Occupational exposures of healthcare personnel (HCP), especially nurses and surgeons, to bloodborne pathogens are frequent events in hospitals.Reference Puro, De Carli, Petrosillo and Ippolito 1 In Italy every day, ~300 HCP sustain an injury involving a contaminated needle or sharp medical device (needlestick and sharps injuries, NSIs), totaling >100,000 accidents per year, but only an estimated 45% are officially reported. 2 Similar figures are available for the United States where, before legislation mandated widespread implementation of needlestick-prevention devices incorporating a safety mechanism to cover the tip after use (safety-engineered devices, SEDs), 385,000 NSIs were estimated to occur annually, 60% of which were unreported.Reference Panlilio, Orelien and Srivastava 3 Available data from other European countries and all continents clearly demonstrate the high NSI burden worldwide.Reference Venier, Vincent and L’Heriteau 4 – Reference Fica, Jemenao and Ruiz 8
These NSIs have the potential to transmit virtually every pathogen present in human blood, either transiently or persistently.Reference De Carli, Abiteboul and Puro 9 , Reference Tarantola, Abiteboul and Rachline 10 However, hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV), all bearing significant morbidity and mortality, together account for the vast majority of occupational infections reported in healthcare. In 2000, in the absence of interventions to prevent NSIs, these agents were estimated to have caused >80,000 new infections among HCP per year worldwide.Reference Prüss-Ustün, Rapiti and Hutin 11
Therefore, administrative, behavioral, and technical interventions aiming to reduce NSI frequency have been progressively introduced, including recommendations to prevent improper needle manipulations, 12 education, 13 provision of sharps containers for appropriate elimination of used devices,Reference Linnemann, Cannon, DeRonde and Lanphear 14 and SEDs.Reference Lamontagne, Abiteboul and Lolom 15 , Reference Tosini, Ciotti and Goyer 16 Each intervention has contributed to decreasing NSI frequency, but the best preventative strategy (ie, incorporating all these elements), requires significant investments of time, resources, and effort, which has limited their widespread implementation.Reference Roudot-Thoraval, Montagne, Schaeffer, Dubreuil-Lemaire, Hachard and Durand-Zaleski 17
Preventive interventions have therefore been included and have thus been reinforced in specific regulations regarding safety at work. In the United States, the Needlestick Safety and Prevention Act was passed in 2000, 18 and the Occupational Safety and Health Administration endorsed the use of safe needles or needleless devices for the collection and/or withdrawal of body fluids and for the administration of fluids and medications. 19 , 20 In Europe, the Council Directive 2010/32/EU, “Prevention from sharp injuries in the hospital and healthcare sector,” fully in force since 2013, protects HCP from NSIs and their consequences, setting up integrated policies regarding risk assessment, risk prevention, training, education, and monitoring. Among the prevention measures, SEDs must be made available based on risk assessment, whereas HBV vaccination must be universally provided free of charge. Monitoring includes investigating the causes and circumstances of the accident and immediate care for the injured HCP that includes post-exposure prophylaxis (PEP), the necessary medical tests, health surveillance, and counseling where appropriate. Additionally, medical treatment is guaranteed. The economic impact of this directive is expected to be significant. 21
Several studies have considered the economic burden of occupational NSIs,Reference Solano, Hernández, Montes and Arribas 22 – Reference Oh, Yoon Chang, Choi, Park and Jin 25 mostly providing direct costs (ie, post-exposure management) and indirect costs (ie, counselling NSI victims, staff absence and compensation) from different perspectives and, more rarely, evaluating NSIs reduction after the introduction of preventive measures and the resulting economic impact. However, these studies vary widely in terms of setting, source of epidemiologic data, and considered costs, making it difficult to define a standardized cost for NSI management and prevention.
Given the increased costs of SEDs compared to conventional devices, in 2012, a Health Technology Assessment project was launched in Italy to evaluate this new technology and its overall impact on health care, including an economic analysis of costs deriving from NSIs and savings arising from their prevention. This systematic review represents the first part of this project.
MATERIALS AND METHODS
A systematic review of economic analyses of occupational NSIs among HCP was performed in accordance with the PRISMA statement.Reference Liberati, Altman and Tetzlaff 26 Independently, 2 authors consulted PubMed and Scopus databases and resolved eventual discrepancies by consulting a third author. The research algorithm used the following keywords: “cost, needlestick injuries” and “cost, occupational exposures, blood injuries.” Inclusion criteria were language (English, Italian, French, or Spanish) and publication date (January 1997–February 2015).
The titles and abstracts of all articles were evaluated, eliminating duplicates. Articles were then assessed for eligibility through a full-text examination, and an article was included in the analysis if it provided an original estimate of either direct or indirect costs of managing a single NSI. The main stages of the systematic review are shown in Figure 1. Categorization and organization of the reference list were performed using JabRef 2.7.2 software.
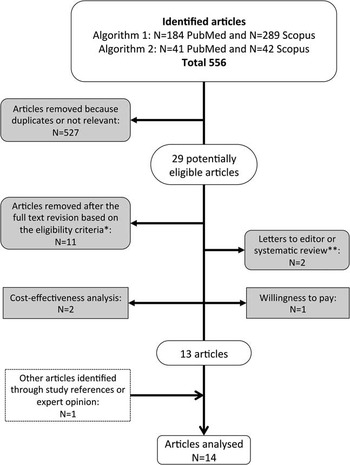
FIGURE 1 Flow-chart of the selection process. *Language other than English, Italian, French or Spanish, or published before January 1997. **Study references were examined to identify other eligible studies.
Data extraction forms included author’s name, publication year, country, source of the occurrence data, study period, population studied, NSI incidence, perspective, cost estimating approach (modeling or data-driven), items considered in cost, type of cost studied (direct and/or indirect), injury management cost (range), and currency equivalent.
To compare monetary values expressed in different currencies and for different years, the national inflation rates provided by the World Bank (http://data.worldbank.org/indicator/fp.cpi.totl.zg) were used to express the injury management costs in national currencies 2015. These values were then converted to 2015 International US dollars (Int$) using purchasing power parity (ppp) exchange rates (http://data.worldbank.org/indicator/pa.nus.ppp). To facilitate comparisons, all costs included in this review were converted to Int$.
Data were analyzed by computing mean, median, and standard deviation (SD) as well as minimum and maximum mean costs abstracted from each article. The weighted mean was used to account for the different proportion of resources used according to the type of event. The findings were stratified by study approach and type of cost (direct and/or indirect). All data used in this analysis were true cost data.
Direct and indirect costs of an NSI were evaluated using the CDC operative definitions:
1. Direct costs: Costs generally borne by a healthcare organization when an NSI occurs: baseline and follow-up laboratory testing, PEP, and other treatment eventually provided, including PEP side-effect management. According to which costs are borne by the organization, in certain circumstances, workers’ compensation or the management of occupationally exposed HCP may be included with direct costs.
2. Indirect costs: These costs include time and wages diverted to receiving or providing exposure-related care: lost productivity associated with reporting and receiving initial and follow-up treatment for the exposure; healthcare provider time to evaluate and treat an employee; and healthcare provider time to evaluate and test the source, including obtaining informed consent for testing if applicable. For occupational health and infection control practitioners, these measures are part of their job responsibilities and therefore are not considered a diversion. Moreover, when staff absence and compensation are borne by a third-party payer, these may not be included with indirect costs. 27
When analyzing in detail the items included within direct and indirect costs in each study, the authors originally assigned baseline and follow-up post-exposure visits differently according to the study context. However, when stratifying the study findings, the post-exposure visits, when disaggregated, were always considered as a direct cost and were assigned accordingly.
The quality of each study was evaluated using Drummond’s checklist modified by La Torre et al.Reference La Torre, Nicolotti, de Waure and Ricciardi 28
RESULTS
In total, 14 studies from Europe, America, Asia, and Australia published from 1997 to 2013 met all the inclusion criteria.Reference Fica, Jemenao and Ruiz 8 , Reference Roudot-Thoraval, Montagne, Schaeffer, Dubreuil-Lemaire, Hachard and Durand-Zaleski 17 , Reference Solano, Hernández, Montes and Arribas 22 , Reference O’Malley, Scott and Gayle 24 , Reference Oh, Yoon Chang, Choi, Park and Jin 25 , Reference Armadans Gil, Fernández Cano and Albero Andrés 29 – Reference Solano Bernad, Rubio Cebrián and Hernández Navarrete 37 Their characteristics are reported in Tables 1 and 2.
TABLE 1 Characteristics of the Studies of Economic Analysis of Occupational Needlestick and Sharps Injuries (NSIs) Among Healthcare Personnel (HCP) Included in the Systematic Review, 1997–2013

NOTE. CH, community hospital; FTE, full-time equivalent; HCP, healthcare personnel (doctors, nurses, technicians, students, etc.); NSIs, needlestick and sharps injuries; UH, university hospital; SEDs, safety-engineered devices.
a NSI incidence rates were standardized according to the available denominator (100 beds; 100 FTE or HCP; 100,000 used devices).
TABLE 2 Economic Characteristics of the Studies of Economic Analysis on Occupational Needlestick and Sharps Injuries Among Healthcare Personnel Included in the Systematic Review, 1997–2013 (Temporal Publication Order)

NOTE. Int$, 2015 International US dollars; HBV, hepatitis B virus; HCP, healthcare personnel; HCV, hepatitis C virus; HIV, human immunodeficiency virus; i.v., intravenous; NA, not applicable; NC, not computable; NHS, National Health System; NSI, needlestick and sharps injury; PEP: post-exposure prophylaxis; SEDs, safety-engineered devices.
a Direct+indirect: aggregated costs; direct/indirect: disaggregated costs.
b Data on categorization of the costs adapted according to Centers for Disease Control (CDC): direct costs include laboratory tests, treatment, medical visits; indirect costs include lost productivity, time-off productivity.
c Injury management costs were expressed in national currencies of 2015 using the national inflation rates provided by the World Bank (http://data.worldbank.org/indicator/fp.cpi.totl.zg), then these were converted to 2015 International US$ (Int$) using purchasing power parity (ppp) exchange rates (http://data.worldbank.org/indicator/pa.nus.ppp).
Cost Analysis
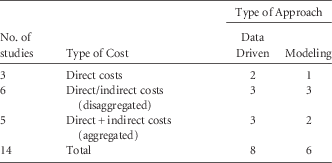
Of the 14 studies estimating costs, 8 studies were data driven, while 6 studies used modeling (Table 3); 11 studies provided both direct and indirect costs (aggregated or disaggregated), while 3 studies only analyzed direct costs (Tables 2 and 3).
TABLE 3 Distribution of Studies of Economic Analysis on Occupational Needlestick and Sharps Injuries Among Healthcare Personnel According to Type of Provided Costs and Study Approach
Costs were analyzed mainly from a hospital or university perspective in 7 studiesReference Fica, Jemenao and Ruiz 8 , Reference Roudot-Thoraval, Montagne, Schaeffer, Dubreuil-Lemaire, Hachard and Durand-Zaleski 17 , Reference O’Malley, Scott and Gayle 24 , Reference Oh, Yoon Chang, Choi, Park and Jin 25 , Reference Armadans Gil, Fernández Cano and Albero Andrés 29 , Reference Hanmore, Maclaine, Garin, Alonso, Leroy and Ruff 32 , Reference Nidegger, Castel and Peltier 36 ; 3 studies adopted the National Health System (NHS) perspectiveReference Solano, Hernández, Montes and Arribas 22 , Reference Glenngård and Persson 31 , Reference Jagger, Bentley and Juillet 33 ; and 1 study adopted both.Reference Cazzaniga, De Carli, Sossai, Mazzei and Puro 30 A societal perspective was considered in 2 studies,Reference Lee, Nicklasson, Cobden, Chen, Conway and Pashos 34 , Reference Leigh, Gillen and Franks 35 and a national insurance perspective was considered in 1 study.Reference Solano Bernad, Rubio Cebrián and Hernández Navarrete 37
Direct costs included testing the source and exposed HCP as well as post-exposure medical visits and treatment (ie, prophylaxis). Costs to treat an occupational infection were included in 2 studies only, which reported annual treatment costsReference Hanmore, Maclaine, Garin, Alonso, Leroy and Ruff 32 and lifetime medical costsReference Leigh, Gillen and Franks 35 for HBV (Int$3,600 and Int$31,306, respectively), HCV (Int$24,424 and Int$23,173, respectively), and HIV infection (Int$35,745 and Int$441,342, respectively). These were excluded from the calculation of average NSI costs.
Indirect costs included lost productivity (time off work) associated with the time required for reporting and receiving initial and follow-up treatment as well as exposure consequences (eg, absence due to emotional distress and anxiety), overhead, compensation, and litigation.
Considering the 11 studies that provided aggregate direct + indirect costs, the overall median of the means of costs for managing an NSI was Int$747 (mean of means, Int$861; range of means, Int$199– Int$1691) (Tables 2 and 4).
TABLE 4 Description of the Distribution of the Means of the Costs for Managing a Single Percutaneous Injury (2015 International US Dollars)
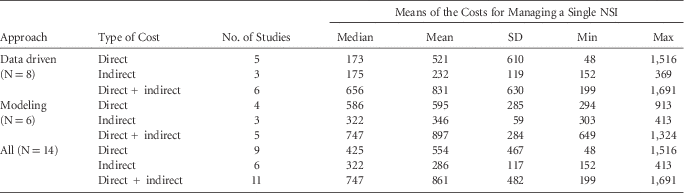
NOTE. NSI, needlestick and sharps injury.
The median of the means of the 9 studies that estimated disaggregated direct costs was Int$425 (mean, Int$554; range, Int$48–Int$1,516), and the median of the means of the 6 studies that estimated disaggregated indirect costs was Int$322 (mean, Int$286; range, Int$152–Int$413).
Considering the type of approach, modeling studies had higher disaggregated and aggregated costs than data-driven studies: direct cost ratio, 3.4:1; indirect cost ratio, 1.84:1; combined direct+indirect cost ratio, 1.13:1. Costs from data-driven studies, however, showed greater variability, with standard deviations more than twice those of modeling studies (Table 4).
When estimating direct and indirect NSI management costs, the included cost items differed among studies (Table 5). Regarding direct costs, while laboratory tests represented the greater proportion in all studies, tests performed on the source and exposed HCP varied greatly. In total, 12 studies provided an itemized serological test menu.Reference Fica, Jemenao and Ruiz 8 , Reference Roudot-Thoraval, Montagne, Schaeffer, Dubreuil-Lemaire, Hachard and Durand-Zaleski 17 , Reference Solano, Hernández, Montes and Arribas 22 , Reference O’Malley, Scott and Gayle 24 , Reference Oh, Yoon Chang, Choi, Park and Jin 25 , Reference Armadans Gil, Fernández Cano and Albero Andrés 29 – Reference Glenngård and Persson 31 , Reference Jagger, Bentley and Juillet 33 , Reference Lee, Nicklasson, Cobden, Chen, Conway and Pashos 34 , Reference Nidegger, Castel and Peltier 36 , Reference Solano Bernad, Rubio Cebrián and Hernández Navarrete 37 In all studies, investigators screened the source for antibodies against HCV (HCV-Ab) and HIV (HIV-Ab). HBV screening was performed mainly using surface antigen (HBsAg),Reference Fica, Jemenao and Ruiz 8 , Reference O’Malley, Scott and Gayle 24 , Reference Oh, Yoon Chang, Choi, Park and Jin 25 , Reference Armadans Gil, Fernández Cano and Albero Andrés 29 , Reference Cazzaniga, De Carli, Sossai, Mazzei and Puro 30 , Reference Glenngård and Persson 31 , Reference Lee, Nicklasson, Cobden, Chen, Conway and Pashos 34 , Reference Nidegger, Castel and Peltier 36 adding surface antibody (HBsAb)Reference Solano, Hernández, Montes and Arribas 22 or core antibody (HBcAb)Reference Jagger, Bentley and Juillet 33 , Reference Solano Bernad, Rubio Cebrián and Hernández Navarrete 37 or through HBcAb alone.Reference Roudot-Thoraval, Montagne, Schaeffer, Dubreuil-Lemaire, Hachard and Durand-Zaleski 17
TABLE 5 Description of Cost Items Included in 14 Studies of Economic Analysis on Occupational Needlestick and Sharps Injuries Among Healthcare Personnel, 1997–2013

NOTE. 3TC, lamivudine; AZT, zidovudine; d4T, stavudine; ddI, didanosine; FU, follow-up; HBIG, hepatitis B immune globulin; HBV, hepatitis B virus; HCV, hepatitis C virus; HCP, healthcare personnel; HIV, human immunodeficiency virus; IDV, indinavir; LPV/r, lopinavir/ritonavir; NFV, nelfinavir; NHS, National Health Service; NSI, needlestick and sharps injury; PEP, post-exposure prophylaxis; PI: protease inhibitor; TDF/FTC: tenofovir/emtricitabine.
Regarding HCP, in 4 studies,Reference Roudot-Thoraval, Montagne, Schaeffer, Dubreuil-Lemaire, Hachard and Durand-Zaleski 17 , Reference Armadans Gil, Fernández Cano and Albero Andrés 29 , Reference Cazzaniga, De Carli, Sossai, Mazzei and Puro 30 , Reference Jagger, Bentley and Juillet 33 HCV-Ab and HIV-Ab were always performed; in 6 studies,Reference Fica, Jemenao and Ruiz 8 , Reference Solano, Hernández, Montes and Arribas 22 , Reference O’Malley, Scott and Gayle 24 , Reference Glenngård and Persson 31 , Reference Nidegger, Castel and Peltier 36 , Reference Solano Bernad, Rubio Cebrián and Hernández Navarrete 37 based on the source serostatus; and in 2 studies,Reference Oh, Yoon Chang, Choi, Park and Jin 25 , Reference Lee, Nicklasson, Cobden, Chen, Conway and Pashos 34 the tests were based on HCP anxiety or other factor. HBV baseline screening varied; HBsAb was performed in vaccinated HCP regardless of the source,Reference Fica, Jemenao and Ruiz 8 in case of a HBsAg-positive source,Reference Armadans Gil, Fernández Cano and Albero Andrés 29 or following exposure to a positive or unknown source.Reference Solano, Hernández, Montes and Arribas 22 , Reference O’Malley, Scott and Gayle 24 , Reference Cazzaniga, De Carli, Sossai, Mazzei and Puro 30 , Reference Solano Bernad, Rubio Cebrián and Hernández Navarrete 37 In 1 study, unvaccinated HCP, or vaccinated HCP with unknown or negative response, were tested for HBsAg, HBcAb, and HBsAb when exposed to an HBsAg-positive or unknown source.Reference Nidegger, Castel and Peltier 36 In another study, all HCP were tested only for HBcAb.Reference Roudot-Thoraval, Montagne, Schaeffer, Dubreuil-Lemaire, Hachard and Durand-Zaleski 17 Finally, in 4 studies, HBV markers (usually HBsAg and Ab) were performed regardless of the HCP vaccinal status or source serostatus.Reference Oh, Yoon Chang, Choi, Park and Jin 25 , Reference Glenngård and Persson 31 , Reference Jagger, Bentley and Juillet 33 , Reference Lee, Nicklasson, Cobden, Chen, Conway and Pashos 34
Furthermore, in a single study, the source and HCP were also screened for syphilis, and in some cases, the source was screened for hepatitis A virus. These tests account respectively for 13.4% and 1.7% of the total tests performed in the study period.Reference Oh, Yoon Chang, Choi, Park and Jin 25
The antiretroviral HIV PEP regimen was specified in 8 of 13 studies. In 6 studies conducted between 1990 and 2003, a zidovudine-based regimen was adopted alone (in 1 study)Reference Lee, Nicklasson, Cobden, Chen, Conway and Pashos 34 or in combination with lamivudine, including a protease inhibitor according to risk (indinavir in 3 studiesReference Roudot-Thoraval, Montagne, Schaeffer, Dubreuil-Lemaire, Hachard and Durand-Zaleski 17 , Reference Solano, Hernández, Montes and Arribas 22 , Reference Jagger, Bentley and Juillet 33 ; nelfinavir in 2 studiesReference Armadans Gil, Fernández Cano and Albero Andrés 29 , Reference Nidegger, Castel and Peltier 36 ). In 2 more recent modeling studies (2006 and 2013), a protease inhibitor-based regimen with lopinavir/ritonavir was considered, adding zidovudine/lamivudineReference Cazzaniga, De Carli, Sossai, Mazzei and Puro 30 or tenofovir/emtricitabineReference Hanmore, Maclaine, Garin, Alonso, Leroy and Ruff 32 (Table 5).
Finally, administration of hepatitis B immune globulin (HBIG) and HBV vaccination were included in the direct costs of an NSI in eightReference Solano, Hernández, Montes and Arribas 22 , Reference O’Malley, Scott and Gayle 24 , Reference Oh, Yoon Chang, Choi, Park and Jin 25 , Reference Armadans Gil, Fernández Cano and Albero Andrés 29 , Reference Cazzaniga, De Carli, Sossai, Mazzei and Puro 30 , Reference Jagger, Bentley and Juillet 33 , Reference Leigh, Gillen and Franks 35 , Reference Solano Bernad, Rubio Cebrián and Hernández Navarrete 37 and sevenReference O’Malley, Scott and Gayle 24 , Reference Oh, Yoon Chang, Choi, Park and Jin 25 , Reference Cazzaniga, De Carli, Sossai, Mazzei and Puro 30 , Reference Jagger, Bentley and Juillet 33 , Reference Leigh, Gillen and Franks 35 studies, respectively (Table 5); in a single hospital study, HBV vaccination was administered but not included in the direct costs because the charges were borne by the NHS.Reference Armadans Gil, Fernández Cano and Albero Andrés 29
Regarding indirect costs, 10 studiesReference Roudot-Thoraval, Montagne, Schaeffer, Dubreuil-Lemaire, Hachard and Durand-Zaleski 17 , Reference Solano, Hernández, Montes and Arribas 22 , Reference O’Malley, Scott and Gayle 24 , Reference Cazzaniga, De Carli, Sossai, Mazzei and Puro 30 , Reference Hanmore, Maclaine, Garin, Alonso, Leroy and Ruff 32 - Reference Solano Bernad, Rubio Cebrián and Hernández Navarrete 37 included lost productivity of an exposed HCP (eg, for initial reporting and treatment, follow-up appointments, and additional work time missed due to PEP side effects); 1 studyReference Hanmore, Maclaine, Garin, Alonso, Leroy and Ruff 32 included also compensation and litigation costs (Table 5).
Quality of the Studies
The quality of the studies is presented in Table 6. For all investigations, the research question (item #1), economic importance of the research question (#2), viewpoints of the analysis (#3), primary outcome measures for the economic evaluation (#11), time horizon of costs and benefits (#22), and conclusions from the data reported (#34) were stated.
TABLE 6 Quality Results of the Selected Studies of Economic Analysis on Occupational Needlestick and Sharps Injuries Among Healthcare Personnel, 1997–2013
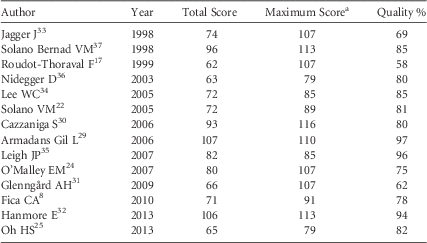
a Maximum score represents the expected score if the study was conducted with optimal practices. Different Maximum scores were shown because different study designs were reviewed according to Drummond’s scale.Reference La Torre, Nicolotti, de Waure and Ricciardi 28
Three studies presented the incremental analysis (#31), provided details of the design and results of effectiveness (#9), and used a synthesis method or meta-analysis of estimates (#10): the data-driven study by Armadans Gil, which had the highest quality score (97%),Reference Armadans Gil, Fernández Cano and Albero Andrés 29 and 2 modeling studies, Leigh (96%)Reference Leigh, Gillen and Franks 35 and Hanmore (94%).Reference Hanmore, Maclaine, Garin, Alonso, Leroy and Ruff 32 These 3 studies reported very different values of aggregate direct + indirect costs, with means of Int$418, Int$747, and Int$1,049, respectively.
DISCUSSION
The overall aggregate direct + indirect costs for managing an NSI ranged from Int$650 to Int$750, considering the median and mean value of the mean costs borne by the hospital or NHS to manage exposures with different scenarios. These values were derived from both modeling and data-driven studies conducted across different countries and continents over a period of approximately 20 years.
Indirect costs are relatively consistent between studies; they mostly refer to lost productivity, which is usually calculated in minutes spent in baseline and follow-up visits by the exposed HCP and more rarely on days of staff absence; the median and mean values of the mean indirect costs ranged between Int$175 and Int$350.
Direct costs vary. The wide range of possible scenarios regarding the infectivity of the source, the susceptibility of the exposed HCP, and the post-exposure diagnostic and prophylactic protocol account for the differences in the average direct costs within and between considered studies, particularly when analyzing studies using a different approach. In modeling studies, the source and exposed HCP are tested according to an optimized protocol. Prophylaxes are provided to all susceptible HCP, and those protocols are always considered to have been completed. All exposed HCP attend follow-up visits and testing. In real life, HCP anxiety can influence the choice of baseline and follow-up testing, or the source situation may be more complicated (eg, multiple possible sources, need for supplemental, confirmatory testing, etc.). Acceptance, adherence, and completion rate of prophylactic treatments may be suboptimal; regimens may vary and cause adverse reactions requiring additional interventions; and compliance with follow-up protocols may differ.
Moreover, regardless of the approach, treatment costs vary between studies: the standard antiretroviral PEP regimen changed significantly over time, including different combinations and newer, more tolerable and expensive drugs in recent studies. Variations in HBIG dosage among protocols increased costs up to 4-fold, and diagnostic tests evolved over time. Two recent modeling studies considering direct and indirect costs and using newer antiretrovirals had a median cost of Int$1,187 (range, Int$1,049–Int$1,324)Reference Cazzaniga, De Carli, Sossai, Mazzei and Puro 30 , Reference Hanmore, Maclaine, Garin, Alonso, Leroy and Ruff 32 ; however, too few recent studies are available to define a real and current overall cost.
Notwithstanding all the observed differences, comparisons between studies also identified significant similarities. Therefore, considering the wide range of possible situations, settings, and behaviors covered in selected studies, this overall amount (including direct and indirect costs) should represent a reliable estimate of the actual average cost for managing an NSI. However, the wide range of observed values should be taken into due account to avoid under- or overestimating the economic costs of managing these incidents or the potential savings resulting from their prevention.
A cautious interpretation of these findings is warranted. The literature search may be incomplete; moreover, different periods, countries, and study designs were compared. Part of the heterogeneity due to the approach and type of estimated costs was controlled by stratifying the studies. Furthermore, weighted means were calculated where disaggregated costs were available. However, this was not possible for all the studies considered. The effect of this heterogeneity is evident in the high variability (SD of the mean and width of the range) of the estimates. In addition, Drummond’s checklist was designed to include all possible aspects concerning economic evaluations, but in this review, different study designs were compared, and it was not possible to apply all items to all articles. To eliminate disparities between the studies, a quality score was calculated based on the items considered appropriate for each study.
Despite these issues, this review confirms that the economic impact of managing NSIs is not negligible, and our analysis provides clues about where to reduce costs.
Protocols should be optimized based on the source; HCP vaccination should increase, and the response should be appropriately evaluated post-vaccination and readily available in case of injury. This recommendation is clearly demonstrated in the high-quality study by Armadans Gil et al,Reference Armadans Gil, Fernández Cano and Albero Andrés 29 where most exposures involved vaccinated HCP exposed to known sources who tested negative for HCV and HIV; the mean NSI management cost (direct + indirect) was Int$418.
Additional strategies to reduce costs could include the following: using rapid HIV tests to assess the source serostatus, thus avoiding unnecessary PEP, tests, and anxiety for the exposed HCP, 38 and implementing tests for the early detection of bloodborne infections, which can shorten follow-up periods (eg, HIV-Ab/Ag test) and allow for early diagnosis at a lower cost (eg, HCV core-antigen testing replacing HCV-RNA).
Even if none of the retrieved studies included HCV-RNA testing in post-exposure protocols, it is recommended as alternative strategy in the United States and is strongly supported in a cost-effectiveness analysis. HCV-RNA testing at 1 month, at an average cost of Int$242 per exposure, leads to earlier detection of HCV transmission and lowers the risk of progression to chronic hepatitis.Reference Deuffic-Burban, Abiteboul, Lot, Branger, Bouvet and Yazdanpanah 39 Consequently, NSI management costs could also increase, and even more in the future, if direct-acting antivirals for hepatitis C are used in PEP, which would add a significant cost to treating these frequent events.
Are these costs truly representative of this issue? The costs of treating an occupational infection were not included in the calculation of average NSI costs, nor were those of litigation and compensation. Bloodborne infections other than HBV, HCV, or HIV were not considered, though many different pathogens can be, and have been, occasionally transmitted through an NSI. Most importantly, intangible costs arising from an NSI were not evaluated. The single willingness-to-pay analysis identified in the literature reported that the high median amount HCP were willing to pay to avoid a sharps-related injury were Int$828 in the base case and Int$1,237 when exposed to HIV or HCV. This finding suggests that the costs of intangible aspects of HCP injuries, such as anxiety and distress, could equal costs associated with the medical evaluation of these injuries.Reference Fisman, Mittleman, Sorock and Harris 40 However, in this study, the median time from injury to interview was 3 days. Thus, the interviewed subjects had not yet experienced the impact of being at risk of developing a bloodborne infection or the effects on personal and family life, sexual relationships, reproductive plans, breastfeeding, or professional expectations, which would increase the amounts reported above.
In conclusion, NSIs generate significant direct, indirect, potential, and intangible costs. While the costs for their prevention may seem high in the beginning, ultimately they prove to be the opposite. The economic expenditures directed toward enhancing interventions to prevent occupational exposures and infections, including provision of SEDs, will likely decrease over time, while enhancing HCP perception of their own value and affecting the quality of the care they provide. As highlighted in the preamble of the EU Directive, “Health and safety of workers is paramount and is closely linked to the health of patients.”
ACKNOWLEDGMENTS
Financial support: This work was supported by the Italian Ministry of Health (grant no. RF-2009-1530527: “Health technology assessment of needlestick-prevention devices to enhance safety of health care workers”) and Ricerca Corrente IRCCS. We are deeply grateful to Andrea Baker for her thoughtful editing of the manuscript.
Potential conflicts of interest: G.D.C., V.P., and V.D.B. have developed educational material for Becton Dickinson. All other authors report no conflicts of interest related to this article.









