The current infection control guidelines recommend isolation protocols when patients are colonized or infected with specific multidrug-resistant organisms (MDROs) (eg, methicillin-resistant Staphylococcus aureus [MRSA], or vancomycin-resistant enterococci [VRE]).1 These isolation protocols, known as contact precautions, include the use of nonsterile gloves during every interaction with colonized or infected patients.Reference Siegel, Rhinehart, Jackson and Chiarello2 Most healthcare facilities use contact precautions as vertical interventions to prevent the spread of specific MDROs from colonized patients to uncolonized patients.Reference Wenzel and Edmond3
The use of contact precautions as a vertical intervention requires the identification of colonization or infection with MDROs before contact precautions are implemented. These may not always be successfully identified. Even when MDROs are identified, a window of time exists between colonization, identification, and implementation of contact precautions that may allow transmission to occur.Reference Agata, Gautam, Green and Tang4 Universal gloving may overcome barriers to effective use of contact precautions and is a horizontal intervention that aims to reduce the transmission of all MDROs.
Universal gloving, as a horizontal intervention, applies the concept of contact precautions to every patient encounter regardless of known MDRO colonization or infection. When universal gloving is implemented on a unit, healthcare workers wear gloves during every patient care activity for every patient on that unit. However, the effectiveness of universal gloving to prevent transmission is unclear; the current literature on this topic is of varying quality and uses a variety of approaches. This meta-analysis aimed to determine whether associations exist between universal gloving and reduction in patient acquisition of HAIs and infection with MDROs in clinical settings.
Methods
Search strategy
This meta-analysis was conducted according to the MOOSE and PRISMA criteria.Reference Stroup, Berlin and Morton5, Reference Liberati, Altman and Tetzlaff6 A systematic search of Ovid MEDLINE, Cochrane Library (including Cochrane Database of Systematic Reviews and Cochrane Central Register of Controlled Trials), Web of Science, EMBASE, and ClinicalTrials.gov was initially conducted in September 2015. The search was updated periodically, with the most recent update conducted in July 9, 2018. The database search strategy was constructed and conducted by a specialized health sciences librarian trained in searching for systematic reviews. No filters for date, language, or any other parameter were used. The database search strategy is provided in Appendix A online. References from included studies were also reviewed for eligible studies.
Inclusion and exclusion
Studies were included if they met the following criteria: (1) healthcare-associated infection was the reported outcome (including but not limited to MRSA, VRE, C. difficile), (2) the intervention being studied included universal gloving during patient care for all patients on that unit regardless of colonization or infection, (3) the study was either a randomized controlled trial, a nonrandomized quasi-experimental study, or an observational study. Studies were excluded if the investigators did not distinguish between universal gloving in the intervention group versus the control group (eg, having universal gloving in both groups). Additionally, all studies concerning interventions implemented because of outbreaks were excluded; outbreak studies are subject to multiple biases including regression to the mean and selection bias.Reference Lin7–Reference Morton and Torgerson9
Data abstraction
Studies that met the inclusion criteria were reviewed, and their characteristics recorded by 2 of the 4 independent reviewers (N.N.C., A.E.K., M.A.W., and M.L.S.). The data abstraction was performed using a standardized form generated for this meta-analysis. Disagreements were resolved by discussion. We collected information on the study location, sample sizes, number of sites, the intervention being studied, the outcome of the study, and the raw data and rates reported by the study.
Assessment of study quality
The revised Downs and Black checklist was used to assess the quality of included studies.Reference Handler, Kennelly and Peacock10, Reference Downs and Black11 For this checklist, 1 point is recorded for each item in the following categories: reporting (12 items), external validity (13 items), and internal validity (7 items). The power of each study was recorded as 0, 1, or 2 points based on the reported sample size. The aggregate score determined the overall quality of the study. The aggregate score for each included study can be found in Table 1.
Table 1. Characteristics of Included Studies

Note. BAQS, before–after quasi-experimental study; RCT, randomized clinical trial; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococcus; CRKP, carbapenem-resistant Klebsiella pneumonia; CAUTI, catheter-associated urinary tract infection.
a Determined using the quality checklist for RCTs and observational studies from Handler et al,Reference Handler, Kennelly and Peacock10 originally by Downs and Black.Reference Downs and Black11
Statistical analysis
Most included studies reported their results using incidence rates and the total number of patient days observed. As such, the incidence rate ratio (IRR) and log standard error of the incidence rate ratio were calculated for each of the studies using the unadjusted data provided. The Woolf test of heterogeneity and the I2 test were performed to evaluate heterogeneity.Reference Woolf12 Random effects models were used to calculate the pooled IRR. Publication bias was evaluated by visual inspection of a funnel plot generated using the data from included studies. Stratified analyses were conducted based on study design, infection type, ward type, and whether universal gloving was implemented as a stand-alone intervention or as part of an intervention bundle. The variables chosen for the stratified analyses was selected a priori based on existing literature and clinical experience on how they could impact the outcomes of the included studies. Cochrane Review Manager (RevMan) version 5.3 was used for the analyses.13
Results
Based on the inclusion and exclusion criteria previously described, 8 of the 781 studies initially identified were included in the final analysis (Fig. 1). The characteristics of included studies can be found in Table 1. The average Downs and Black score of the included studies was 21. Of these 8 studies, 4 were before-and-after quasi-experimental studies and 4 were randomized controlled trials (RCTs), 2 of which were cluster randomized.Reference Kaufman, Blackman, Conaway and Sinkin14–Reference Yin, Schweizer, Herwaldt, Pottinger and Perencevich21 Reported outcomes differed among the included studies: 3 studies reported incidence of MRSA and VRE only, 2 reported incidence of MRSA, VRE, and an additional infection type (carbapenem-resistant Klebsiella pneumonia [CRKP]Reference Furuya, Cohen, Jia and Larson16 and CAUTIReference Mody, Krein and Saint20), and the remaining 3 studies reported the incidence of all HAIs. The duration of the included studies varied greatly depending on study design, ranging from 6 months to 9 years.

Fig. 1. Data search and abstraction flow diagram.
Furthermore, 4 studies implemented universal gloving as a stand-alone intervention, 2 studies evaluated universal gown use in addition to gloving, 1 study evaluated universal glove and gown use and modified universal contact precautions, and the remaining study evaluated universal gloving as a part of an intervention bundle. Also, 6 studies were conducted in ICU settings, 1 was conducted in nursing homes, and the remaining study was conducted in acute treatment and transplant wards. In addition, 3 studies implemented universal gloving in a neonatal or pediatric setting, and the remaining 5 studies implemented universal gloving in an adult setting. All studies used standard practice as the control group.
The results of the stratified analyses are listed in Table 2. We first stratified the included studies based on study design. When the results of the 4 before-and-after quasi-experimental studies were pooled, significant association between universal gloving and HAIs was detected (IRR, 0.77; 95% CI, 0.67–0.89), and these studies were homogeneous (P = 1.00; I2 = 0%). Although a similar association emerged when the 4 RCTs were pooled, it was no longer statistically significant (IRR, 0.78; 95% CI, 0.57–1.07) and there was significant heterogeneity among the RCTs (P = .007; I2 = 75%). We then stratified the studies by how universal gloving was implemented. The pooled results of the 4 studies that implemented universal gloving as a stand-alone intervention showed a significant association between universal gloving and reduced incidence of all HAI (IRR, 0.77; 95% CI, 0.67–0.89). The 4 studies that implemented universal gloving as part of an intervention bundle did not show a statistically significant effect on the incidence of HAI (IRR, 0.95; 95% CI, 0.86–1.05).
Table 2. Pooled Incidence Rate Ratios of Stratified Analyses

Note. IRR, incidence rate ratio; CI, confidence interval; BAQS, before-after quasi-experimental study; RCT, randomized clinical trials; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococcus; ICU, intensive care unit.
We also stratified the included studies by ward type. With the exception of the Mody study, the included studies were conducted either in adult ICUs or pediatric/neonatal ICUs and special care wards. In the adult ICUs, there was no significant association between universal gloving and any changes in the incidence of HAI (IRR, 1.01; 95% CI, 0.91–1.13). However, in pediatric/neonatal ICUs and special care wards, a significant reduction in incidence of HAI was associated with universal gloving (IRR, 0.75; 95% CI, 0.65–0.87). Lastly, we stratified the results by the type of infections reported. The pooled results did not show a statistically significant association between universal gloving and the incidence of MRSA (IRR, 0.94; 95% CI, 0.79–1.11), and there was no statistically significant association between universal gloving and the incidence of VRE (IRR, 0.94; 95% CI, 0.69–1.28). Of the 8 included studies, only the studies reported by Kaufman et alReference Kaufman, Blackman, Conaway and Sinkin14 and Yin et al.Reference Yin, Schweizer, Herwaldt, Pottinger and Perencevich21 provided results for BSI and CLABSI. The results from the Kaufman study indicated a nonsignificant association between universal gloving and decreased incidence of BSI (IRR, 0.70; 95% CI, 0.35–1.39) and CLABSI (IRR, 0.90; 95% CI, 0.22–3.58). The Yin study reported a statistically significant association between universal gloving and decreased incidence of BSI (IRR, 0.72; 95% CI, 0.60–0.87) and CLABSI (IRR, 0.68; 95% CI, 0.54–0.88).
When the 8 included studies were pooled, there was a significant association between universal gloving and the incidence of HAI (pooled IRR, 0.80; 95% CI, 0.67–0.96) (Fig. 2). However, there was moderate-to-substantial heterogeneity between studies (P = .02; I2 = 59%; 95% CI, 10%–81%). Visual inspection of the funnel plot showed a potential for publication bias: 6 of the 8 studies were clustered on 1 side of the pooled effect estimate (Fig. 3).
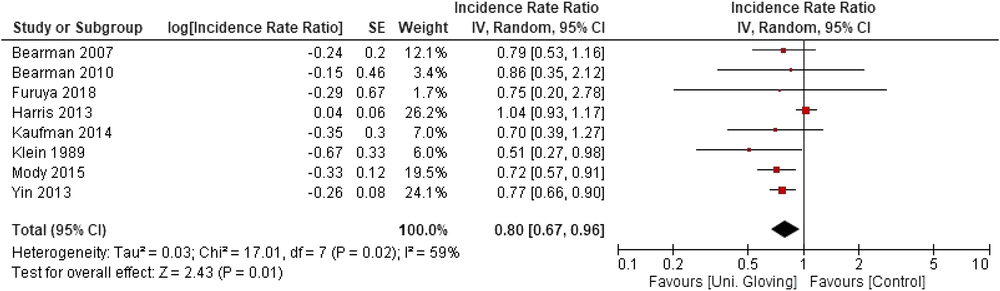
Fig. 2. Forest plot of all included studies for the association between universal gloving and incidence of all Healthcare-associated infections.
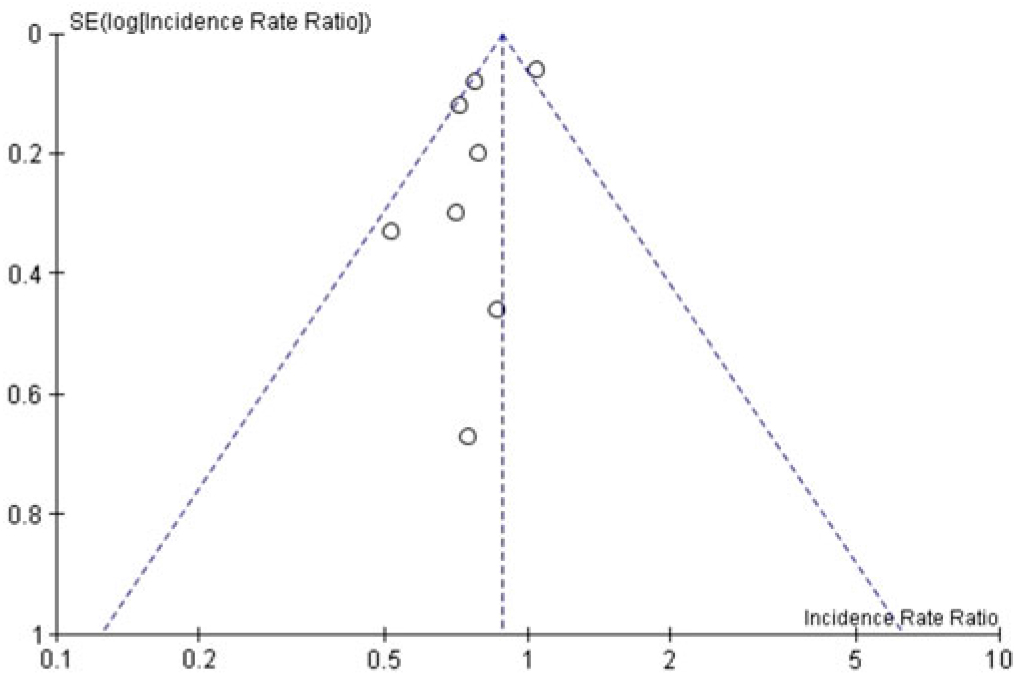
Fig. 3. Funnel plot of all included studies.
Discussion
In this meta-analysis, we aimed to assess the association between implementation of universal gloving and the incidence of HAI. When we pooled the results of the included studies, there was a statistically significant association between universal gloving and reduction of all HAIs. However, the heterogeneity and the low number of included studies suggests that while there may be possible benefits, it is not accurately represented by existing studies. These studies were heterogeneous by study design, patient population, and whether universal gloving was implemented alone or as part of a bundle. Due to these sources of heterogeneity, the stratified analyses likely provide more meaningful information than the overall pooled analysis of all 8 studies. Additionally, we could not rule out the possibility for publication bias, in which studies that confirm a hypothesis are more likely to be published than negative studies.
When the analysis was limited to studies with the before-after quasi-experimental design, a significant association was observed. Although we observed a similar effect when we limited the analysis to RCTs only, the results were no longer statistically significant. Stratification by infection type did not change the results. There was no significant association between universal gloving and decreased incidence of MRSA or VRE when they were analyzed separately. We also found that when universal gloving was implemented as a stand-alone intervention, it had a more significant effect in reducing incident HAI than when it was implemented as a part of intervention bundle. These results may be a statistical anomaly or they may be due to how universal gloving was implemented and evaluated. When universal gloving was implemented, healthcare workers wore gloves while caring for all patients instead of only when indicated by the presence of MRSA or VRE. Because the outcome of the study is also the incidence of those pathogens, it may be difficult to identify any effects, given that the behavior of healthcare workers when treating those pathogens would be the same in the exposure and the control group. In addition, many of the included studies evaluated the bundled interventions as vertical interventions, reporting incidence rates of specific pathogens. As a horizontal intervention, universal gloving should have decreased transmission of multiple pathogens including both antibiotic-susceptible and antibiotic-resistant pathogens, as well as gram-positive and gram-negative pathogens. Of the included studies, only those focused on universal gloving alone included incidence rates of infections caused by pathogens other than MRSA and VRE. Therefore, future studies should evaluate overall transmission rather than limiting the assessment to just MRSA and VRE.
Additionally, some of the studies did not randomize patients or units to the intervention or statistically adjust for important patient characteristics nor did they report certain patient characteristics. Our results showed a significant difference between the adult ICUs and pediatric/neonatal ICUs, which may have been due to increased vulnerability of the patients being treated in pediatric/neonatal ICUs. Pediatric and neonatal care settings may have different care patterns than adult settings, as well as increased awareness from the healthcare workers to reduce HAIs.Reference Balkhy and Zingg22 Although we were able to identify literature on universal glove use in nursing homes, only 1 study met our inclusion criteria. As the setting and care patterns differs greatly between acute-care and long-term care facilities, we do not believe the effects observed by the nursing home study, while significant, are applicable to acute-care settings.
Failure to adjust for important characteristics could significantly modify the results of these studies because these characteristics could have a significant impact on the ability of pathogens to be transmitted. In some of the included studies, the types of ward are specified for certain conditions, such as pediatric special care wards, where the patient assigned to those wards are likely have similar characteristic, despite the primary diagnoses. However, in wards such as ICUs, where the condition of the patients may vary greatly, it is difficult to identify possible patient-level risk factors. Furthermore, these patient characteristics may result in different levels of hand hygiene practices. Additionally, the duration of each study varied. It is possible that the varying study duration may have contributed significantly to the observed heterogeneity of the included studies. Studies conducted only for short periods of time, on a significantly smaller sample may not capture the true, representative state of infection incidence at the facility, both for the intervention and control groups.
This meta-analysis has several limitations. First, only eight published studies met our inclusion criteria, and they were heterogeneous, which may have affected our ability to interpret the results. Thus, our overall pooled results should be interpreted with caution and more studies should be performed. Second, meta-analyses are limited by the limitations of the studies included in the meta-analysis, which often lack description of important factors beyond the intervention, referring to existing practices as ‘standard care.’ The included studies were of moderate quality; most of the studies did not report power calculations. All but 2 of the studies were single-center studies, and only half of the included studies were randomized. The sample sizes of the studies greatly varied. The results of our meta-analysis may have been driven by the 4 largest studies, which contributed 81.9% of the weight in the analysis. Third, only 3 of the studies reported hand hygiene compliance and gloving practices of the healthcare staff, which may have affected the results of the individual studies as well as the overall analysis. Lastly, the majority of the included studies were conducted within the United States; therefore, the applicability of the results to facilities outside United States and resource-limited facilities may be limited.
Based on the results of this analysis, universal gloving was associated with reduced incidence of HAIs. However, the results were not statistically significant when only RCTs were pooled. More multicenter, high-quality studies of universal gloving using broad horizontal outcomes (eg, including gram-negative pathogens and other HAIs) and different settings (eg, general wards and long-term care centers) should be conducted to definitively answer this question. However, despite limited data, universal gloving could be considered in high-risk settings such as pediatric ICUs.
Author ORCIDs
Marin L. Schweizer, 0000-0002-3604-0093
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2019.123
Acknowledgments
Financial support
This work was supported by a Department of Veterans Affairs, Health Services Research and Development (HSR&D) VA Career Development Award (no. CDA 11-215; principle investigator, Schweizer) and a Centers for Disease Control and Prevention (CDC) Prevention Epicenters Program (grant no. 1U54CK000448-01; principle investigator, Perencevich).
Conflicts of interest
All authors report no conflicts of interest relevant to this article.