Maternal gestational weight gain (GWG), as an important indicator of maternal nutrition during pregnancy, includes the developed weight of the fetus, placenta, amniotic fluid, uterus, maternal blood volume, mammary gland and maternal adipose tissue during pregnancy. It has been well established that maternal GWG is associated with infant birth weight, and suboptimal GWG is associated with a series of adverse perinatal outcomes(Reference Voerman and Santos1–Reference Ukah, Bayrampour and Sabr3), maternal postpartum weight retention(Reference Nehring, Schmoll and Beyerlein4,Reference Widen, Whyatt and Hoepner5) and children’s overweight/obesity(Reference Oken, Taveras and Kleinman6,Reference Hinkle, Sharma and Swan7) . Moreover, mounting studies have evaluated the association between the timing of maternal GWG and infant birth weight, while the results remain controversial.
Some studies assessing the association between trimester-specific GWG and infant birth weight indicated that maternal GWG in the first two trimesters predicted birth weight(Reference Feghali, Catov and Zantow8–Reference Brown, Murtaugh and Jacobs11), while others suggested that GWG in all three trimesters was associated with birth weight(Reference Mao, Wang and Li12,Reference Margerison-Zilko, Shrimali and Eskenazi13) . As well as, some studies reported that GWG in the first 18 weeks only(Reference Retnakaran, Wen and Tan14) or in the late two trimesters(Reference Karachaliou, Georgiou and Roumeliotaki15) was associated with birth weight. Furthermore, few studies have evaluated the sex differences in the association between timing of GWG and infant birth weight, though sex-specific intra-uterine growth patterns have been reported(Reference Melamed, Meizner and Mashiach16). Additionally, the guidelines on optimal ranges of total GWG have been extensively studied and recommended. However, no studies have evaluated the association between timing of GWG and birth weight among women with adequate total GWG and explored whether suboptimal GWG in a critical time window is associated with the risk of adverse birth weight outcomes among them. This information is needed to determine the importance of having adequate GWG in a particular gestation period.
In this regard, the current study aimed to evaluate the association between timing of maternal GWG and infant birth weight by infant sex, especially among women with adequate total GWG, in a large prospective cohort study in Wuhan, China.
Methods
Participants and study design
A total of 5274 pregnant women with serial weight measurements were identified from the Tongji Maternal and Child Health Cohort, a prospective cohort aimed to investigate the effects of maternal nutritional, environmental and lifestyle exposures on the health outcomes of mother and child pairs in Wuhan, China(Reference Zhang, Wu and Zhong17). Pregnant women in the Tongji Maternal and Child Health Cohort were enrolled before 16 weeks of gestation from three hospitals between January 2013 and May 2016. After excluding those with diabetes (n 18) or hypertension (n 6) before pregnancy, missing data on birth outcomes (n 16) or preterm birth (n 185), 5049 pregnant women were included in the current study (online Supplementary Fig. S1).
Ethical approval
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the ethics review committee of Tongji Medical College of Huazhong University of Science and Technology (No. 201302). Written informed consent was obtained from all subjects/patients.
Anthropometric measurements and gestational weight gain assessment
Pregnant women’s pre-pregnancy weight was self-reported at enrolment (mean (sd): 12·9 (1·8) weeks of gestation), and their current weight and height were measured with light clothing and no shoes by trained nurses. Their weights were routinely measured in the same way during follow-up prenatal cares, with a median of 10 (ranged from 3 to 20) weight measurements per woman. Pre-pregnancy BMI was calculated as pre-pregnancy weight (kg) divided by square of height (m), categorised as underweight (< 18·5 kg/m2), normal weight (18·5 to < 24·0 kg/m2), overweight (24·0 to < 28·0 kg/m2) and obesity (≥ 28·0 kg/m2) according to the Chinese standards(Reference Zhou18).
Because pregnant women’s weight was measured during routine antenatal visits, the number of weight measurements varied by gestational week. Based on the characteristics of weight measurements, ten gestational intervals were identified in the current study: the first 13, 14–18, 19–23, 24–28, 29–30, 31–32, 33–34, 35–36, 37–38 and 39–40 weeks(Reference Retnakaran, Wen and Tan14). Then, weight and its gestational weeks in these intervals were determined. Mean weight and gestational weeks were used if a participant had two or more weight measurements within one interval. Subsequently, weekly GWG in these intervals was calculated as the difference of weight between the indicated interval and the preceding one divided by the weeks between, while that in the first 13 weeks (the first interval), weekly GWG was calculated as the difference between the weight prior to 13 weeks of gestation and before pregnancy divided by the weeks between. Finally, the numbers (percentages) of weight measurements for these ten gestational intervals were 4061 (80·4 %), 3562 (70·5 %), 3788 (75·0 %), 3658 (72·4 %), 2760 (54·7 %), 2088 (41·4 %), 2430 (48·1 %), 3034 (60·1 %), 3546 (70·2 %) and 2123 (42·0 %), respectively. Also, cumulative GWG up to these intervals was calculated as the difference in weight between the indicated interval and pre-pregnancy.
Total GWG was defined as the difference between the latest weight before delivery (within 4 weeks) and pre-pregnancy weight, and it was categorised as inadequate, adequate or excessive according to the recommended GWG on Chinese women (11·0–16·0 kg for women categorised as underweight, 8·0–14·0 kg for normal weight, 7·0–11·0 kg for overweight and 5·0–9·0 kg for obesity) (online Supplementary Table S1)(19).
Among women with adequate total GWG, GWG percent for each of the ten intervals was calculated as weekly GWG in the indicated interval divided by total GWG.
Birth outcomes
Information on date of birth, delivery mode, infant sex, birth weight and birth length was derived from hospital records. Small-for-gestational-age (SGA) and large-for-gestational-age (LGA) were defined as birth weight < 10th and > 90th percentile of the Chinese reference, respectively(Reference Dai, Deng and Li20).
Covariates
Maternal baseline information was collected by face-to-face questionnaire at enrolment, including age, ethnicity, parity, average monthly income, current smoking at enrolment, alcohol intake before and in early pregnancy, and date of last menstrual period. Gestational age was initially calculated from the last menstrual period. If an ultrasound in early pregnancy indicated a different gestational age, the estimated date of confinement and gestational age were amended. Gestational diabetes mellitus was diagnosed based on the results of a routine 75 g of oral glucose tolerance test at 24–28 weeks of gestation, according to the criteria of the International Association of the Diabetes and Pregnancy Study Groups(Reference Metzger and Gabbe21). Hypertensive disorders of pregnancy including gestational hypertension and preeclampsia were diagnosed based on blood pressure and urine protein after 20 weeks of gestation, according to the criteria of the American College of Obstetricians and Gynecologists task force on hypertension in pregnancy(22).
Statistical analysis
Continuous variables were presented as means and standard deviation and categorical variables as frequencies (percentages). We conducted ten discrete generalized linear models with Gaussian distribution to examine the associations of GWG in each of the ten intervals with birth weight. Covariates included maternal pre-pregnancy BMI (continuous), height (continuous), ethnicity (Han Chinese or others), age (continuous), parity (0 or ≥ 1), average monthly income (< 5000, 5000–9999 or ≥ 10 000 Chinese Yuan), current smoking at enrolment (yes or no), alcohol intake before and in early pregnancy (yes or no), cumulative GWG prior to the current interval (continuous), gestational age at delivery (continuous) and infant sex (male or female). We imputed the missing value on average monthly income (2·0 %) using the multiple imputation (m = 5 imputations) with chained equations(Reference Sterne, White and Carlin23). To explore whether the association of GWG in each of the ten intervals with birth weight was modified by infant sex, maternal pre-pregnancy BMI and maternal vomiting in early pregnancy, the interactions were tested by including cross-product terms in the models, and stratified analyses by these variables were then conducted.
Among women with adequate total GWG, ten discrete generalized linear models with Gaussian distribution were conducted to evaluate the association of GWG percent in each of the ten intervals with birth weight, after adjustment for covariates mentioned above (except for cumulative GWG prior to the current interval). The critical time window was defined as gestational intervals during which GWG percent was positively associated with birth weight. If a gestational interval with a non-significant association was between two intervals with a positive association, then that interval was included in the critical time window.
Subsequently, we categorised participants as inadequate, adequate and excessive according to the GWG in the critical time window, using robust Poisson models to evaluate their associations with the risk of SGA and LGA after multivariate adjustment(Reference Zou24). In sensitivity analyses, the analyses were performed among women without gestational diabetes mellitus and hypertensive disorders of pregnancy. Also, we repeated these analyses when the GWG was grouped according to the National Academy of Medicine (NAM, formerly known as Institute of Medicine) guidelines(25).
Additionally, based on the area under the receiver operating characteristic curve, we compared the discriminative performance of GWG in the critical time window and in other intervals for SGA and LGA in all participants separately.
With a statistical power of 80 % and a significance level of 0·05, a sample size of 2088 (minimum number of weight measurements in the ten intervals) could detect a target correlation of 0·06, more precise than the predicted correlation of GWG and birth weight at 0·26(Reference Thorsdottir, Torfadottir and Birgisdottir26).
Two-sided P values < 0·05 were considered statistically significant. All analyses were performed using Stata, version 15.0 (Stata Corporation).
Results
The characteristics of participants
Among the 5049 pregnant women, the mean (sd) values were 20·8 (2·7) kg/m2 for pre-pregnancy BMI and 15·9 (4·5) kg for total GWG. Of them, 1713 (33·9 %), 183 (3·6 %) and 3153 (62·4 %) had adequate, inadequate and excessive total GWG, respectively. Women with inadequate total GWG were slightly older, less likely to be primiparous and more likely to have gestational diabetes mellitus; those with excessive total GWG had a higher pre-pregnancy BMI, were slightly younger, less likely to have gestational diabetes mellitus and more likely to be primiparous and have hypertensive disorders of pregnancy (Table 1).
Table 1. Characteristics of the study population according to total GWG
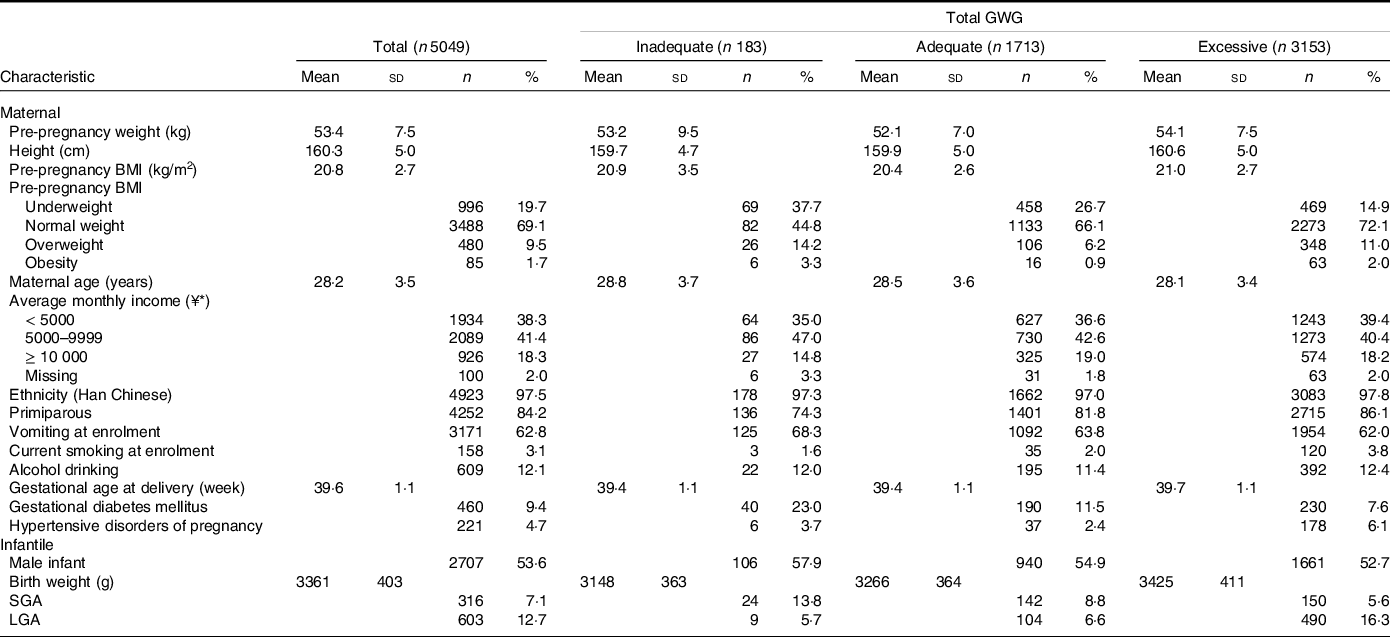
GWG, gestational weight gain; SGA, small-for-gestational-age; LGA, large-for-gestational-age.
* ¥, Chinese Yuan; ¥1 ≈ USA $ 0·16.
Timing of gestational weight gain and birth weight
In all participants, maternal GWG in the first 30 weeks (i.e. the first 13, 14–18, 19–23, 24–28 and 29–30 weeks) was positively associated with birth weight after multivariate adjustment. Birth weight increased by 56·54 (95 % CI 30·68, 82·40), 94·51 (74·46, 114·55), 86·16 (65·40, 106·93), 65·80 (44·95, 86·65) and 36·32 (18·17, 54·47) g for 0·5 kg/week increase in GWG during the first 13, 14–18, 19–23, 24–28 and 29–30 weeks, respectively (Table 2).
Table 2. Association of GWG in each gestational interval with birth weight
(β and 95 % confidence intervals)
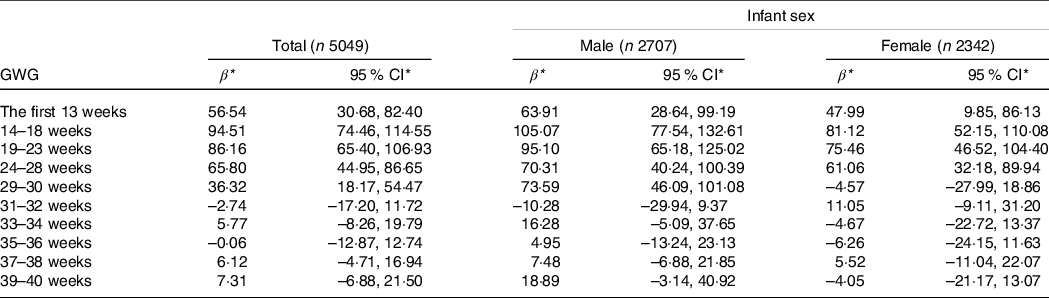
GWG, gestational weight gain.
* Values represent the change in birth weight (g) when GWG in the indicated gestational interval changed by 0·50 kg/week.
All models were adjusted for maternal pre-pregnancy BMI, height, ethnicity, age, parity, average monthly income, current smoking at enrolment, alcohol intake before and in early pregnancy, cumulative GWG prior to the current interval, gestational age at delivery and infant sex (expect for the stratified analyses).
The interaction between GWG in 29–30 weeks and infant sex was significant in relation to birth weight (P < 0·001). When stratified by infant sex, GWG in the first 30 weeks was positively associated with birth weight among male infants (birth weight increased by 63·91 (95 % CI 28·64, 99·19), 105·07 (77·54, 132·61), 95·10 (65·18, 125·02), 70·31 (40·24, 100·39) and 73·59 (46·09, 101·08) g for 0·5 kg/week increase in GWG during the first five intervals, respectively), and GWG in the first 28 weeks was positively associated with birth weight among female infants (birth weight increased by 47·99 (95 % CI 9·85, 86·13), 81·12 (52·15, 110·08), 75·46 (46·52, 104·40) and 61·06 (32·18, 89·94) g for 0·5 kg/week increase in GWG during the first four intervals, respectively) (Table 2). Consistency results were found in stratified analyses by maternal pre-pregnancy BMI or vomiting in early pregnancy (online Supplementary Table S2 and S3). In sensitivity analyses, similar results were found among women without gestational diabetes mellitus and hypertensive disorders of pregnancy (online Supplementary Table S4).
Gestational weight gain in the critical time window and birth weight outcomes
Among 1713 women with adequate total GWG, GWG percent between 14 and 23 weeks (i.e. 14–18 and 19–23 weeks) was positively associated with birth weight after multivariate adjustment (Table 3). Similar results were found among male and female infants (Table 3).
Table 3. Association of GWG percent in each gestational interval with birth weight among women with adequate total GWG
(β and 95 % confidence intervals)
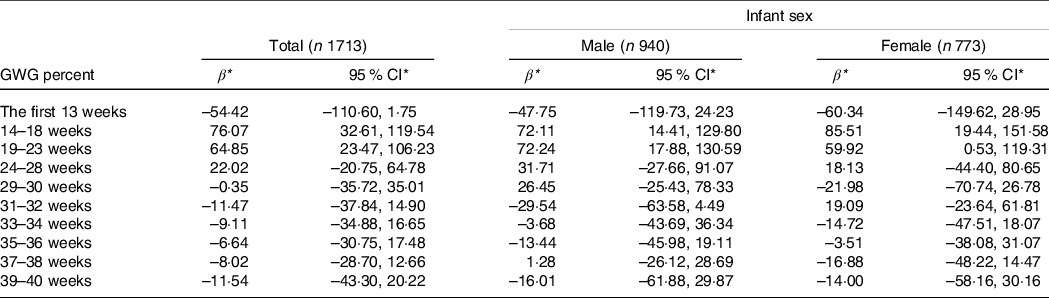
GWG, gestational weight gain.
* Values represent the change in birth weight (g) when GWG percent in the indicated gestational interval changed by 5 % per week.
All models were adjusted for maternal pre-pregnancy BMI, height, ethnicity, age, parity, average monthly income, current smoking at enrolment, alcohol intake before and in early pregnancy, gestational age at delivery and infant sex (expect for the stratified analyses).
Then, weekly GWG between 14 and 23 weeks was calculated and categorised as inadequate, adequate and excessive (0·37–0·56 kg/week for women categorised as underweight, 0·26–0·48 kg/week for normal weight, 0·22–0·37 kg/week for overweight and 0·15–0·30 kg/week for obesity) (Table 4).Women with inadequate GWG between 14 and 23 weeks, compared with those with the adequate GWG, were associated with an increased risk of SGA (43 (13·7 %) v. 42 (7·2 %); relative risk 1·83, 95 % CI 1·21, 2·76), but not LGA (17 (5·9 %) v. 33 (5·7 %); relative risk 1·05, 95 % CI 0·60, 1·85). These results were unchanged among women without gestational diabetes mellitus and hypertensive disorders of pregnancy (Table 4). Also, similar results were observed when total GWG and GWG between 14 and 23 weeks were grouped according to the National Academy of Medicine guidelines (online Supplementary Table S5).
Table 4. Associations of GWG between 14 and 23 weeks with the risk of SGA and LGA among women with adequate total GWG
(Relative risks and 95 % confidence intervals)
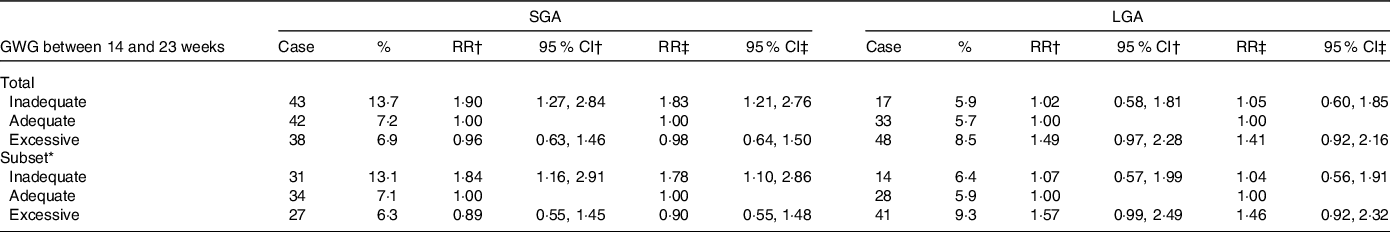
GWG, gestational weight gain; SGA, small-for-gestational-age; LGA, large-for-gestational-age; RR, relative risks.
* Among women without gestational diabetes mellitus and hypertensive disorders of pregnancy.
† Models were crude models.
‡ Models were adjusted for maternal pre-pregnancy BMI, height, ethnicity, age, parity, average monthly income, current smoking at enrolment and alcohol intake before and in early pregnancy.
Additionally, in all participants, for SGA, the area under the receiver operating characteristic curve was larger for GWG at 14–23 weeks (0·58, 95 % CI 0·55, 0·62) than GWG in other intervals (0·52, 95 % CI 0·49, 0·55) (P = 0·013); while for LGA, the area under the receiver operating characteristic curve was similar between GWG at 14–23 weeks (0·58, 95 % CI 0·56, 0·61) and GWG in other intervals (0·60, 95 % CI 0·57, 0·62) (P = 0·483) (online Supplementary Fig. S2).
Discussion
In the current prospective cohort study, maternal GWG in the first 30 weeks was positively associated with birth weight among male infants, and maternal GWG in the first 28 weeks was positively associated with birth weight among female infants. Among women with adequate total GWG, increased GWG percent between 14 and 23 weeks was associated with increased birth weight. Moreover, inadequate GWG between 14 and 23 weeks among women with adequate total GWG was significantly associated with an increased risk of SGA.
Some studies on trimester-specific GWG suggested that maternal GWG in the first two trimesters predicted birth weight(Reference Feghali, Catov and Zantow8–Reference Brown, Murtaugh and Jacobs11), the current study extended the finding to that maternal GWG in the first 30 weeks was associated with birth weight in male infants and maternal GWG in the first 28 weeks was associated with birth weight in females. Maternal GWG may influence birth weight by mediating maternal fat mass. Theoretically, pregnant women with pre-pregnancy normal weight will gain 12·5 kg weight throughout pregnancy, and 3·8 kg of that is the fat mass(Reference Butte, Ellis and Wong27). The first two trimesters of pregnancy are considered to be a period of accumulation of fat stores, while the third trimester is a catabolic period(Reference Ghio, Bertolotto and Resi28). Moreover, a study suggested that maternal changes in thigh skin folds and fat gain before the 30 weeks of gestation were associated with infant birth weight(Reference Villar, Cogswell and Kestler29). Additionally, the sex-specific difference found in the association between timing of GWG and birth weight may be related to the fact that male fetuses grow faster and require more energy than females in utero(Reference Villar, Cheikh Ismail and Victora30,Reference Tamimi, Lagiou and Mucci31) , and mothers of male fetuses require a relatively longer gestation period to accumulate fat than mothers of female fetuses.
Among women with adequate total GWG, 14–23 weeks of gestation was a critical time window during which increased GWG percent was found associated with increased birth weight. This finding suggests that the pattern of GWG may be an important indicator of infant birth weight, and pregnant women should be recommended to have adequate GWG throughout gestation, especially at 14–23 weeks of gestation. A possible explanation for the observed association is that the 14–23 weeks of gestation is a sensitive period of fetal fat lobule development, during which the number of lobules increases(Reference Poissonnet, Burdi and Bookstein32). As an indicator of maternal nutrition, GWG in this critical time window may influence birth weight by regulating this process.
Moreover, inadequate GWG between 14 and 23 weeks was found associated with a higher risk of SGA among women with adequate total GWG, which was supported by results showing that GWG between 14 and 23 weeks of gestation had a greater predictive effect on SGA than GWG in other intervals. These findings were generally consistent with some prior studies which suggested that GWG in the second trimester, but not GWG in other trimesters, was associated with the risk of SGA(Reference Feghali, Catov and Zantow8,Reference Sridhar, Xu and Hedderson10) , and these findings further limited the critical time window to the 14–23 weeks of gestation and added that the higher risk of SGA associated with inadequate GWG in the critical time window might not be offset by adequate total GWG. Additionally, some studies reported that maternal GWG prior to glucose screening (at 24–28 weeks of gestation) was positively associated with the risk of gestational diabetes mellitus, emphasising that pregnant women should prevent more GWG before 24 weeks of gestation(Reference Brunner, Stecher and Ziebarth33). Under the circumstances, the findings in the current study have important clinical implications.
The prospective longitudinal and accurate measurement of pregnant women’s body weight to ensure high-quality measurements are major strengths of the current study. Other strengths include a large sample size and detailed information on covariates. Moreover, considering the pattern of GWG during the whole pregnancy may be affected by gestational diabetes mellitus and hypertensive disorders of pregnancy, the analyses were also performed among women without these pregnancy complications to test the robustness of the findings.
Our study has some limitations. First, the participants’ pre-pregnancy weight was self-reported, which might introduce reporting bias. However, the reporting bias in pre-pregnancy weight is small, and self-reported is a cost-effective and common measurement approach for pre-pregnancy weight(Reference Headen, Cohen and Mujahid34). In addition, pre-pregnancy weight of the current study was self-reported at enrolment in early pregnancy (about 12 weeks of gestation) when the magnitude of change in body weight was small and the recall period was short. Second, since most pregnant women in the current study are Han Chinese, the association of timing of GWG with birth weight deserves a further investigation in other ethnic groups. Third, there is only a small percentage of participants with pre-pregnancy overweight or obesity in the current study; thus, the applicability of the findings to them remains to be evaluated.
Conclusion
In conclusion, findings from this longitudinal cohort study indicate that maternal GWG up to 30 weeks of gestation may predict birth weight among male infants and maternal GWG up to 28 weeks of gestation may predict birth weight among female infants. Pregnant women with inadequate GWG between 14 and 23 weeks of gestation may be at higher risk of delivering SGA infants, despite having adequate total GWG. These findings highlight the importance of having adequate GWG between 14 and 23 weeks of gestation for improving infant birth weight outcomes.
Acknowledgements
The authors thank all participants who took part in this study and all members of the Tongji Maternal and Child Health Cohort study group.
This work was supported by the National Natural Science Foundation of China (NSFC 81673159), the Fundamental Research Funds for the Central Universities (HUST2019kfyXMPY008) and the National Program on Basic Research Project of China (NO. 2013FY114200). These founders had no role in the design, analysis or writing of this article.
L. L., X. C., C. Z., L. H., Q. L. and N. Y. designed the research; L. L., X. Z., M. W., H. W., S. Y. and X. C. were responsible for data collection under the supervision of G. X., G. S., X. Y., L. H. and N. Y.; L. L. and C. Z. analysed data; L. L. and X. C. wrote the first draft of the manuscript, which was critically reviewed and improved by all authors. All authors read and approved the final version of the manuscript.
There are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114522001921







