Recent research carried out by our group and others suggests that higher protein intake may be associated with the preservation of lean body mass( Reference Houston, Nicklas and Ding 1 ) and reduced frailty( Reference Beasley, LaCroix and Neuhouser 2 ) in postmenopausal women. The characterisation of mechanisms through which higher protein intake may be related to successful ageing phenotypes could inform dietary guidelines. The insulin-like growth factor (IGF) axis constitutes an evolutionarily conserved system involved in the regulation of cell growth, proliferation and survival that affects nearly every organ system in the body. The axis consists of two phylogenetically conserved peptide ligands, IGF-I and IGF-II, with potent anabolic effects and six high-affinity binding proteins (insulin-like growth factor-binding protein (IGFBP)-1 to IGFBP-6)( Reference Froesch, Schmid and Schwander 3 ).
IGF-I is the primary mediator of the effects of growth hormone and is thought to be the major IGF affecting growth, health and disease following fetal development( Reference Yu and Rohan 4 , Reference Rajpathak, Gunter and Wylie-Rosett 5 ). IGF-I shares extensive sequence homology and downstream signalling pathways with insulin and has insulin-like effects on glucose and fat uptake in peripheral tissues. However, IGF-I exhibits stronger mitogenic and anti-apoptotic activity than insulin( Reference Zapf, Schmid and Froesch 6 ). Previous research has suggested that circulating IGF-I levels may influence the risk of cancer( Reference Yu and Rohan 4 ), diabetes( Reference Rajpathak, Gunter and Wylie-Rosett 5 ) and other conditions related to healthy ageing( Reference Kawai and Rosen 7 ). Most of the IGF-I in the circulation is produced by the liver and is bound to IGFBP, with IGFBP-3 binding 75 % or more of all IGF-I in the blood. Only approximately 1 % of total serum IGF-I is unbound, and this free fraction may be the most biologically active component of total IGF-I( Reference Juul, Holm and Kastrup 8 ).
It is well known that diets deficient in energy and/or protein lead to substantive reductions in serum IGF-I levels( Reference Thissen, Ketelslegers and Underwood 9 ); however, the role of protein and other dietary factors under conditions of weight maintenance is not well characterised. Significant increases in serum IGF-I levels (51·5 (95 % CI 18·6, 84·4) % after 6 months and 8 % (P= 0·016) after 2 years, respectively) have been reported by two randomised, controlled trials of 20 g( Reference Schurch, Rizzoli and Slosman 10 ) and 30 g( Reference Zhu, Meng and Kerr 11 ) of daily protein supplementation compared with isoenergetic placebo. A randomised trial comparing three daily servings of milk with control has reported a 10 % increase in IGF-I levels (P< 0·001)( Reference Heaney, McCarron and Dawson-Hughes 12 ). Observational studies have suggested that animal protein( Reference Holmes, Pollak and Willett 13 ), particularly from dairy sources( Reference Ma, Giovannucci and Pollak 14 ), is specifically associated with higher serum IGF-I levels; such studies can help to discern whether the putative increases in IGF-I levels are sustained with habitually higher protein intake than with lower protein intake.
We are unaware of any published study that has addressed the relationship of protein intake with free IGF-I levels, and a few dietary studies have examined that with IGFBP-3 levels, though some data from two cross-sectional studies indicate that IGFBP-3 levels are not associated with protein consumption( Reference Holmes, Pollak and Willett 13 , Reference Kaklamani, Linos and Kaklamani 15 ). The present study also has the potential advantage of having data regarding biomarker-calibrated total protein intake, thought to be a better measure of dietary intake compared with self-report alone, from the Women's Health Initiative (WHI). Therefore, the present study examined the cross-sectional associations of total IGF-I, free IGF-I and IGFBP-3 levels with biomarker-calibrated total protein intake, along with uncalibrated dairy product and milk consumption, among 747 women in the WHI – Observational Study (WHI-OS)( Reference Prentice, Mossavar-Rahmani and Huang 16 , Reference Neuhouser, Tinker and Shaw 17 ).
Methods
Study population
The WHI-OS is a prospective cohort study that enrolled 93 676 women aged 50–79 years at forty US clinical centres between 1993 and 1998, as has been described in detail elsewhere( 18 ). The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the institutional review boards at each of the WHI clinical centres and at the Clinical Coordinating Center at the Fred Hutchinson Cancer Research Center (Seattle, Washington, USA). Written informed consent was obtained from all the participants. The participants of the present study were controls from an ancillary nested case–cohort study designed to evaluate the relationship between the IGF axis and colorectal, breast and endometrial cancers, as reported previously( Reference Gunter, Hoover and Yu 19 – Reference Gunter, Hoover and Yu 21 ). Of the 816 randomly selected controls from the WHI-OS, the analytical sample consisted of 747 women who reported plausible energy intake in FFQ (i.e. excluding values < 2510·4 or >20 920 kJ/d) and having complete information on covariates needed to calculate biomarker-calibrated protein intake (age, BMI, smoking, race/ethnicity, education, income and physical activity).
Measurement of insulin-like growth factor levels
Total IGF-I, free IGF-I and IGFBP-3 levels were determined in serum samples collected at baseline using commercially available ELISA kits (Diagnostic Systems Laboratories)( Reference Gunter, Hoover and Yu 19 – Reference Gunter, Hoover and Yu 21 ). Inter-assay CV were 8·2, 11·2 and 3·6 % for total IGF-I, free IGF-I and IGFBP-3, respectively.
Dietary protein assessment
All the participants completed the WHI FFQ at WHI-OS baseline. The self-administered FFQ included 122 items for individual foods/food groups, nineteen adjustment items and summary questions( Reference Patterson, Kristal and Tinker 22 ). Protein intake was characterised as absolute energy intake (total intake in grams) and relative to energy intake (as a percentage of total energy intake) and relative to body weight (as a ratio of 0·1 g of daily protein intake per kg of body weight)( 23 ). Milk consumption was captured from FFQ questions of frequency and portion of milk as beverage, on cereals and in coffee or tea. Dairy product consumption was computed by summing milk consumption and twenty items from the FFQ querying weekly servings of cheese, yogurt and combination foods containing these items (i.e. quesadilla and cream soups).
Calibrated energy and protein estimation
As has been described previously( Reference Neuhouser, Tinker and Shaw 17 ), the WHI Nutritional Biomarkers Study used objective biomarkers of total energy expenditure (equivalent to energy intake in weight-stable persons) and protein intake to assess the measurement properties of the FFQ. Briefly, 544 women from twelve clinical centres of the Dietary Modification Trial were enrolled in a doubly labelled water protocol to estimate total energy expenditure over a 2-week period and a urinary N protocol to estimate protein consumption over a 24 h period to be compared with concurrent self-reported dietary intake data. Calibrated energy and protein estimates were obtained by introducing baseline FFQ consumption estimates and other participant characteristics obtained at baseline from the WHI-OS into linear regression calibration equations( Reference Gunter, Hoover and Yu 19 ). For example:
log-calibrated percentage of energy obtained from protein = 2·66+0·439 × log FFQ percentage of energy obtained from protein − 0·004 × BMI − 0·005 × age − 0·129 × current smoker (1 = yes, 0 = no).
Covariate measurements
Data pertaining to demographic characteristics, medical history and other health-related characteristics were obtained by self-report at WHI-OS baseline. BMI was computed using measured height and weight at baseline (kg/m2).
Statistical analysis
IGF levels and protein, dairy product and milk intake were ln-transformed both to better approximate a normal distribution and to allow coefficients of regression models to be interpreted as percentage difference. Spearman's correlations were calculated between the levels of total IGF-I, free IGF-I and IGFBP-3. A series of linear regression models were examined to evaluate the influence of energy intake, BMI and other potential confounders on observed associations between IGF axis component levels and protein, dairy product and milk intake. Age, energy intake and BMI were modelled as continuous variables( Reference Harrell 24 ). Exposures of interest were modelled both continuously and categorically. Standard errors for regression coefficients where total protein was the exposure variable were estimated using a bootstrapping procedure (1000 replicates) to acknowledge the uncertainty in the regression calibration coefficients as well as that due to the sampling from the study population. To compare results with those of intervention studies, for the dairy product and milk intake analyses, both the outcome and exposure variables were log-transformed and scaled to express associations in terms of percent difference in the outcome per three-serving increase in the exposure. Logistic regression models were used to measure associations between calibrated protein intake categorised into quartiles and the odds of high serum total IGF-I, free IGF-I and IGFBP-3 levels (defined as top 10 %). Additional analyses were carried out modelling the percentage of energy obtained from protein sources (animal, vegetable and milk) as the exposure variable, adjusting for Ca intake, restricted to participants with no history of hormone therapy use and without prevalent diabetes (n 201). Analyses stratified by BMI category ( < 25, 25–29·9 and ≥ 30 kg/m2), protein source (animal/vegetable) and milk fat content (whole/2 %, 1 % and skimmed) were also carried out, and the P for interaction term was estimated by multiplying the exposure variable by the interaction term. All analyses were carried out using SAS version 9.3 (SAS Institute)
Results
The majority of the women were White (85 %), completed at least high school education (79 %), were current users of postmenopausal hormone therapy at baseline (49 %) and were overweight (BMI 27·4 (sd 5·7) kg/m2) (Table 1). Women who were current hormone therapy users had lower free IGF-I levels (P< 0·05), as reported previously( Reference Gunter, Hoover and Yu 19 – Reference Gunter, Hoover and Yu 21 ). Each of the IGF axis proteins was positively correlated with one another, with the strongest association between total IGF-I and IGFBP-3 (r 0·46 and P< 0·05) (Table 2).
Table 1 Demographic and health characteristics by free insulin-like growth factor-I tertiles (n 747), Women's Health Initiative – Observational Study (Mean values and standard deviations; number of participants and percentages)

MET, metabolic equivalent; % energy, percentage of total energy intake.
Table 2 Geometric mean levels and Spearman correlations between insulin-like growth factor (IGF) axis proteins (Geometric means and 95 % confidence intervals)
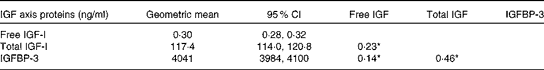
IGFBP, insulin-like growth factor-binding protein. *P< 0·05.
Mean calibrated protein intake was 1·1 (sd 0·2) g/kg body weight per d, exceeding the current RDA for protein (0·8 g/kg body weight per d)( 23 ). Expressed as a percentage of biomarker-calibrated energy intake, median protein intake was 14·3 % across a narrow range (interquartile range 13·3–15·2 %). Dairy product and milk intake also did not vary widely (i.e. median of 1·6 servings of dairy products daily (interquartile range 0·9–2·6)).
Calibrated protein intake, expressed in absolute terms, relative to calibrated energy intake, or relative to body weight, was not associated with free IGF, IGF-I or IGFBP-3 levels (Table 3). However, there was a positive association between free IGF-I levels and milk intake (Table 4). Specifically, a three-serving increase in milk intake per d was associated with an estimated average 18·6 % increase in free IGF-I levels (95 % CI 0·9, 39·3 %) (Table 4).
Table 3 Multivariate linear regression associations between free insulin-like growth factor (IGF)-I, total IGF-I and insulin-like growth factor-binding protein (IGFBP)-3 levels and calibrated protein intake†‡ (β-Coefficients and 95 % confidence intervals)

% energy, percentage of energy intake.
* Scaled to 0·1 g/kg body weight per d.
† Adjusted for age, BMI, race/ethnicity, education, biomarker-calibrated energy intake, alcohol intake, smoking, physical activity and hormone therapy use.
‡ Primary exposure and outcome variables ln-transformed to represent the expected percentage difference in IGF levels per percentage difference in protein intake.
Table 4 Percent difference in insulin-like growth factor (IGF) levels per three-serving increase in daily dairy product and milk intake estimated from linear regression models† (Percent differences and 95 % confidence intervals)

IGFBP-3, insulin-like growth factor-binding protein-3.
* P< 0·05.
† Estimated from ln-transformed outcome and primary exposure variables in multivariate linear regression models adjusted for age, BMI, race/ethnicity, education, energy intake, alcohol intake, smoking, physical activity and hormone therapy use. Estimates were scaled (base = 2·58 and 4·3 for dairy products and milk, respectively) to reflect intervention amount of three servings (average dairy product intake is 1·9 servings per d, so a three-serving increase per d is equal to 158 % increase, and average milk intake is 0·8 servings per d, so a three-serving increase is equal to 375 % increase).
IGFBP-3 levels were not associated with dairy product or milk intake in continuous or categorical analyses. Results obtained from logistic regression models between calibrated protein intake, dairy product intake and milk intake categorised into quartiles and the odds of high serum total IGF-I, free IGF-I and IGFBP-3 levels (defined as top 10 %) were consistent with those obtained from linear regression models (data not shown). The results of analyses that modelled the exposure variable as a categorical or continuous variable were similar, and there was no statistically significant or discernible difference in estimates restricted to (1) non-HT users and (2) women without prevalent diabetes. Nor were the results meaningfully affected by stratification by BMI category ( < 25, 25–29·9, and ≥ 30 kg/m2), protein source (percentage of energy obtained from animal vegetable and milk protein) or milk fat content (whole/2 %, 1 % and skimmed) (all P>0·05 for interaction, data not shown). Analyses including uncalibrated energy and protein intake instead of calibrated one were also similar (data not shown). Lastly, we assessed whether the relationship of milk consumption with free IGF-I levels might be explained by Ca intake. There was no association between free IGF-I levels and Ca intake (β = 0, 95 % CI − 0·002, 0·0003) for Ca from foods alone as well as from supplements (β = 0, 95 % CI − 0·001, 0).
Discussion
The present study supports evidence from clinical trials and observational studies of a specific relationship between milk consumption and IGF-I levels, although in the present study this relationship was only statistically significant for free IGF-I levels. To our knowledge, this is the first study of the relationship between protein intake and milk consumption and free IGF-I levels. Our data, however, did not support reports from other epidemiological studies suggesting a positive relationship between higher overall total protein intake and higher total IGF-I levels( Reference Schurch, Rizzoli and Slosman 10 – Reference Heaney, McCarron and Dawson-Hughes 12 , Reference Hoppe, Kristensen and Boiesen 25 ). Biomarker calibration of protein intake did not appreciably affect the results.
Prior observational studies( Reference Holmes, Pollak and Willett 13 , Reference Crowe, Key and Allen 26 – Reference Norat, Dossus and Rinaldi 28 ) also support a positive association between protein intake, particularly from dairy sources, and total IGF-I levels( Reference Schurch, Rizzoli and Slosman 10 – Reference Heaney, McCarron and Dawson-Hughes 12 , Reference Hoppe, Kristensen and Boiesen 25 ). Data from the European Prospective Investigation into Cancer and Nutrition (EPIC, n 4731 men and women) indicate 2·5 and 2·4 % increases in IGF-I levels per 3 and 2 % increase in energy derived from total protein and dairy protein, respectively( Reference Crowe, Key and Allen 26 ). Given that each serving of dairy products equates to 601 kJ( Reference Fulgoni, Keast and Auestad 29 ) and mean energy intake was 9876 kJ in the EPIC, 2 % energy equates to 198 kJ or approximately 1/3 dairy product serving. Assuming a linear dose response, this would equate to a 22·5 % increase in IGF-I levels with a three-serving dairy product increase per d, which is consistent with the strength of the association observed in the present study (17·3 %) and in clinical trials (range 8–52 %). Moreover, of the protein sources examined (plant, meat, dairy products, fish and eggs) in the EPIC, only dairy products were found to be significantly associated with IGF-I levels. The Nurses' Health Study, examining a comprehensive list of dietary correlates, has reported 9·3 and 7·7 % higher IGF-I levels by comparing highest v. lowest quintiles of total protein and dairy protein, respectively( Reference Holmes, Pollak and Willett 13 ). Similar to the present study, prior studies examining correlates of IGFBP-3 levels( Reference Kaklamani, Linos and Kaklamani 15 , Reference Crowe, Key and Allen 26 , Reference Vrieling, Voskuil and Bueno de Mesquita 27 ) have reported no significant associations with either total dietary protein intake or dairy protein intake.
In prior clinical trials, an additional 20–30 g of protein, often whey protein, or two to three milk servings daily have been shown to result in increases in IGF-I levels of 8–10 % that were sustained for up to 2 years( Reference Schurch, Rizzoli and Slosman 10 – Reference Heaney, McCarron and Dawson-Hughes 12 , Reference Hoppe, Kristensen and Boiesen 25 ). This is well above typical average daily milk consumption of 0·7 servings for the US population aged 2 years and above (National Health and Nutrition Examination Survey (NHANES) 2003–8 data)( Reference Fulgoni, Keast and Auestad 29 ). In the present study, mean daily milk consumption was 0·8 (sd 1·0) servings, less than 25 % of women reported consuming three milk servings per d, and the upper limit of intake was 7·2 servings per d. Thus, the magnitude of the effect size observed in the present study is in keeping with that found in clinical trials and, for the first time, indicates that dairy product intake within the upper range of the typical American diet increases free IGF-I levels. Furthermore, a meta-analysis of eight randomised trials has reported a weighted mean difference of circulating IGF-I levels between milk intervention and control groups of 13·8 ng/ml (95 % CI 6·1, 21·5), and postulated components of milk that may increase IGF-I levels include its nutrient composition of high protein, fat, and Ca and/or contaminants or hormones such as oestrogens and IGF( Reference Qin, He and Xu 30 ).
Collectively, the present study and prior reports are supportive of a particular association between IGF-I levels and milk consumption, which may involve components of milk other than protein. That is, the consistent positive association between IGF-I levels and milk consumption might be explained by a single non-protein nutrient that, nonetheless, may be correlated with total protein or milk protein (since these two factors were variably significant in some prior studies, but not in the present study or several others) or possibly a combination of milk components that include protein. For example, the Singapore Chinese Health Study has reported a positive association between Ca intake from foods and supplements with IGF-I and IGFBP-3 levels( Reference Probst-Hensch, Wang and Goh 31 ). However, Ca intake, both from foods alone and in combination with Ca supplement use, was not significantly associated with free IGF-I, total IGF-I or IGFBP-3 levels in the present study.
The limitations of the present study should be considered while interpreting the data. Diet was characterised using a FFQ, which is limited by both differential and non-differential measurement errors( Reference Subar, Kipnis and Troiano 32 ). For example, the participants were not queried about some items that could contribute to protein intake, such as protein supplements. However, using biomarkers of energy and protein intake, we were able to mitigate some important sources of measurement errors associated with energy and total protein intake( Reference Neuhouser, Tinker and Shaw 17 ). Similar to representative samples of the US population( Reference Fulgoni 33 ), the range of protein, and particularly dairy product and milk, intake was not wide in this population. Since the dairy, cheese and yogurt food group variables are a summary of several FFQ items having different weights and protein content, we could not compute with certainty the protein intake for dairy, cheese and yogurt groups. However, our analyses of IGF axis protein levels and their relationship with protein consumption were null even when examined separately by source (animal, vegetable or milk protein), suggesting that protein consumption alone is not responsible for the observed positive association between milk consumption and free IGF-I levels.
In conclusion, the study data suggest that a three-serving increase in milk consumption per d is associated with an 18·6 % (95 % CI 0·9, 39·3 %) increase in free IGF-I levels. These data provide new insight into the factors that influence free IGF-I, presumably the most bioactive component of IGF-I, a hormone peptide that is the primary mediator of the effects of growth hormone and has been reported to be associated with several major health conditions, including diabetes, CVD and cognitive function in the elderly.
Acknowledgements
The WHI programme is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services, through contracts N01WH22110, 24152, 32100–2, 32105–6, 32108–9, 32111–13, 32115, 32118–32119, 32122, 42107–26, 42129–32 and 44221. This work was also supported by PO1 CA53996. Dr Beasley's involvement was supported by R00AG035002 by the National Institute of Aging, Dr Strickler's involvement was supported by R01DK080792, and Dr Prentice's involvement was supported by R01CA119171 and P01CA53996. None of the funding sources had a role in the design and analysis of the study or in the writing of this article.
The authors' contributions are as follows: J. M. B. analysed the data and drafted the initial manuscript; M. J. G., R. L. P. and H. D. S. were responsible for the study design and implementation and contributed substantively to the writing of the manuscript; M. L. N., L. F. T., M. Z. V. and A. Z. L. reviewed and edited the manuscript.
None of the authors has any conflicts of interest to declare.






