Introduction
Hepatic alveolar echinococcosis (HAE) is a potentially fatal zoonosis that occurs in the northern hemisphere (Konyaev et al., Reference Konyaev, Yanagida, Nakao, Ingovatova, Shoykhet, Bondarev, Odnokurtsev, Losutova, Lukmanova, Dokuchaev, Spiridonov, Alshinecky, Sivkova, Andreyanov, Abramov, Krivopaliv, Karpenko, Lopatina, Dupal, Sako and Ito2013; Moss et al., Reference Moss, Chen, Li, Qiu, Wang, Giraudoux, Ito, Torgerson and Craig2013; Alishani et al., Reference Alishani, Sherifi, Rexhepi, Hamidi, Armua-Ternandez, Grimm, Hegglin and Deplazfs2016; Knapp et al., Reference Knapp, Giraudoux, Comnes, Umhang, Boue, Said-Ali, Aknouche, Garcia, Vacheyrou, Laboissiere, Raton, Comte, Favier, Demerson, Caillot, Millon and Raoul2018). The majority of human AE cases, in China, have been confirmed from the autonomous region of Tibet, Xinjiang, Ningxia and provinces of Sichuan, Qinghai and Gansu.
Echinococcosis is acquired by the ingestion of the eggs of the parasite. The larvae are liberated in the duodenum and pass through the intestinal wall to enter the portal system and reach the liver. Morphological pattern of HAE is an early microcyst but later a more partially solid liver lesion with malignant appearance, calcification and also pseudocystic appearance, especially in large lesions with central necrosis. Although the liver is the organ most commonly involved (Nunnari et al., Reference Nunnari, Pinzone, Gruttadauria, Celesia, Madeddu, Malagurnera, Pavone, Cappellani and Cacopardo2012), primary involvement of extrahepatic organs is rare (<1%) and mainly bones, spleen, retroperitoneum and CNs may be affected, but the lungs are more involved in N and M stages (Kern et al., Reference Kern, Wen, Sato, Vuitton, Gruener, Shao, Delabrousse, Kratzer and Bresson-Hadni2006).
HAE progresses slowly, and the patient may be asymptomatic for many years, until the enlarging lesions give rise to complications. The imaging for screening and additional serology can detect lesions early if they are done. Diagnosis can be confirmed by histology or PCR from the liver biopsy specimen. However, in marginal areas, early diagnosis is difficult due to limited medical conditions and people's awareness of the disease. Often, the condition is only diagnosed incidentally on imaging or when complication gives rise to symptoms.
Non-surgical treatment is primarily with anthelmintic agents such as albendazole. Percutaneous treatments such as radiofrequency ablation and microwave ablation were shelved after initial attempts proved to be ineffective (Zhang et al., Reference Zhang, Ye, Kong, Cai, Zhao, Han and Li2013; Tamarozzi et al., Reference Tamarozzi, Vuitton, Brunetti, Vuitton and Koch2014). Treatment with high-intensity focused ultrasound appears promising but is still in preclinical trials (Cairang et al., Reference Cairang, Zhang, Ren, Ren, Hou, Wang, Zhou, Zhang, Shao and Fan2017). Radiotherapy and heavy-ion therapies have been used in experimental models but whether they can be used in humans is questionable and, in any case, they are not curative treatments (Zhou et al., Reference Zhou, Zhao, Zhou and Zhang2013).
In HAE, complete surgical resection is recommended with albendazol treatment for 2 years afterwards, and if complete surgical resection cannot be achieved, long-term albendazol treatment is recommended (Brunetti et al., Reference Brunetti, Kern and Vuitton2010). Beside the medical and surgical treatment, there are quite some interventional procedures, which have to be mentioned, especially biliary complications can be handled by endoscopic interventions, without major surgery (Ambregna et al., Reference Ambregna, Koch, Sulz, Grüner, Ozturk, Chevaux, Sulima, de Gottardi, Napoleon, Aberqel, Bichard, Boytchev, Deprez, Dumortier, Frossard, Kull, Meny, Moradpor, Prate, Vanbiervliet, Thevenot, Vuitton, Bresson-Hadni and Vuitton2017). However, it should be noted that these therapeutic strategies have not undergone systematic evaluation of efficacy and safety (Kern et al., Reference Kern, Menezes, Akhan, Mullhaupt, Vizcaychipi, Budke and Vuitton2017). Surgery can extirpate the parasite and offer a permanent cure, although there are associated surgical risks (Pohnan et al., Reference Pohnan, Ryska, Hytych, Matei, Hrabal and Pudil2017; Yang et al., Reference Yang, Su, Deng, Deng, Li and Li2017). In complicated disease, however, surgical management is unavoidable.
The aim of this study was to present our experience with the surgical management of HAE. Different surgical techniques and the long-term outcomes of patients are reported.
Methods
Patients
Between January 2012 and February 2018, we have reviewed 262 patients who were diagnosed with HAE, and 187 patients treated with radical hepatectomy at the West China Hospital. These patients included 75 (42.1%) men and 103 (57.9%) women in the age range of 10–60 years. Patients were eligible for inclusion in this study if (1) they had confirmed diagnosis of HAE, (2) they were ⩽60 years old, (3) they were treated with radical resection, (4) they had predicted postoperative remnant liver volume >40% and (5) they had liver function graded as Child–Pugh class A. Patients with the involvement of distant metastasis received palliative resection and were therefore excluded from this study.
Preoperative assessment
Computed tomography (CT) and magnetic resonance imaging (MRI) were used to identify the involvement of adjacent structures. Biologically, the HAE lesions behave like a slow-growing liver cancer, without sharp boundaries between the involved tissue and the normal liver parenchyma.
It is known and described that HAE appearance can be like a malignancy, so biopsy has to be performed, especially when Echi serology is negative (Grüner et al., Reference Grüner, Schmidberger, Drews, Kratzer and Grater2017). The hepatitis screening for viral hepatitis was done in all patients. The liver biopsy was performed with albendazol treatment peri-intervention.
High-resolution contrast-enhanced CT scan of the chest and abdomen was performed for all patients to assess the liver volume and vascular anatomy, to measure the extent of the hepatic lesion and to rule out extra-hepatic disease. CT data were used for three-dimensional reconstruction to improve the success of the procedures and reduce surgical complications.
Surgical management
All patients underwent radical excision. Radical excision was defined as complete resection of the lesion, with pathologically confirmed negative margins and confirmed the absence of lesions in the residual liver. The treatment approach was modified according to the preoperative features, such as size and extent of the lesion, presence of obstructive jaundice, expected postoperative residual volume, presence of vessel invasion and so on.
The surgical approach varied with the stage of disease as classified by the World Health Organization's PNM system for alveolar echinococcosis (Kern et al., Reference Kern, Wen, Sato, Vuitton, Gruener, Shao, Delabrousse, Kratzer and Bresson-Hadni2006). The PNM staging pays attention to the involvement of vessels, biliary tract and therefore can be helpful in the HAE management. Usually P4 lesions with the involvement of liver hilus/interior caval veins are supposed to be not radically resectable (Brunetti et al., Reference Brunetti, Kern and Vuitton2010). Nevertheless, with the development of technology for autologous liver transplantation (ALT), lesions invasion into critical veins can be used for ex vivo liver resection and autotransplantation (Cheng et al., Reference Cheng, Yang, Zeng, Gu, Cui, Ning and Yi2018; Hwang et al., Reference Hwang, Liou and Kato2018).
The study patients could be divided into four groups according to the type of surgical procedure undertaken: group A patients directly underwent hepatic resection (Fig. 1A); group B patients underwent percutaneous puncture external drainage, followed by radical hepatic resection 2 months later (Fig. 1B); group C patients underwent a two-step hepatic resection (Fig. 1C); and group D patients underwent liver transplantation (Fig. 1D). All patients were treated with albendazole upon admission.
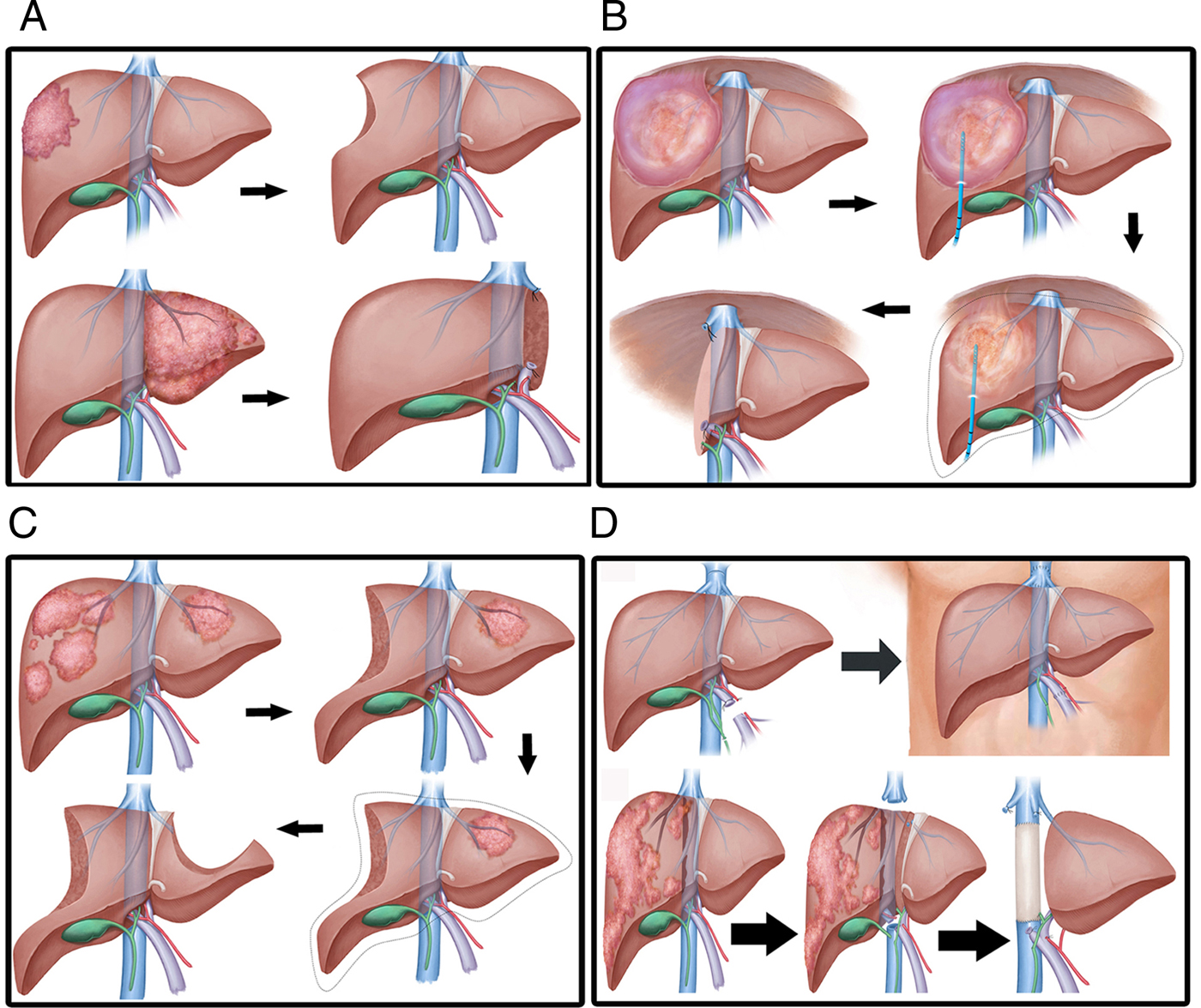
Fig. 1. Different approaches for radical hepatic resection. (A) Lesion that can be removed in a one-stage surgery, i.e. suitable for direct radical hepatic resection. (B) Huge cystic and solid lesions adherent to surrounding tissue; such lesions are best treated by initial percutaneous puncture and external drainage, followed by radical hepatic resection. (C) Multiple liver lesions, with preoperative assessment showing insufficient residual liver volume after surgery; such lesions call for two-step hepatic resection. (D) Invasion of vital vessels by lesion requiring treatment by liver transplantation.
Intraoperative ultrasound was routinely used to determine the extent of the lesion and its relationship with the surrounding vascular structures, and also to detect any lesions that may have been overlooked during preoperative assessment.
Following surgery, all patients received albendazole (15 mg kg−1 day−1 in two divided doses) for 2 years. The drug was stopped only if there were any adverse events (such as severe liver or kidney functional damage).
Follow-up and long-term management
Postoperative complications were graded according to the Clavien–Dindo classification (Martel et al., Reference Martel, Ismail, Beqin, Vandenbroucke-Menu and Lapointe2014). During follow-up visits, patients underwent physical examinations, liver function tests, serological examination and ultrasonography. After 3 months, CT or MRI was performed to evaluate liver regeneration, vascular thrombopoiesis and recurrence of parasitic lesions. CT/MRI or serological examination is recommended for 6 months or 1 year if there are no special circumstances.
Statistical analysis
Statistical analysis was performed with SPSS 21.0 (IBM Corp., Armonk, NY, USA). Normally distributed continuous variables were summarized as means ± s.d. deviations, and non-normally distributed variables as medians. Categorical variables were summarized as frequencies and as proportions of the total cohort. Statistical significance was at P < 0.05.
Results
Demographic and clinical characteristics of patients
Demographic and clinical characteristics of patients were shown in Table 1. Symptoms were present in 79.8% (142/178) of patients; these included non-specific abdominal pain, abdominal distension, jaundice and a palpable mass. Reason of abdominal pain was to make the diagnosis by ultrasonography and CT of the abdomen. Preoperative echinococcal serology was tested in 107/178 (60.1%) patients and was positive in all.
Table 1. Clinical characteristic of study patients
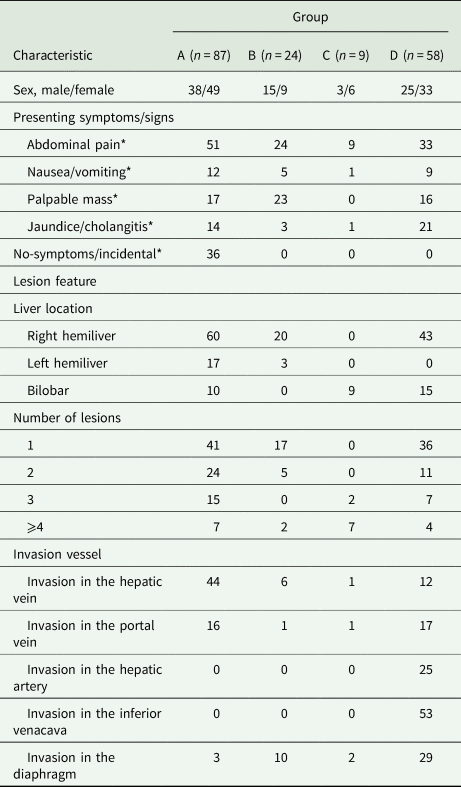
* P < 0.05, compared to each group, respectively.
Preoperative assessment
On preoperative assessment with CT or MRI scan, or with 3D reconstruction, the majority of patients (94/178) had a single confluent alveolar lesion; the others had two or more separate lesions. Among cases, preoperative imaging revealed that the largest lesion was over 20 cm in size and two or multiple liver segments were most frequently involved. Bilobar lesion was found in 34/178 (19.1%) patients; 84/178 (47.2%) patients had two or more lesions. However, part of lesion invasion into the hepatic vein (63/178), hepatic artery (5/178), portal vein (25/178), diaphragm (44/178) and inferior vena cava (53/178) are presented (Table 1). PNM classification of AE of each group was shown in Table 2.
Table 2. PNM classification of each group
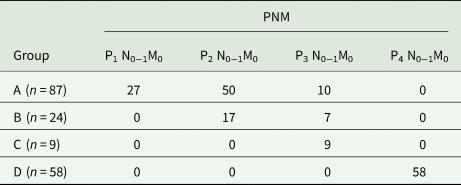
Surgical procedures
Preoperative evaluation of residual liver volume is necessary to decide whether it will meet the body's needs. Group A comprised 87/178 (48.9%) patients; all underwent radical hepatectomy directly. Patients received left hemihepatectomy, right hemihepatectomy, extended left hemihepatectomy, right hemihepatectomy or limited hepatectomy (Table 3). Group B comprised 24/178 (13.5%) patients. These patients had an enormous cyst size and so first underwent percutaneous transhepatic puncture and external drainage; this was followed by radical hepatectomy 2 months later. Group C comprised 9/178 (5.1%) patients. Direct radical hepatectomy was not performed because preoperative assessment indicated that the residual liver volume after surgery would be too low and there would be a high risk of postoperative liver failure. These patients therefore underwent a two-stage hepatectomy. During the first stage, patients underwent right-sided liver parenchyma partition (n=6) or a left-sided liver parenchyma partition (n = 3) (Table 3). This was followed by radical hepatectomy 3 months later. Group D comprised 58/178 (32.6%) patients. These patients presented with a tumour syndrome, with the invasion of both lobes of the liver, secondary sclerozing cholangitis and invasion of hilum and hepatic veins or inferior vena cava. Autotransplantation was performed in 100% patients (58/58).
Table 3. Surgical treatment and complication
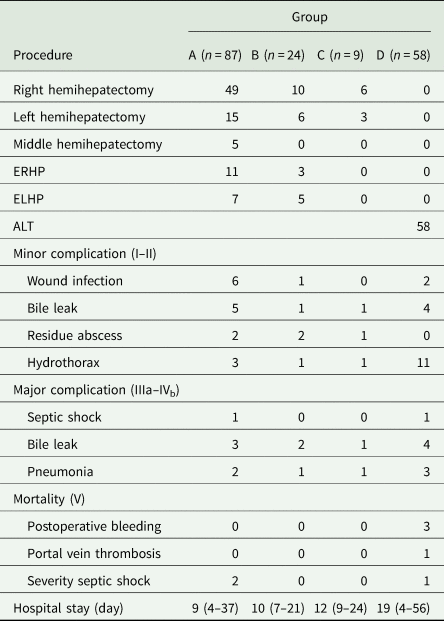
ERHP, extended right hemihepatectomy; ELHP, extended left hemihepatectomy; ALT, autogeneic liver transplantation.
Outcomes
Surgery was technically successful in all 178 patients; there were no intraoperative deaths and no patient required reoperation. A total of 7/178 (3.9%) patients died during the postoperative period: 2/87 (2.29%) patients in group A and 5/58 (8.62%) patients in group D, respectively. There were no deaths in groups B and C. The deaths were mostly due to serious postoperative complications. Major complications (Clavien–Dindo grades III–V) occurred in 19 patients, and mortality (V) occurred in seven patients. Table 3 lists the postoperative complications. There was a considerable difference in the incidence of postoperative complications between the groups. The major complications included septic shock following lung infection and pleural effusion, postoperative biliary leaks and postoperative hydrothorax. Obviously, bile leakage is the most common complication.
No recurrences of HAE were documented during follow-up, a median of 35.8 months (8–72). The postoperative survival rates were 97.7% (85/87) in group A, 95.8% (23/24) in group B, 100% (9/9) in group C and 91.4% in group D (53/58), respectively. The difference between the groups was not considered significant. The 5-year survival rate is not yet available for partly patients. Here, it needs more time for follow-up.
Discussion
This study examined the different surgical approaches for the management of HAE in the West China Hospital, Sichuan University. This work demonstrates the usefulness of surgery for definitive management of echinococcal disease and the high incidence of complicated HAE. The study results show that long-term cure of HAE can be achieved with radical liver surgery, and that major postoperative complications and mortality are within acceptable limits. The number of surgically treated patients is high; west china is an important endemic area. Moreover, patients are younger when presenting with huge AE lesions (compared to Europe) (Du et al., Reference Du, Liu, Yang, Yang, Li, Wen, Yang, Xu, Chen and Wang2016; Aii et al., Reference Aii, Dong, Shao, Zhao, Li, Tuxun, Shalayiadang, Ran, Jinag, Zhang, He, Huang and Wen2018), so the surgical approach to the disease in this region seems to be really important.
The introduction of albendazole treatment has revolutionized the treatment of AE, because life expectancy and outcome of AE has shown to be improving with albendazole treatment, data from Switzerland, France and Germany (Torqerson et al., Reference Torqerson, Schweiger, Deplazes, Pohar, Rechen, Ammann, Tarr, Haikik and Mullhaupt2008; Piarroux et al., Reference Piarroux, Piarroux, Giorqi, Knapp, Bardonnet, Sudre, Watelet, Dumortier, Gerard, Beytout, Abergel, Mantion, Vuitton and Bresson-Handni2011; Schmidberger et al., Reference Schmidberger, Kratzer, Stark and Grüner2018). For the living patients, postoperative BMZ treatment is mandatory. Our patients have received albendazole treatment.
HAE exhibits tumour-like behaviour, with features such as liver invasion (Du et al., Reference Du, Zhang, Hou, Ren, Wang, Guo and Fan2017), destruction of the hepatic parenchyma and infiltration of adjacent vascular or biliary structures (Vogel et al., Reference Vogel, Görich, Kramme, Kramme, Merkle, Sokiranski, Kern and Bramhs1996; Caballero-Mateos et al., Reference Caballero-Mateos, Martine-Cara and Redondo-Cerezo2017; Yang et al., Reference Yang, Qiu, Huang, Wang, Shen, Feng, Wei, Lei, Zhao, Li, Wen and Yan2018). Most of these features were observed in our patient population. The main symptom was abdominal pain, most often located in the right upper quadrant. Other clinical symptoms included pernicious vomiting, jaundice, indigestion, abdominal distention and so on. Asymptomatic patients were seen only in group A (36/87), which indicates that HAE may not be easily diagnosed in the early stages, though the symptoms of progression or end-stage are obvious, and also shows that liver disease is not easy to find in the early stage (Bresson-Hadni et al., Reference Bresson-Hadni, Vutton, Bartholomot, Heyd, Meyer, Hrusovsky, Becker, Mantion, Lenys and Miquet2000; Sezqin et al., Reference Sezqin, Altintas, Saritas and Sahin2005; Connor et al., Reference Connor, Yawathe and Harrison2015). Unfortunately, in the absence of early symptoms, a proportion of patients may have missed the opportunity for early radical resection and needed to undergo more complicated procedures. In our study sample, only 48.9% of patients (group A patients; 87/178) were suitable for direct radical hepatectomy. Patients in the other groups needed other procedures.
In group B, percutaneous puncture and drainage of the pseudocystic lesions were performed 2 months before radical resection. This was necessary to avoid the risk of the semiliquid necrotic tissue entering the biliary tree and causing a bacterial infection or even life-threatening sepsis (Sato et al., Reference Sato, Namieno, Takahashi, Yamashita, Aoki and Uchino1996). The imaging data in these patients also showed dense adhesions in the abdominal cavity, which would have made surgery difficult (Fig. 2). With very large pseudocyst, there is an increased risk of rupture during the operation (Xia et al., Reference Xia, Yan, Li, Zeng, Cheng, Wen, Yang, Li, Wang, Yan, Wang, Xie, Pan and Liu2005; Nunnari et al., Reference Nunnari, Pinzone, Gruttadauria, Celesia, Madeddu, Malagurnera, Pavone, Cappellani and Cacopardo2012), which can lead to peritoneal cavity seeding. An advantage is that drainage of the pseudocyst relieves pressure on the liver parenchyma and promotes liver tissue regeneration and residual liver volume increase. Two months after drainage, liver volume was assessed by 3D.
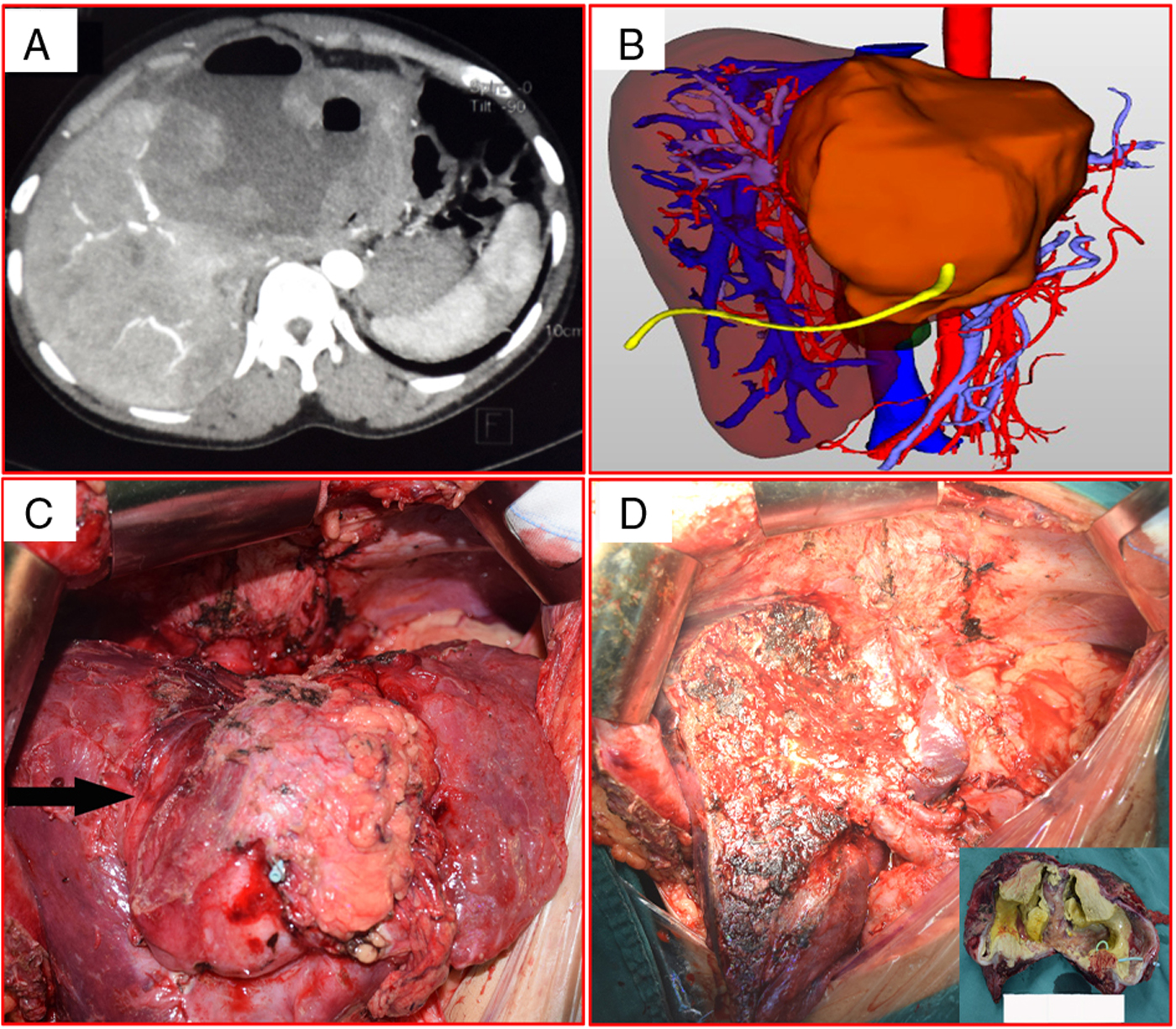
Fig. 2. Representative images of enormous pseudocystic alveolar echinococcosis. (A) Abdominal computed tomography shows huge alveolar echinococcosis lesions in the left lobe, with lesions adhering closely to surrounding tissue. (B) Three-dimensional imaging after drainage of the pseudocyst. (C) Intraoperative photograph shows massive lesions in the left lobe; dense adhesions can be seen around the liver. (D) The cut surface of the liver during hepatectomy, and the resected specimen (inset).
In group C, a two-stage procedure was adopted because the residual liver volume was assessed as insufficient due to the presence of large or multiple HAE lesions (Fig. 3). In such cases, direct radical resection can result in liver failure. Our study showed that the two-step process is safe and feasible for patients with multiple giant alveolar echinococcosis. No patients died during operation or after surgery.
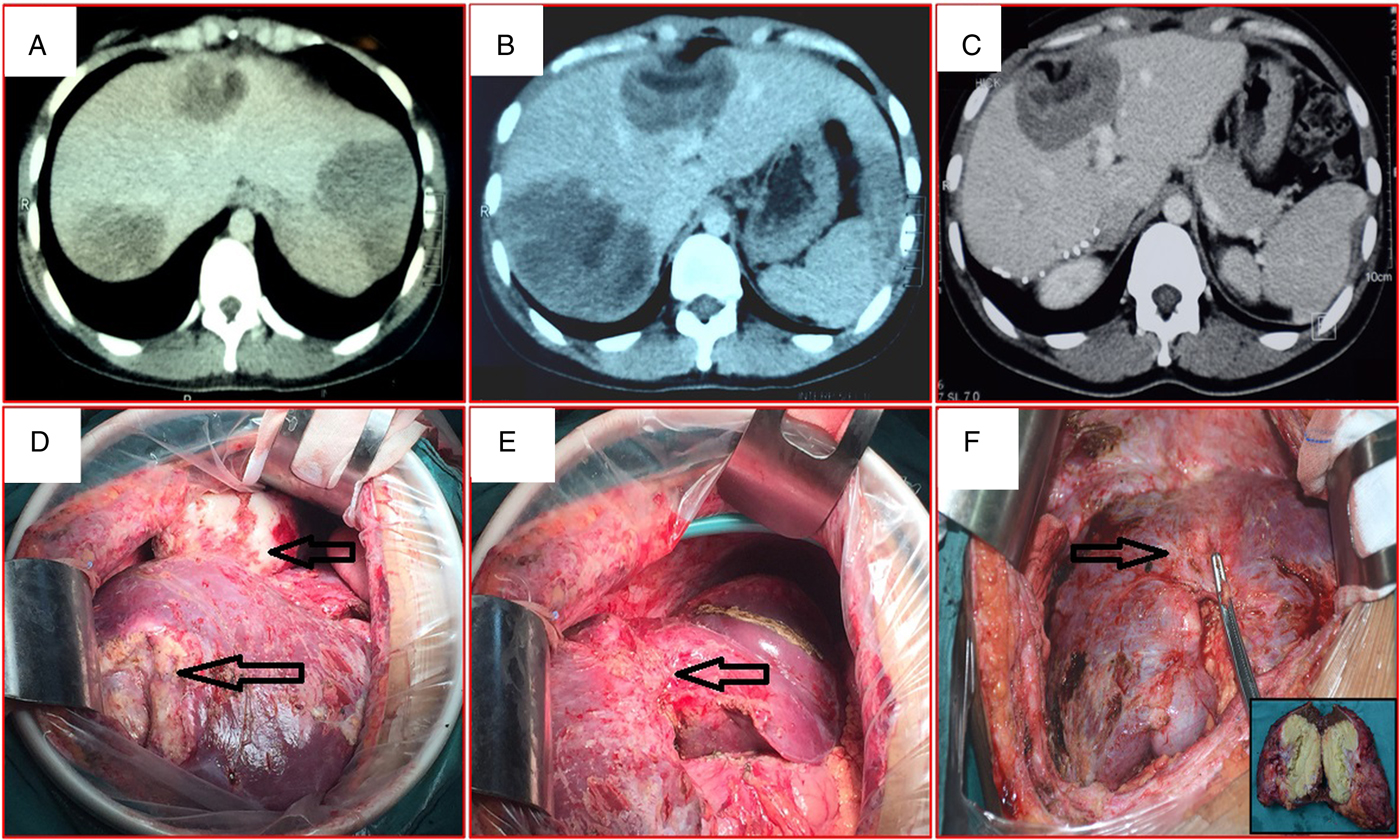
Fig. 3. Representative images of multiple lesions of alveolar echinococcosis. (A and B) Abdominal computed tomography shows multiple lesions in the liver. (C) After the first-stage surgery, computed tomography shows postoperative liver regeneration and the remaining lesions. (D and E) Open surgery shows multiple lesions in the liver during the first-stage hepatectomy. (F) The cut surface of the liver after the second-stage hepatectomy shows evidence of compensatory regeneration in the liver remnant; the resection specimen (inset).
Group D patients had end-stage alveolar echinococcosis and were therefore treated with liver transplantation (ALT). End-stage HAE is one of the accepted indications for liver transplantation. Orthotopic liver transplantation has been used for the treatment of end-stage HAE since 2002 (Bresson-Hadni et al., Reference Bresson-Hadni, Franza, Miguet, Vuitton, Lyenys, Monnet, Landecy, Paintaud, Rohmer, Becker, Christophe, Mantion and Gillet1991; Palma et al., Reference Palma, Oberkofler, Rapitis, Eshmuminov, de Rouqemont, Schnyder, Dimitroulis, Lesurtel, Dutkowski and Clavien2014). The recent development of ALT technology has brought great relief to patients with end-stage liver disease. Ex vivo liver resection and autotransplantation are now often performed in patients with complicated end-stage liver disease (He et al., Reference He, Bai, Jiang, Ji, Tai, Zhao, Zhang, Liu and Wen2015; Jianyong et al., Reference Jianyong, Jingcheng, Wentao, Lunan, Jichun, Bing and Ding2015; Wen et al., Reference Wen, Dongle, Zhang, Duan, Zhao, Liang, Shao, Ji, Tai, Li, Gu, Tuxun, He and Huang2016). The long wait for a donor liver inevitably results in the growth of the lesion and worsening of the patient's condition, which only makes surgery more difficult. On the other hand, with the development of transplanting technology, the improvement of surgical technique, the invasion of inferior vena cava and even the heart ear segment vessels, which are not an untouchable location (Jianyong et al., Reference Jianyong, Jingcheng, Wentao, Lunan, Jichun, Bing and Ding2015; Li and Wu, Reference Li and Wu2016; Letouzé et al., Reference Letouzé, Shinde, Renault, Couchy, Blanc, Tubacher, Bayard, Bacq, Meyer, Semhoun, Bioulac-Saqe, Prevot, Azoulay, Paradis, Imbeaud, Deleuze and Zucman-Rossi2017), and the early postoperative mortality was low with acceptable morbidity. In our sample, overall mortality was 8.62% (5/58) in patients undergoing autologous transplant. These rates are similar to those reported by others (Palma et al., Reference Palma, Oberkofler, Rapitis, Eshmuminov, de Rouqemont, Schnyder, Dimitroulis, Lesurtel, Dutkowski and Clavien2014; Jianyong et al., Reference Jianyong, Jingcheng, Wentao, Lunan, Jichun, Bing and Ding2015).
As we all know, there are different mechanisms of the liver lesion between HAE and liver cancer. Hepatocellular carcinoma (HCC) generally arises against a background of liver cirrhosis, and so surgery can be performed only if there is an assurance of at least 50% residual liver volume after resection (Ricard-Blum et al., Reference Ricard-Blum, Bresson-Hani, Guerret, Grenard, Volle, Risteli, Grimaud and Vuitton1996; Huang and Lu, Reference Huang and Lu2017). However, patients with HAE have shown lower cirrhosis background, compared with HCC caused by virus infection. In fact, partly HAE patients presented secondary biliary cirrhosis (mainly due to biliary tract chronic obstruction) at the time of ALT (Koch et al., Reference Koch, Bressons-Hadni, Miguet, Crumbach, Gillet, Mantion, Heyd, Vuitton, Minello and Kurtz2003). Yet, with the application of percutaneous transhepatic cholangial drainage, the risk of hepatic lesion was effectively reduced for biliary tract obstruction (Takada, Reference Takada2001), and so the expected postoperative residual liver volume only need to be >40%; this makes the ALT easier in these patients. Ex vivo liver resection and autotransplantation are now an effective solution to the problem of donor shortage.
The PNM staging system of WHO for alveolar echinococcosis has helped standardize the classification of HAE. It takes into consideration the anatomical location of the primary parasitic lesion and the anatomical extent (Cheng et al., Reference Cheng, Yang, Zeng, Gu, Cui, Ning and Yi2018). However, when judging the resectability of the lesion, the primary consideration is the postoperative remnant liver volume and liver function. It is like liver cancer surgery.
Postoperative complication (by the Clavien–Dindo classification) was acceptable in our series. Other series have reported postoperative complication rates of 14–40% (Birnbaum et al., Reference Birnbaum, Hardwigsen, Barbier, Bouchiba and Le Treut2012; Qu et al., Reference Qu, Guo, Sheng, Yu, Chen, Wang, Shi, Zhang, Yang and Du2017). Although the overall complication rate identified in this study (33.1%, 59/178) was on the higher end of the previously published range (He et al., Reference He, Bai, Jiang, Ji, Tai, Zhao, Zhang, Liu and Wen2015), we argue that this was still an acceptable morbidity rate. In our study, Clavien–Dindo grade 1 or 2 complications constituted 22.5% (40/178) of all complications; these included pleural effusion, bile leakage, infection, etc. Pleural effusion (reactivity) was relatively common and was probably related to surgical trauma. This incidence of complications was related to the extensive nature of the surgery. Major complication resulted in a relatively longer hospital stay. The longest post-operative stay duration of patients was 56 days. Nevertheless, the shortest was 4 days due to fatal complication (Table 3).
As for recurrence after surgery in HAE, it is related to incomplete resection of the lesion, metastatic lesions not detected and no timely anthelmintic agents treatment, especially effective albendazole treatment after surgery. All of our patients were given albendazole as long-term treatment (15 mg kg−1, lasting over 2 years), and recurrence was not observed in the patients who survived.
Adequate preoperative evaluation is required for the surgical treatment. Figure 4 illustrates this process in a simplified flow diagram, showing a guiding principle for HAE. The limitations of this study are that the number of patients enrolled may be not sufficient enough, follow-up recall bias and selection bias possibly exists. So, large cases of randomized controlled long-term observation researches are expected in the future.
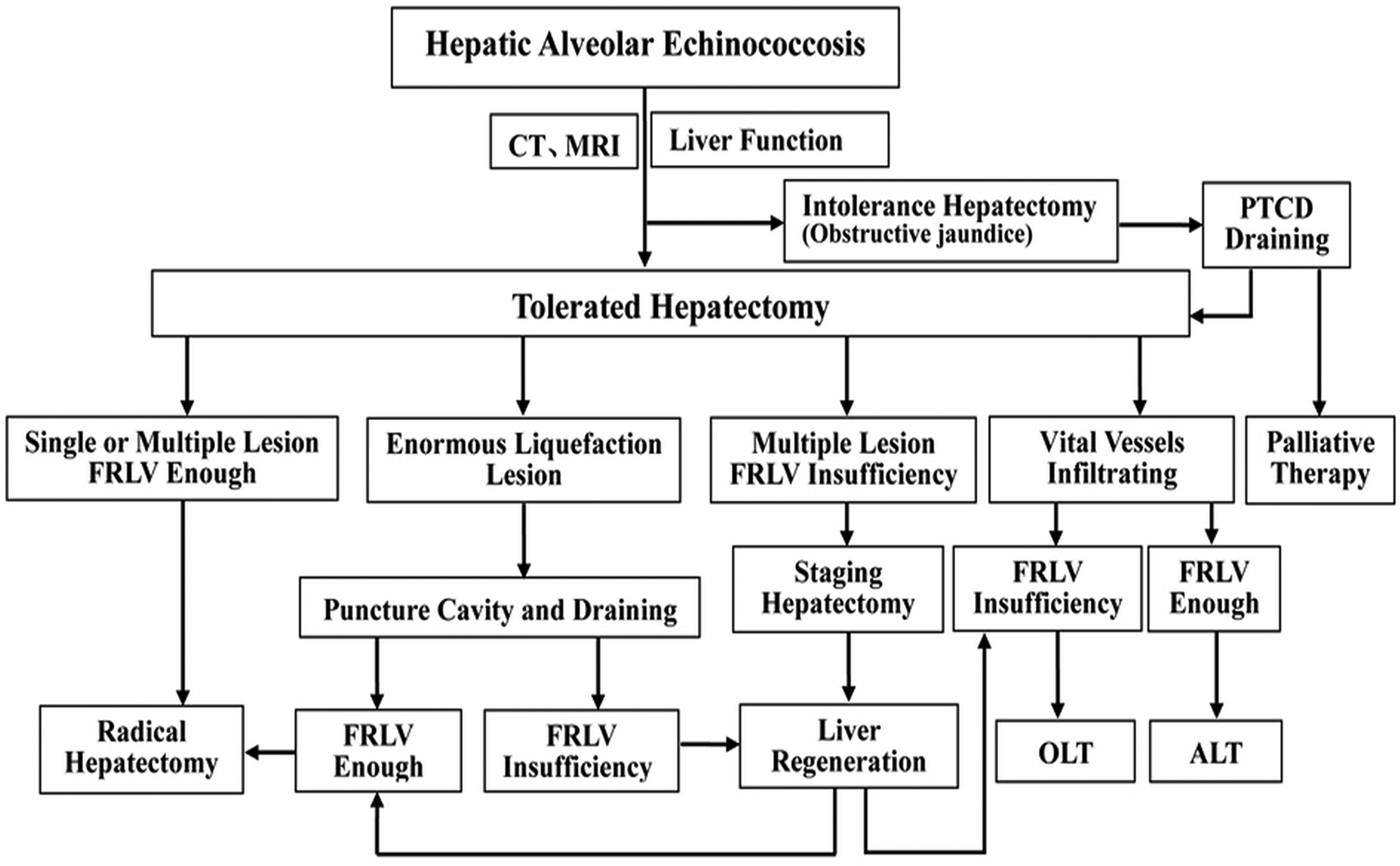
Fig. 4. Flow diagrams summarizing the surgery strategies of hepatectomy procedure and guiding principle for hepatic alveolar echinococcosis. ※, No neighbouring organ or distant metastasis; CT, computed tomography; MRI, magnetic resonance imaging; FRLV, future remnant liver volume; PTCD, percutaneous transhepatic cholangial drainage; OLT, orthotopic liver transplantation; ALT, autologous liver transplantation.
Conclusion
The surgical management of HAE remains challenging because of the associated lesion invasion. Decisions on resectability of a lesion depend primarily on estimated postoperative residual liver volume and liver function. Active HAE should be treated by radical liver resection, and the complicated alveolar echinococcosis of the liver has been managed whenever possible using principles of radical liver resection by experienced hepatic surgeons.
Financial support
This work was supported by the National Science Foundation (No. 81770566). This work was also funded by the Sichuan Science and Technology Program (No. 19ZDYF1682).
Conflicts of interest
None.
Ethical standards
The West China Hospital Research Ethics Committee approved this retrospective analysis of anonymized data. The need for informed consent was waived because of the retrospective nature of the study. We were confirmed that all methods have performed in accordance with the relevant guidelines.










