calculated as weight (kg)/height2 (m2).
Overweight and obesity were defined according to BMI-specific percentiles for age and sex in the reference population. The cut-off for overweight was the 85th percentile and for obesity the 97th percentile(Reference Hernández, Castellet and Narvaiza15, Reference Rolland-Cachera, Deheeger and Bellisle16).
Waist and hip measurements were determined using a Holtain flexible metallic tape (range 0–150 cm, precision 1 mm; Holtain Limited, Crymych, Wales, UK). Measurements were carried out while the subject was standing relaxed and with the tape held snugly around the body, although not tight enough to compress the subcutaneous adipose tissue. The waist was measured midway between the inferior margin of the last rib and the crest of the ileum, in the horizontal plane. The hip circumference was measured in the horizontal plane of maximum circumference encircling the buttocks and the pubic symphysis. An assistant helped to hold the tape on the side of the subject's body opposite to the measurer. The mean of the three measurements was used for analysis. The waist:hip ratio was then calculated.
Dietary survey
A ‘weighed food record’ was kept for three consecutive days including a Sunday (from Sunday to Tuesday)(Reference Ortega, Requejo, López-Sobaler, Requejo and Ortega17). Parents were instructed to record the weights if possible – and to record the household measurements (spoonfuls, cups, etc.) if not – of all the food consumed outside school by their children. On Monday and Tuesday of the aforementioned 3 d, trained personnel visited the school canteen, recorded the foods in the menu and weighed the amounts of food served to each child plus those each left on the plate.
The energy and nutrient contents of the ingested foods were then calculated using food composition tables(Reference Ortega, López-Sobaler, Requejo, Ortega, López-Sobaler, Requejo and Andrés18). The values obtained were compared with those recommended(Reference Ortega, Requejo, Navia, Ortega, López-Sobaler, Requejo and Andrés19) to determine the adequacy of the diets. Special attention was paid to the intake of energy and Zn. Recommended intake (RI) of Zn used to estimate the percentage coverage of the RI was 10 mg/d for children under 10 years old, and 12 mg/d for girls and 15 mg/d for boys older than 10 years(Reference Ortega, Requejo, Navia, Ortega, López-Sobaler, Requejo and Andrés19). DIAL software (Alce Ingeniería, Madrid, Spain) was used to process all the data(Reference Ortega, López-Sobaler, Andrés, Ortega, López-Sobaler, Andrés, Requejo, Aparicio and Molinero20).
Theoretical energy expenditure was established by taking into account the body weight, age and physical activity of all the children, using equations proposed by the Institute of Medicine(21).
To validate the results of the dietetic study, we compared energy intake to the theoretical energy expenditure. The percentage discrepancy between the energy expenditure and the sum of the measured and declared intakes was determined using the following formula:
A negative value indicates the component involving the declared energy intake to be greater than that of the theoretical energy expenditure (probable over-reporting), while a positive value indicates it to be lower than that of the theoretical energy expenditure (probable under-reporting)(Reference Ortega, Requejo and Andrés22).
The index of nutritional quality of Zn was calculated as follows:
Physical activity
The subjects’ physical activity was examined by a questionnaire, registering the length of time spent sleeping, eating, playing sports, etc. An activity coefficient was established for each subject(Reference Ortega, Requejo, López-Sobaler, Requejo and Ortega23).
Biochemical survey
Blood samples were drawn by venipuncture between 08.00 and 09.00 hours after 12 h of fasting. The adequacy of the fasting period was checked by nurses before blood was collected.
Fasting insulin was measured by immunochemiluminometric assay(Reference El Kenz and Bergmann24) (Abbott Diagnostics, Madrid, Spain; CV = 4·8 %). Plasma glucose was determined colorimetrically using the glucose oxidase–peroxidase method (Vitros GLU Slides, Rochester, NY, USA; CV = 2·8 %)(Reference Neese, Duncan and Bayse25).
The homoeostasis model assessment (HOMA) value was used to reflect the degree of IR. This was estimated from the fasting plasma glucose and serum insulin concentrations using the following formula(Reference Albareda, Rodriguez-Espinosa and Murugo26, Reference Tripathy, Carlsson and Almgren27):
The HOMA cut-off point for the diagnosis of IR was taken as 3·16(Reference Keskin, Kurtoglu and Kendirci28).
For determination of Zn, blood was collected in trace-element-controlled Vacutainer tubes (Becton Dickinson, Le Pont de Claix, France). Serum Zn concentrations were determined by flame atomic absorption spectrometry using a Perkin-Elmer 3100 apparatus (Norwalk, CT, USA; CV = 4·1 %)(Reference Smith, Butrimovitz and Purdy29).
The cut-off level for moderate Zn deficiency was 10·7 μmol/l(Reference Pilch and Senti30, Reference Wallach and Wallach31).
Statistical analysis
Descriptive data were expressed as means and standard deviations. The Student's t test (or the Mann–Whitney test if the distribution of the results was not homogeneous) was used to compare means. The ANCOVA was used to adjust the Zn intake for under-reporting energy intake (percentage). The χ2 test was used to determine the significance of differences between proportions. Relationships between variables were quantified using multiple linear regressions controlling for potential confounders. Logistic regression analysis was used to identify risk or protection factors, expressing the OR and the 95 % CI. All calculations were performed using RSIGMA BABEL Software (Horus Hardward, Madrid, Spain). Significance was set at P < 0·05.
Results
Girls had a larger hip circumference, a higher mean serum insulin concentration, a higher mean HOMA value, a lower waist:hip ratio and a lower mean plasma glucose concentration than boys (Table 1).
Table 1 Age, blood data and anthropometric results for the studied children†
(Mean values and standard deviations)

Mean values were significantly different between boys and girls: *P < 0·05, **P < 0·01, ***P < 0·001.
† The Student's t test (or the Mann–Whitney test if the distribution of results was not homogeneous) was used to compare variables between boys and girls. The χ2 test was used to determine the significance of differences between proportions.
Absolute Zn intake was lower in girls, but the contribution of their intake to the coverage of the RI was greater; the RI for Zn is lower in girls 10 years or older (72 % of study subjects) (12 mg/d compared with 15 mg/d in boys)(Reference Ortega, Requejo, Navia, Ortega, López-Sobaler, Requejo and Andrés19) (Table 2). On the other hand, ANCOVA showed that Zn intake was still different between males (9·8 (sd2·1) mg/d) and females (8·54 (sd 1·8) mg/d; P < 0·001; data adjusted for percentage of under-reporting).
Table 2 Intake of energy and zinc in the studied schoolchildren†
(Mean values and standard deviations)
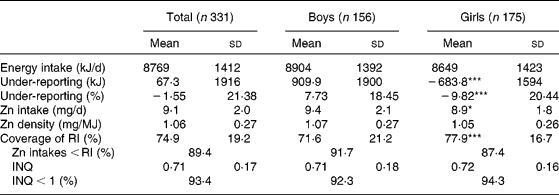
RI, recommended intake (10 mg/d for children under 10 years old and 12 mg/d for girls and 15 mg/d for boys older than 10 years(Reference Ortega, Requejo, Navia, Ortega, López-Sobaler, Requejo and Andrés19)); INQ, index of nutritional quality (Zn density (mg/MJ)/density recommended (mg/MJ)).
Mean values were significantly different between boys and girls: ***P < 0·001.
† The Student's t test (or the Mann–Whitney test if the distribution of results was not homogeneous) was used to compare variables between boys and girls. The χ2 test was used to determine the significance of differences between proportions.
Children were divided into those with a serum Zn concentration of < 10·7 and ≥ 10·7 μmol/l (Table 3). No differences were observed between these two groups in terms of the sex ratio; thus, the results for boys and girls are shown together. No differences were observed in terms of any of the anthropometric or dietetic variables measured between the concentration of < 10·7 and ≥ 10·7 μmol/l groups, although the insulin and HOMA levels were lower in the ≥ 10·7 μmol/l group (Table 3).
Table 3 Differences observed between children with a zinc deficiency and those with no such deficiency†
(Mean values and standard deviations)

HOMA, homoeostatic model assessment; RI, recommended intake (10 mg/d for children under 10 years old and 12 mg/d for girls and 15 mg/d for boys older than 10 years(19)); INQ, index of nutritional quality (Zn density (mg/MJ)/density recommended (mg/MJ)).
Mean values were significantly different between boys and girls: *P < 0·05, ***P < 0·001.
† The Student's t test (or the Mann–Whitney test if the distribution of results was not homogeneous) was used to compare variables between boys and girls. The χ2 test was used to determine the significance of differences between proportions.
A positive correlation was found between the HOMA value and age (r 0·237), body weight (r 0·504), height (r 0·343), BMI (r 0·463), waist circumference (r 0·510), hip circumference (r 0·478), the waist:hip ratio (r 0·180), serum insulin (r 0·984) and glucose (r 0·162), and the under-reporting intake (r 0·231). Inverse relationships were observed between the HOMA value and the serum Zn concentration (r − 0·149) and Zn dietary density (r − 0·122; P < 0·05 in all cases).
Logistic regression showed the risk of IR (i.e. a HOMA value greater than the 75th percentile) to increase with age (OR 1·438; 95 % CI 1·021, 2·027) and BMI (OR 1·448; 95 % CI 1·294, 1·619) and to diminish with increase in serum Zn concentration (OR 0·908; 95 % CI 0·835, 0·987; P < 0·001). Sex had no significant influence on this risk.
No correlation was found between Zn intake and the serum Zn concentration, although among boys with a HOMA value of >3·16, the contribution of Zn intake to the coverage of the RI (59·7 (sd 14·7) %) was significantly lower than in boys with lower HOMA values (73·6 (sd 18·2) %; P < 0·05).
Taking all the children together, when the dietary density of Zn was < p25 (0·87 mg/MJ), significantly higher HOMA values were observed than when the dietary density was higher (1·73 (sd 1·09) compared with 1·33 (sd 0·82); P < 0·01).
Discussion
The present dietetic and anthropometric results are similar to those obtained in other studies(Reference Ortega, Aparicio, Ortega, Requejo and Martínez32–Reference Serra, Ribas and Aranceta34). The serum Zn concentrations and the percentage of subjects with deficient serum Zn concentrations are also similar to those previously reported(Reference Hotz, Peerson and Brown35–Reference Sayeg Porto, Oliveira and Cunha37).
The percentage of children with IR in the present study (6·5 %) agrees with that reported for schoolchildren and adolescents by other authors in other countries(Reference Eisenmann, DuBose and Donnelly38–Reference Manios, Moschonis and Kourlaba40). The girls had higher HOMA values than the boys (Table 1), which also agrees with that reported by other authors(Reference Manios, Moschonis and Kourlaba40, Reference Hirschler, Maccallini and Karam41), perhaps because of hormonal differences(Reference Moran, Jacobs and Steinberger42).
Children with serum Zn deficiency (concentration of < 10·7 μmol/l) had poorer glycaemia, insulin and HOMA results than the members of the ≥ 10·7 μmol/l group (Table 3). In fact, an inverse correlation was observed between serum Zn concentration and that of insulin (r − 0·1793) and the HOMA value (r − 0·149; P < 0·05 in all cases).
The risk of suffering IR (defined as a HOMA value greater than the 75th percentile) increased with age and BMI but fell with increasing serum Zn concentration (P < 0·001). Thus, Zn would appear to protect against IR, while increasing BMI and age raise the risk of IR. This influence of Zn on the HOMA value has been reported in other studies(Reference Hashemipour, Kelishadi and Shapouri2, Reference Jansen, Karges and Rink3, Reference Wiernsperger and Rapin7, Reference Sun, van Dam and Willet9).
Some authors have suggested that supplementing with Zn might help to reduce the BMI in children(Reference Lee, Lee and Bae43), and thus help reduce HOMA values. However, no such trend was observed in the present study; the children with better Zn status did not have a lower BMI than those deficient in Zn (Table 3).
As found in all previous studies(Reference Eisenmann, DuBose and Donnelly38, Reference Manios, Moschonis and Kourlaba40, Reference Chiarelli and Marcovecchio44), overweight/obese children had higher HOMA values (1·96 (sd 1·01)) than those of normal weight (1·22 (sd 0·78); P < 0·001). However, no perturbation of Zn metabolism or the very low plasma Zn concentrations that some authors associate with obesity(Reference Marreiro, Fisberg and Cozzolino45) were observed. In fact, in the present study, the overweight/obese children had serum Zn concentrations similar to those of lighter children (15·2 (sd 3·6) μmol/l compared with 15·7 (sd 3·5) μmol/l).
No correlation was found between Zn intake and serum Zn concentrations. This agrees with that reported by other authors, who indicate that homoeostatic mechanisms maintain the serum Zn concentration, with no notable change occurring when Zn intake is restricted(Reference Gibson, Hess and Hotz46, 47). However, this can be only applicable for moderate intake restrictions, which is observed in the present study and other similar studies from developed societies, since serum Zn definitely declines under severe restrictions(Reference Hashemipour, Kelishadi and Shapouri2, Reference Hotz, Peerson and Brown35, Reference Brown, Peerson and Rivera36).
Zn intake did, however, influence the HOMA value. An inverse correlation was found between the HOMA value and the dietary density of Zn (r − 0·122, P < 0·05). In addition, in children with a dietary density of Zn less than the 25th percentile, the HOMA values were significantly higher than in children with greater dietary densities. Further, boys with a HOMA value of >3·16 had Zn intakes that contributed less to the coverage of the RI than observed in those with more adequate HOMA values.
It would appear that Zn status plays a role in modulating the HOMA value and the risk of IR in children. Hashemipour et al. (Reference Hashemipour, Kelishadi and Shapouri2) earlier reported that, compared with children administered a placebo, those receiving Zn supplementation (20 mg/d) experienced significant and favourable changes in BMI, certain cardiometabolic risk factors and IR.
Some authors suggest that, given the increase in the proportion of children with IR and the metabolic syndrome, providing Zn supplementation or increasing the Zn intake through the diet might help control this emerging health problem(Reference Hashemipour, Kelishadi and Shapouri2, Reference Jansen, Karges and Rink3, Reference Marreiro, Geloneze and Tambascia12).
These studies support the results obtained in the present investigation, since we have found that children with serum Zn deficiency had poorer glycaemia, insulin and HOMA results than children with adequate serum Zn. Also, we have found that there is an inverse correlation between serum Zn concentration and HOMA value (r − 0·149) and that the risk of having HOMA greater than the 75th percentile increased with age and BMI, but fell with increasing serum Zn concentration (P < 0·001). The results of this study indicate that the risk of having greater IR reduced as serum Zn levels increased, probably due to the fact that Zn status is not optimal and can be improved.
As reported in other studies, the children's diets provided marginal Zn amounts(Reference Marreiro, Fisberg and Cozzolino45, Reference Hotz48); indeed, 89·4 % of the children in our study had Zn intakes below those recommended (Table 2).
The present study has several limitations. First, we used serum Zn as a Zn status indicator. Although its validity as a Zn status indicator can be questioned, it remains one of the best tools that we have for this purpose and is the parameter used in most studies(Reference Hashemipour, Kelishadi and Shapouri2, Reference Jansen, Karges and Rink3, Reference Marreiro, Geloneze and Tambascia12, Reference Hotz, Peerson and Brown35, Reference Brown, Peerson and Rivera36). Also, the interpretation of the association between serum Zn concentrations and the HOMA value is complex. The serum Zn concentration is sensitive to various factors that have not been determined(Reference Hashemipour, Kelishadi and Shapouri2), and further studies are needed in this issue. In any case, we consider that improving the Zn status of children is desirable from a nutritional point of view, and might help fight against IR.
Acknowledgements
This study was supported by the project FISS (PI060318) and the ‘Creation and Consolidation Program Research Group at the Complutense University of Madrid, Madrid’ (ref. GR58/08; code 4120787). None of the authors had any personal or financial conflict of interest. R. M. O., A. M. L.-S., E. R.-R. and A. A. contributed to the study design and E. R.-R., A. A., A. I. J. and L. G. G. R. performed the data collection. R. M. O., A. M. L.-S., E. R.-R., A. A. and P. A. were involved in data analysis and the interpretation of results. R. M. O., A. A., A. I. J. and P. A. contributed to the writing of the manuscript.





