Red and processed meat consumption increases the risk of colorectal cancer, according to meta-analyses of epidemiological studies(Reference Larsson and Wolk1, Reference Norat, Lukanova and Ferrari2). In its 2007 report, the World Cancer Research Fund panel judges that ‘the evidence that red meat and processed meat are a cause of colorectal cancer is convincing’(3). The panel thus recommends: ‘Limit intake of red meat and avoid processed meat’. Several meat components have been speculated to explain why meat eaters are at a higher risk for colorectal cancer. Meat cooked at a high temperature contains mutagenic heterocyclic amines that induce colon, mammary and prostate tumours in rodents and monkeys(Reference Sugimura, Wakabayashi and Nakagama4). However, heterocyclic amines might not play an important role in colorectal cancer incidence, since (i) chicken intake is a major contributor of heterocyclic amines, but it is not associated with the risk(Reference Sinha, Rothman and Brown5), and (ii) doses of heterocyclic amines that induce cancer in animals are 1000–100 000 times higher than doses ingested by humans(Reference Stavric6). Red meat enhances the formation of putative carcinogenic N-nitroso compounds in human and rodent faeces(Reference Bingham, Pignatelli and Pollock7–Reference Lunn, Kuhnle and Mai9). N-nitroso compounds level is associated with the promotion of colon carcinogenesis by cured meat(Reference Santarelli, Vendeuvre and Naud10). But N-nitroso compounds brought into rat faeces by a bacon-based diet do not initiate or promote preneoplastic lesions in the colon of rats(Reference Parnaud, Pignatelli and Peiffer11). Red meat also contains haem, the Fe-bearing prosthetic group of myoglobin. Intake of haem Fe is associated with an increased risk of colon cancer among women of the Iowa Women's Health Study(Reference Lee, Anderson and Harnack12). Intake of black pudding, a blood sausage overloaded with high haem Fe, is associated with an increased risk of colorectal cancer among women of the Swedish Mammography Cohort(Reference Larsson, Rafter and Holmberg13).
Experimental animal studies support the hypothesis that haem Fe promotes colorectal carcinogenesis(Reference Sesink, Termont and Kleibeuker14). We have shown that dietary haem, in the form of haemin (haem stabilised by a Cl atom, also called ferriprotoporphyrin IX chloride), Hb or red meat, promotes putative precancerous lesions, aberrant crypt foci (ACF) and mucin-depleted foci (MDF), in the colon of rats fed a low-Ca diet(Reference Pierre, Freeman and Tache15–Reference Pierre, Tache and Petit17). This haem-induced promotion is associated with increased lipoperoxidation and cytotoxicity of faecal water, and linked to urinary 1,4-dihydroxynonane mercapturic acid excretion, a fat peroxidation biomarker(Reference Pierre, Peiro and Tache18). The addition of calcium phosphate to the diet inhibits haemin-induced lipoperoxidation, cytotoxicity and promotion of carcinogenesis in rats(Reference Pierre, Tache and Petit17), and we can explain the lack of effect of meat diets on colon carcinogenesis in previous study(Reference Parnaud, Peiffer and Tache19) by a high concentration of Ca in the diet. The addition of calcium phosphate to beef meat diet fully prevents red meat promotion of colon carcinogenesis, but, unexpectedly, the rats fed the no-meat high-calcium phosphate high-fat diet had more ACF and more MDF than the rats fed the no-meat low-calcium phosphate diet(Reference Pierre, Santarelli and Tache16). In the absence of meat, dietary calcium phosphate appears to promote carcinogenesis in this rodent model, which precludes its use in human volunteers.
The present study was designed to determine a Ca supplementation with no adverse effect by testing different types of Ca salts and finding the minimum effective Ca dose.
Materials and methods
Animals and diets
Eighty-five Fischer 344 female rats were purchased at 4 weeks of age from Iffa Credo (St Germain sur l'Arbresle, France). Animal care was in accordance with the guidelines of the European Council on animals used in experimental studies. The animal colony and staff got official agreement no. 31-121 for animal studies by the French government.
Short-term studies
Two short-term studies were designed to find the most effective Ca salt and the minimum effective Ca dose to suppress early biomarkers of haem toxicity. Rats were housed individually in metabolism cages. The room was kept at a temperature of 22°C on a 12 h light–dark cycle. After 5 d of acclimatisation to the AIN76-powdered diet, rats were provided with the experimental diets. Body weights and food intake were monitored at the start and at the end of each study. In the first study, forty rats were randomly allocated to eight dietary groups (five rats per group) for 1 week. Faeces were collected at day 7 and were frozen at − 20°C. Animals were killed 7 d after the start of the experimental diets. In the second study, twenty-five rats were randomly allocated to five dietary groups (five rats per group) for 2 weeks. Faeces were collected at day 14 and were frozen at − 20°C. Animals were killed 14 d after the start of the experimental diets.
Long-term study
A 3-month study was designed to test the hypothesis that the minimum effective dose of the most effective Ca salt, selected from the results of the short-term studies, does not promote carcinogenesis in rats. Twenty rats were housed in pairs in stainless steel wire-bottomed cages. The room was kept at a temperature of 22°C on a 12 h light–dark cycle. Rats were allowed 7 d of acclimatisation to the room and to the control diet, before being injected intraperitoneally with the carcinogen 1,2-dimethylhydrazine (190 mg/kg body weight; Sigma Chemical, St Quentin, France) in NaCl (9 g/l). In most published studies, several carcinogen injections are administered to rats. We reasoned here that the first shot initiates carcinogenesis, but that the following shots would promote carcinogenesis, blurring diet-induced promotion. We thus chose a single-shot protocol, following Karkare et al.(Reference Karkare, Clark and Glauert20). After 7 d, rats were randomly allocated to two groups of ten, and allowed free access to calcium carbonate-based diets for 94 d. We chose to initiate with the carcinogen in all rats, since the study was designed to show dietary promotion of carcinogenesis.
Diets
Experimental diets shown in Table 1 were based on a modified standard AIN76 diet(21), prepared and formulated in a powdered form by UPAE (INRA, Jouy-en-Josas, France). For the first short-term study, high-beef meat diets were formulated to contain 60 % meat (w/w, freeze dried, 0·6 mmol haem/g of meat), and dibasic calcium phosphate was included in all the diets at a low concentration of 20 μmol/g. To determine the minimum effective dose of calcium phosphate, the effect of six concentrations of Ca was tested in six groups of rats fed the low-Ca beef meat diet supplemented with calcium phosphate (doses range: 33–250 μmol/g, diet names: Phos33 … Phos250). To compare the efficacy of calcium phosphate with other salts, the effect of two Ca salts was tested in two groups of rats fed a calcium carbonate (Carb250) or calcium gluconate (Gluc250) diet at the highest tested dose (250 μmol/g). For the second short-term study, the effect of calcium carbonate was tested in five groups of rats fed a Hb diet, supplying 0·6 mmol/g haem to mimic the 60 % beef meat diet, supplemented with calcium carbonate (doses range: 33–155 μmol/g, diet names: Carb33 … Carb155). For the long-term carcinogenesis study, two experimental diets were formulated to contain two levels of calcium carbonate (33 and 100 μmol/g, diet names: control diet and Carb100) added into the control low-calcium phosphate diet.
Table 1 Formulation of diets (g/100 g)*

* Phos20–Phos250 are low-Ca beef diets supplemented with calcium phosphate from 20 to 250 μmol/g. Carb250 and Gluc250 are low-Ca beef diets supplemented, respectively, with calcium carbonate and calcium gluconate at 250 μmol/g. Carb33–Carb155 are low-Ca Hb diets supplemented with calcium carbonate from 33 to 155 μmol/g. Control diet and Carb100 low-Ca Hb diet supplemented with calcium phosphate from 33 to 100 μmol/g.
† Low-Ca casein.
‡ AIN76 mix, but 500 g/kg of dibasic calcium phosphate were replaced by sucrose in mineral mix.
Preparation of faecal water
Faecal pellets were collected for 24 h under each cage (one rat/cage, short-term studies; two rats/cage, long-term study), thus leading to five samples per diet. Freeze-dried faeces were used to calculate dry faecal mass and to prepare faecal water by adding 1 ml of sterilised water to 0·3 g of dry faeces. Samples were then incubated at 37°C for 1 h, stirring thoroughly every 20 min, followed by centrifugation at 20 000 g for 10 min. The aqueous phase was re-centrifuged at the same speed and duration, and the subsequent supernatant (faecal water) was collected and stored at − 20°C until use.
Thiobarbituric acid-reactive substances and haem assay
Thiobarbituric acid-reactive substances (TBARS) were measured in faecal water according to Ohkawa et al. (Reference Ohkawa, Ohishi and Yagi22), exactly as described previously, and results are expressed as malondialdehyde equivalent(Reference Pierre, Freeman and Tache15). Haem contents of faecal water were measured by fluorescence according to Van den Berg et al. (Reference Van den Berg, Koole-Lesuis and Edixhoven-Bosdijk23) and Sesink et al. (Reference Sesink, Termont and Kleibeuker14), respectively, as described previously(Reference Pierre, Freeman and Tache15). Both TBARS and haem in faeces were measured on faecal samples from both the short-term studies.
In vitro experiment
The ability of five different Ca salts (1 – calcium phosphate, 2 – calcium chloride, 3 – calcium gluconate, 4 – calcium carbonate, 5 – calcium lactate) to bind and to precipitate haem from bovine Hb solution was studied in vitro. Hb (0·36 mmol; Sigma Chemical) was acidified at pH 1·4 with 2 mol HCl and then heated 1 h at 105°C to dissociate haem from Hb. This solution was centrifuged at 20 000 g for 10 min. Supernatant with free haem was collected.
Six concentrations of each of five Ca salts were prepared (25, 50, 75, 100, 125 and 150 mmol/l). According to Sesink et al. (Reference Sesink, Termont and Kleibeuker24), 1 ml Hb added to 1 ml Ca salt was incubated for 90 min at 37·5°C in the dark and mixed every 10 min. The mixture was centrifuged for 2 min at 10 000 g. Supernatant (100 μl) was assayed for the remaining haem after twenty-fold dilution in distilled water, by absorbance being measured at 400 nm with a Lambda 2 spectrophotometer (PYE Unicam-PU8600 UV/VIS-spectrophotometer Philips). Different Hb concentrations were prepared as standard solutions: 1/1 (100 %), 1/5, 1/10, 1/20 and 1/50 in water. The assay was repeated three times, and the mean values were recorded.
Aberrant crypt foci assay
Rats were killed by CO2 asphyxiation in a random order at day 94 of the long-term study. Colons were excised from rats immediately post-mortem, flushed with cold Krebs solution (Sigma Chemical), opened longitudinally and fixed flat between two sheets of filter paper in 10 % formalin (Sigma Chemical), marked with a two-digit blinding code. Colons picked up in random order were stained for 6 min in a 0·05 % filtered solution of methylene blue(Reference Bird25). The number of ACF per colon and number of crypts in each ACF were counted under a light microscope at 40 × magnification in duplicate by two readers, blinded for the origin of the colon.
Statistical analysis
Results were analysed using SYSTAT 10 software for Windows and reported as means and standard deviations. Long-term study data were analysed by Student's t test, except ACF data that were analysed by two-way ANOVA (dietary group and reader). Short-term study data were considered first using one-way ANOVA. If a significant difference was found between the groups (P < 0·05), then pairwise comparisons were made with Fisher's least significant difference multiple comparison test.
The meta-analysis of the relation between calcium carbonate and colon cancer in carcinogen-injected rats was done as a sub-analysis of a study that we published in 2005(Reference Corpet and Pierre26). From this previous meta-analysis, we selected studies where calcium carbonate had been fed to experimental groups of rats. The studies were homogeneous (Q Cochran P>0·05); we thus used the ‘Fixed Effects’ model, using EasyMA DOS software (2001 version) to compute data.
Results
Weight and food intake during in vivo studies
Final body weight of rats was 107 (sd 2) g after 7 d on the experimental diets in the first short-term study, without significant differences between the groups. Food intake was not significantly different (data not shown), but Gluc250-fed rats tended to eat less than the other groups (Gluc250: 7·2 (sd 2·0) g/d, all other groups: 9·1 (sd 1·0) g/d, P = 0·09). No difference in body weight and diet intake between the groups was observed in the second short-term study or in the long-term study. Mean body weights were, respectively, 166 (sd 2) g after 14 d and 201 (sd 8) g after 94 d on the experimental diets.
First short-term study: thiobarbituric acid-reactive substances of faecal water
Haem can induce the formation of peroxyl radicals in rats, which may be cytotoxic and cleave DNA in vivo. This might explain the promoting effect of haem on colon carcinogenesis. Lipid peroxidation was thus measured in faecal water by TBARS assay. Ca can prevent haem-induced promotion of colon carcinogenesis by precipitating haem in the colonic content; thus haem concentration in faecal water was measured.
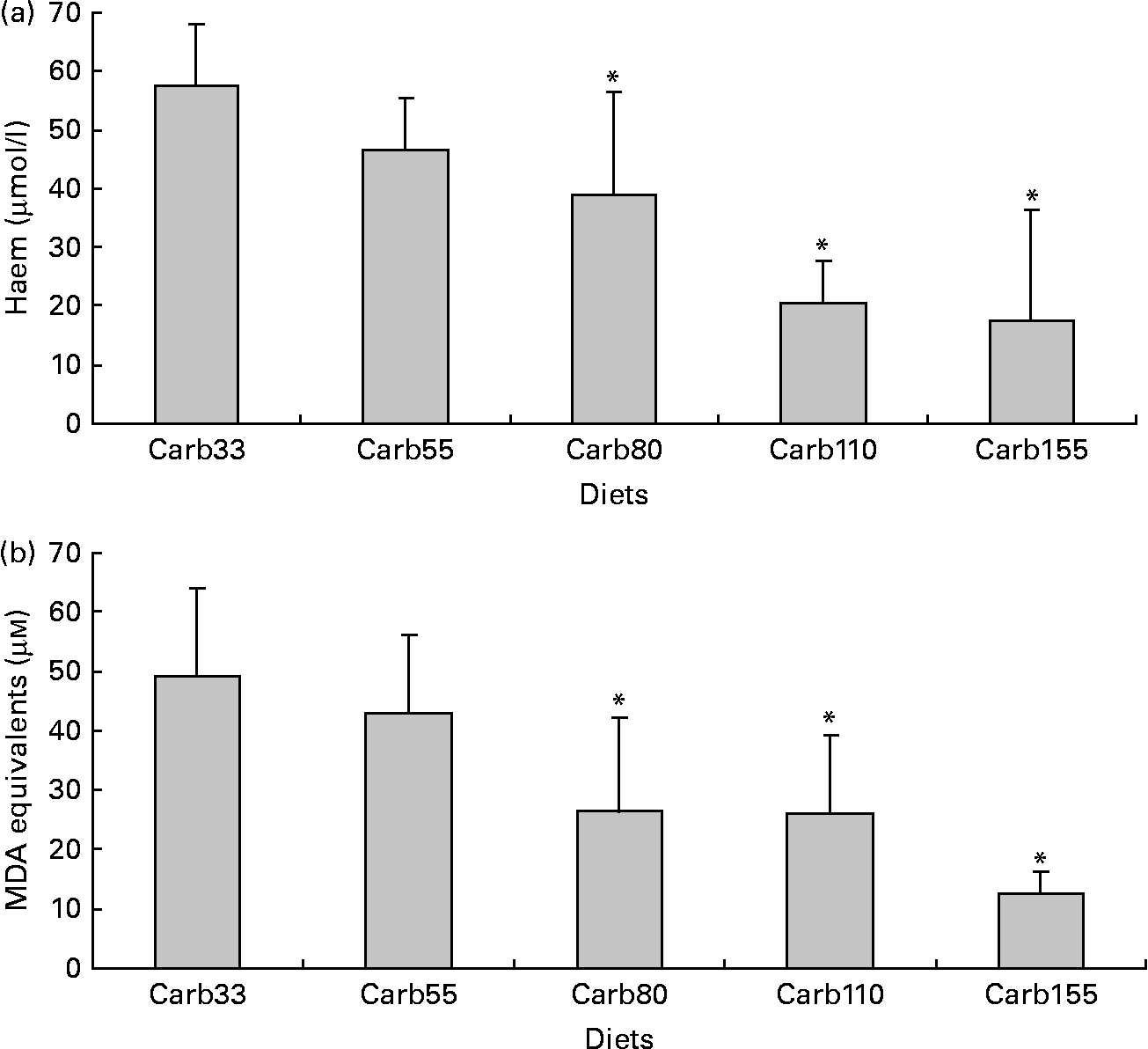
Haem concentration in faecal water from beef meat-fed rats was significantly reduced in rats fed 150 and 250 μmol/g calcium phosphate (P < 0·01; Fig. 1(a)), but not in rats fed lower levels of calcium phosphate. Dietary calcium carbonate and 250 μmol/g gluconate produced the same effect on haem than calcium phosphate (Fig. 1(a)). Surprisingly, haem-induced lipid peroxidation measured by TBARS level was not significantly reduced by the calcium phosphate diets, but only the high-calcium carbonate diet decreases TBARS level in faecal water (P < 0·01; Fig. 1(b)).

Fig. 1 Effect of diets on haem and thiobarbituric acid-reactive substances (TBARS) in faecal water after the first short-term study. (a) Haem in faecal water. (b) TBARS (malondialdehyde (MDA) equivalents) in faecal water as marker for lipid luminal peroxidation. * Mean values were significantly different from Phos20 (P < 0·01, by Fisher's least significant difference test). Data are means, and bars are standard deviations (n 5 in each group). Note: Phos20–Phos250 are low-Ca beef diets supplemented with calcium phosphate from 20 to 250 μmol/g. Carb250 and Gluc250 are low-Ca beef diets supplemented, respectively, with calcium carbonate and calcium gluconate at 250 μmol/g.
In vitro study
Any tested concentration of calcium carbonate higher than 25 mmol was much more effective to precipitate haem than any other tested Ca salt. Proportion of trapped haem was more than three times higher with calcium carbonate than with calcium phosphate (100 mmol) (P < 0·001; Fig. 2).
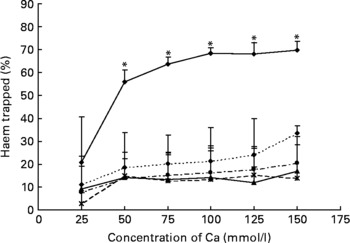
Fig. 2 Effect of Ca salts on haem solubility in vitro. Haem (0·36 mm) was incubated with six different concentrations of Ca salts (150, 125, 100, 75, 50 and 25 mmol/l). Data are means, and bars are standard deviations (n 3). * Mean values were significantly different from other salts. –♦–, Calcium carbonate; - -♦- -, calcium phosphate; –▲–, calcium chloride; – -●– -, calcium gluconate; ![]() , calcium lactate.
, calcium lactate.
Second short-term study: thiobarbituric acid-reactive substances of faecal water
Beef meat was replaced by Hb in the second study diets, since we have demonstrated previously that they produce similar effects on colon carcinogenesis and on faecal water peroxidation(Reference Pierre, Freeman and Tache15). The high-calcium phosphate diet (250 μmol/g) did not prevent haem toxicity in the 7 d study, in contrast with our previous 50 d study(Reference Pierre, Tache and Petit17). We suggest that this duration was too short to reduce the level of TBARS; thus we chose the second short-term study to last for 14 d. As calcium carbonate was the only effective salt against haem-induced lipid peroxidation in vivo and the only effective salt to precipitate haem in vitro, this salt was used to determine the smallest effective Ca dose.
Haem concentration was lower in faecal water from rats fed a diet containing at least 80 μmol/g calcium carbonate (P < 0·001; Fig. 3(a)). Accordingly, diets containing at least 80 μmol/g calcium carbonate reduced haem-induced lipid peroxidation of faecal water (P < 0·001; Fig. 3(b)).
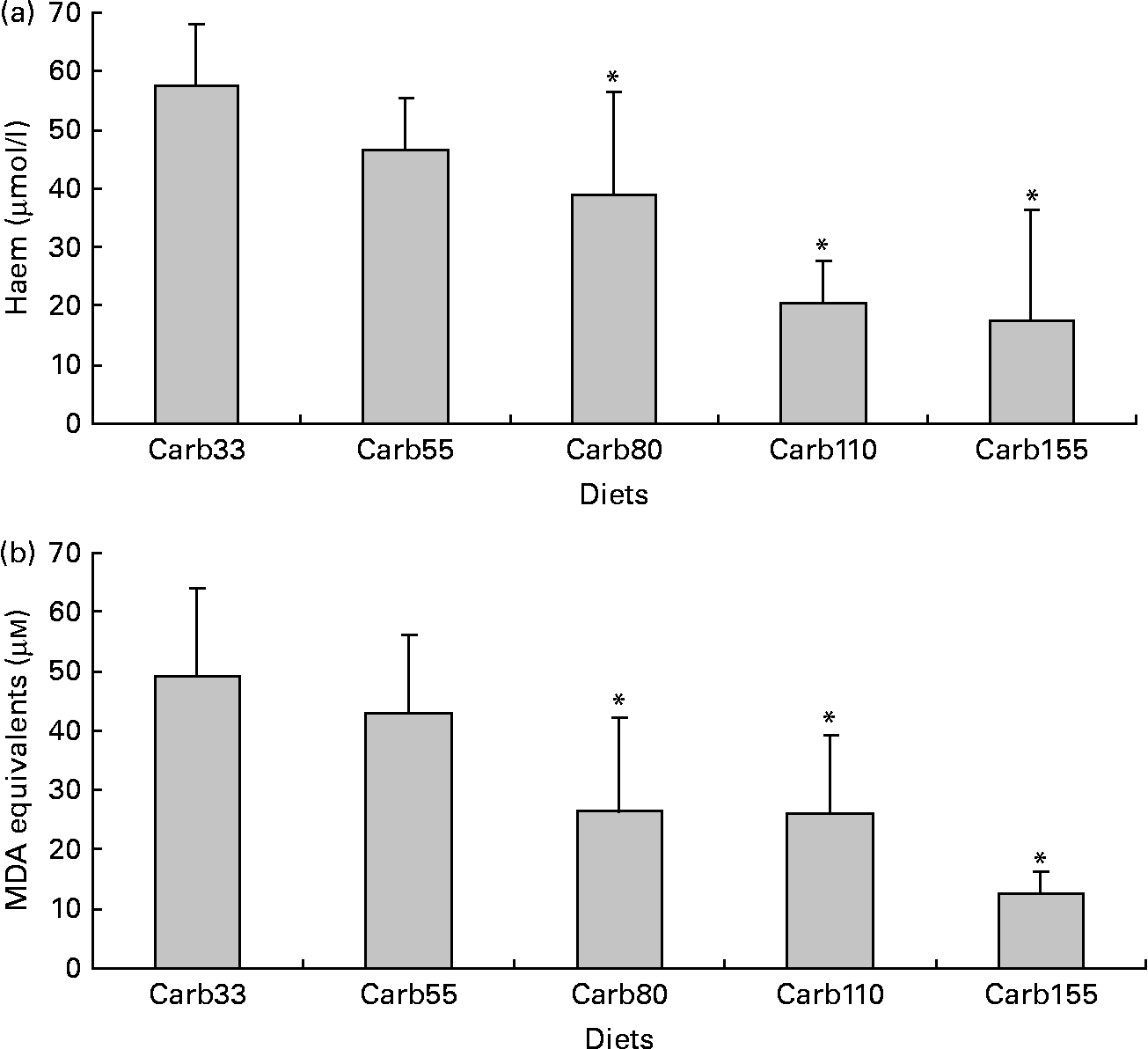
Fig. 3 Effect of calcium carbonate-based diets (30–155 μmol/g) on faecal water values after the second short-term study. (a) Haem concentration in faecal water (μmol/l). (b) Thiobarbituric acid-reactive substances concentration in faecal water, expressed as μmol/l malondialdehyde (MDA) equivalents. * Mean values were significantly different from Carb30 by Fisher's least significant difference test (P < 0·05). Data are means, and bars are standard deviations (n 5 in each group). Note: Carb33–Carb155 are low-Ca Hb diets supplemented with calcium carbonate from 33 to 155 μmol/g.
Aberrant crypt foci assay
We chose to test if a diet containing 100 μmol/g calcium carbonate would promote colon carcinogenesis because the second short-term study showed that a 80 μmol/g calcium carbonate diet reduces faecal TBARS concentration, and that 110 μmol/g seemed twice more potent than 80 μmol/g to bind faecal haem. In contrast with a calcium phosphate diet(Reference Pierre, Santarelli and Tache16), the consumption of a diet containing 100 μmol/g calcium carbonate for 94 d did not increase significantly the number of ACF per colon, 101 d after a dimethylhydrazine injection (Fig. 4).
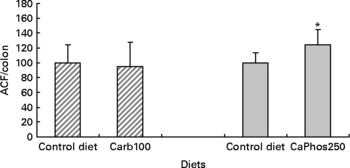
Fig. 4 Effect of calcium salts in diet on putative precancerous lesions (aberrant crypt foci (ACF)) per rat colon 101 d after the injection of dimethylhydrazine. Results of the present study with 100 μmol/g calcium carbonate are represented by ![]() . Results of the previous study with 250 μmol/g calcium phosphate (Pierre et al. (Reference Pierre, Santarelli and Tache16)) are represented by
. Results of the previous study with 250 μmol/g calcium phosphate (Pierre et al. (Reference Pierre, Santarelli and Tache16)) are represented by ![]() . Results are expressed after normalisation of the control diet groups to 100. * Mean values were significantly different control group. Note: the control diet was a low-Ca Hb diet supplemented with calcium carbonate at 33 μmol/g. Carb100 and CaPhos250 are low-Ca Hb diets supplemented, respectively, with calcium carbonate (100 μmol/g) and calcium gluconate (230 μmol/g).
. Results are expressed after normalisation of the control diet groups to 100. * Mean values were significantly different control group. Note: the control diet was a low-Ca Hb diet supplemented with calcium carbonate at 33 μmol/g. Carb100 and CaPhos250 are low-Ca Hb diets supplemented, respectively, with calcium carbonate (100 μmol/g) and calcium gluconate (230 μmol/g).
Discussion
These data confirm that dietary Ca reduces faecal water lipoperoxides level, a biomarker linked with haem-induced carcinogenesis promotion. They also establish that the type of Ca salt is paramount in this protection, and that calcium carbonate is effective without promoting the side effect of calcium phosphate.
Red meat promotes ACF and MDF in the colon of carcinogen-induced rats, and haem-induced promotion by meat is associated with faecal water cytotoxicity and TBARS(Reference Pierre, Freeman and Tache15, Reference Pierre, Santarelli and Tache16). The mechanism of promotion by haem is not known and may be linked to lipid peroxidation. We have explored the effect of faecal water from beef meat-fed rats on normal (Apc+/+) and premalignant colonic cells (Apc − /+). Results show that Apc-mutated cells survive in haem-induced faecal lipoperoxides, notably 4-hydroxynonenal that is toxic to normal cells. Selection of mutated cells by cytotoxic lipoperoxides may thus explain haem-induced promotion of colon carcinogenesis(Reference Pierre, Tache and Gueraud27). Ca added on top of beef meat or haemin diets normalises the number of ACF and MDF at the same low level as in the control group(Reference Pierre, Tache and Petit17). Calcium phosphate precipitates haem in the gut, and little haem remains available to induce lipid peroxidation(Reference Pierre, Tache and Petit17, Reference Sesink, Termont and Kleibeuker24). The trapping of haem by Ca thus abolishes carcinogenesis promotion. In the present study, we established that different Ca salts are able to precipitate haem in vitro and in faecal water of haem-fed rats and by consequence are able to reduce faecal water peroxidation (Figs. 1 and 2). We observed that only high concentrations of calcium phosphate were able to precipitate haem (Fig. 1(a)). Furthermore, the ability of the three tested salts (phosphate, carbonate and gluconate) to trap haem was very similar in high-Ca diets (Fig. 1(a)). However, only calcium carbonate decreased significantly the level of lipid peroxidation in faecal water (Fig. 1(b)). So, to screen more precisely the efficiency of different Ca salts, we measured the in vitro trapping of haem: only calcium carbonate precipitated haem significantly. In conclusion, the present study shows for the first time a difference of efficiency between Ca salts in vitro (Fig. 2) and in vivo, in the trapping of haem and the limitation of peroxidation (Fig. 1(b)). We have no explanation for the relatively high TBARS level in controls and in rats administered with high doses of calcium phosphate or gluconate: we speculate that the beef meat in the first study may have contained already oxidised fat or oxidising agents different from haem Fe (Fig. 1(b)).
Our previous study showed that rats consuming a high-Ca control diet had more ACF and more MDF than rats consuming a low-Ca control diet(Reference Pierre, Santarelli and Tache16). This promotion was observed with calcium phosphate in a high-fat diet context, but was not observed in a low-fat diet context without a clear explanation for the high-fat/low-fat discrepancy(Reference Pierre, Tache and Petit17). Bull et al. (Reference Bull, Bird and Bruce28) also showed that the promotion of tumours by calcium phosphate was more important in 30 %-fat diet-fed rats than in 3 %-fat diet-fed rats. Phosphate, not Ca, might be the promoting nutrient, an issue already discussed by Bruce et al. (Reference Bruce, Giacca and Medline29). Since our in vitro study indicated that calcium carbonate was more effective than calcium phosphate to trap haem (Fig. 2) and since the first in vivo study indicated that a high concentration of calcium carbonate was able to precipitate haem and to limit peroxidation in vivo, we determined the lower effective dose of calcium carbonate in a second short-term study (Fig. 3). We showed that 80 μmol calcium carbonate/g diet is enough to trap haem and to reduce peroxidation. The first aim of the present study was to identify the lower dose and type of Ca sufficient to limit the effect of haem in the colon without side effects. We thus tested the effect of an addition of 100 μmol calcium carbonate/g diet on the promotion of colon carcinogenesis at the level of ACF (Fig. 4). In contrast with the promoting effect of calcium phosphate at 250 μmol/g(Reference Pierre, Santarelli and Tache16), we did not observe a promoting effect of calcium carbonate on ACF. The present study shows for the first time that a Ca salt can inhibit the toxic effect of haem in the colon without side effect on the promotion of colon carcinogenesis.
To confirm that calcium carbonate does not promote carcinogenesis in rats, we performed a meta-analysis of published data on calcium carbonate and colon carcinogenesis (Fig. 5). Our previous meta-analysis study showed that Ca (all salts) modestly decreases tumour incidence in rats, and that some Ca salts are more protective than others(Reference Corpet and Pierre26): calcium lactate, but not calcium phosphate, reduces chemically induced carcinogenesis. Fig. 5 shows data from a meta-analysis of eleven studies of high-calcium carbonate diets on colon carcinogenesis in chemically injected rats; the combined relative risk from all of these studies was 0·983 (95 % CI 0·902, 1·071; P = 0·69), compared with low Ca controls. This meta-analysis confirms the lack of promoting side effect by calcium carbonate on colon carcinogenesis. These results on rodents are coherent with two randomised, double-blinded, placebo-controlled trials. Calcium carbonate supplementation was associated with a significant – though moderate – reduction in the risk of recurrent colorectal adenomas in the Calcium Polyp Prevention Study(Reference Baron, Beach and Mandel30). In contrast, daily supplementation of calcium carbonate (with vitamin D) for 7 years to 36 282 postmenopausal women from forty Women's Health Initiative centres had no effect on the incidence of colorectal cancer among postmenopausal women(Reference Wactawski-Wende, Kotchen and Anderson31). Altogether, these results suggest that calcium carbonate supplementation to neutralise haem does not bear any risk. However, chelation of dietary haem Fe bears the theoretical risk of Fe deficiency leading to anaemia: recommendations should take this in account.
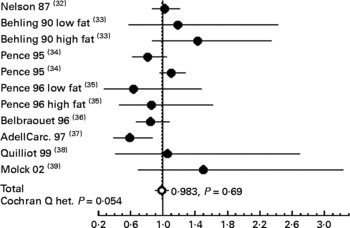
Fig. 5 Meta-analysis of the relation between calcium carbonate and colon cancer in carcinogen-injected rats. The common relative risk with 95 % CI was calculated from eleven studies. The study-specific relative risk is represented by ●. The common relative risk is represented by ○. Horizontal lines are 95 % CI. References are indicated between brackets. Het., heterogeneity.
The study also supports the concept that nutrients such as Ca may inhibit the toxicity associated with the excess of another nutrient like haem. This concept differs from the classical chemoprevention concept, since calcium carbonate does not reduce carcinogenesis in rats that are not fed with dietary haem. This specific Ca-preventive effect, by interaction, should be looked for in a cohort study by crossing haem and Ca intake with adenoma or cancer risk.
Acknowledgements
The authors disclosed no potential conflicts of interest. The present study was supported in part by the INRA, the DGER and the French region Midi-Pyrénées. We thank Xavier Blanc (UPAE) for the preparation of experimental diets, Raymond Gazel and Florence Blas Y. Estrada for the care of the animals. O. A. had made substantial contributions to the in vitro study, the second short-term in vivo study and the long-term study (acquisition of data, analysis and interpretation of data). D. B. had made substantial contributions to the first in vivo study (acquisition of data or analysis and interpretation of data). S. T. and N. N. had made substantial contributions to the three in vivo studies. D. E. C. and F. H. F. P. had made substantial contributions to the conception and design of the present study and by drafting the article.