Bipolar disorder affects approximately 1% of the population, Reference Kleinman, Lowin, Flood, Gandhi, Edgell and Revicki1 and is a chronic disorder characterised by manic and depressive episodes. Reference Belmaker2 According to DSM-IV, the diagnosis of bipolar disorder requires a history of at least one manic or hypomanic episode which causes clinically significant distress and impairment in social and occupational functioning, and is neither caused by psychotic disorders such as schizophrenia nor the result of substance abuse, medication or a medical condition (e.g. hyperthyroidism). 3 Furthermore, most patients with bipolar disorder also suffer from recurrent depressive episodes. The nature of the disease, the frequent relapses and the chronic course of the disease has considerable consequences for patients, including impaired social functioning, decreased ability to work, poor quality of life, high risk of somatic and psychiatric comorbidities, and increased use of in-patient and out-patient medical services. Reference Kleinman, Lowin, Flood, Gandhi, Edgell and Revicki1
Although inflammation has previously been associated with depression and schizophrenia, Reference Wium-Andersen, Ørsted, Nielsen and Nordestgaard4 fewer studies have examined the role of inflammation in bipolar disorder. Reference Goldstein, Kemp, Soczynska and McIntyre5 A recent national Danish study of 3.56 million individuals including 13 034 individuals with bipolar disorder reported that prior admission to hospital for autoimmune diseases or severe infections, i.e. diseases resulting in persistent or acutely increased inflammation, was associated with a 25–61% increased risk of bipolar disorder. Reference Benros, Waltoft, Nordentoft, Østergaard, Eaton and Krogh6 Furthermore, an increasing number of studies including two meta-analyses with 18 and 30 studies respectively have reported elevated levels of pro-inflammatory markers (cytokines and acute-phase proteins) in individuals with bipolar disorder. Reference Modabbernia, Taslimi, Brietzke and Ashrafi7,Reference Munkholm, Brauner, Kessing and Vinberg8
C-reactive protein (CRP) is an acute-phase protein and a reliable marker of inflammation. In apparently healthy individuals, plasma levels of CRP are usually below 3 mg/L but may be up to 10 mg/L. Reference Pepys and Hirschfield9 During acute inflammation, levels of CRP may increase up to 300-fold. Reference Gabay and Kushner10 Previous studies have primarily compared plasma CRP levels in patients with and without bipolar disorder, Reference Cunha, Andreazza, Gomes, Frey, da Silveira and Gonçalves11–Reference Wadee, Kuschke, Wood, Berk, Ichim and Maes20 and many have reported elevated CRP levels in individuals with bipolar disorder compared with healthy controls. Reference Cunha, Andreazza, Gomes, Frey, da Silveira and Gonçalves11–Reference Dickerson, Stallings, Origoni, Boronow and Yolken13,Reference Huang and Lin16,Reference Tsai, Chung, Wu, Kuo, Lee and Huang18 However, most of these studies did not adjust analyses for important confounders such as lifestyle factors (e.g. smoking and body mass index (BMI), socioeconomic status and chronic disease). One of the few studies adjusting for such confounders included 420 participants and showed no significant CRP elevation in patients with bipolar disorder after adjustment for age, gender, race, education, smoking and BMI. Reference Dickerson, Stallings, Origoni, Vaughan, Khushalani and Yang14 Furthermore, all previous studies have used a cross-sectional design which may have been influenced by reverse causation, that is, bipolar disorder may be the cause of elevated CRP rather than vice versa. Thus, no studies to date have examined the prospective association between elevated CRP levels and late-onset bipolar disorder.
In this study, we tested the hypothesis that elevated plasma levels of CRP are associated cross-sectionally and prospectively with late-onset bipolar disorder in the general population. To examine this we measured CRP in 78 809 participants from two large independent general population studies, the Copenhagen General Population Study and the Copenhagen City Heart Study, and followed participants for up to 20 years. To ascertain late-onset bipolar disorder we used data on admission to hospital with bipolar disorder from the national Danish Patient Registry and death with bipolar disorder from the national Danish Causes of Death Registry. We also tested the hypothesis that an association between elevated CRP and late-onset bipolar disorder possibly is causal, by using four single nucleotide polymorphisms (SNPs) in the CRP gene. We chose four SNPs that capture most of the variation in the CRP gene, including the most important 3-allelic promoter SNP (rs3091244). Reference Zacho, Tybjaerg-Hansen, Jensen, Grande, Sillesen and Nordestgaard21 All four SNPs cause lifelong elevated but fully functional CRP levels which have been replicated in different independent samples, Reference Hage and Szalai22–Reference Naitza, Porcu, Steri, Taub, Mulas and Xiao24 and together they explain approximately 2% of the variation in plasma CRP levels. Reference Zacho, Tybjaerg-Hansen, Jensen, Grande, Sillesen and Nordestgaard21 We chose not to include SNPs outside the CRP gene that influence CRP levels, as such SNPs typically will have pleotropic effects, making interpretation of data more difficult than when using SNPs solely influencing CRP levels, Reference Zacho, Tybjaerg-Hansen, Jensen, Grande, Sillesen and Nordestgaard21 as done in the present study.
Method
Participants
We included 78 809 men and women from two independent prospective cohort studies of the Danish general population, the Copenhagen General Population Study (n = 68 779) 2003–2011 and the Copenhagen City Heart Study (n = 10 030) 1991–94 and 2001–2003; no individual appeared in both studies (if a participant appeared in more than one study, only data from the first examination was included) and the two studies were combined to achieve maximal statistical power. Participants from both studies were 20–100 years old and were randomly selected from the national Danish Civil Registration System to represent the Danish general population. All participants were White and of Danish descent (the participant and both parents were born in Denmark and were Danish citizens). Participants without data on CRP (n = 1626) were excluded, leaving 78 809 for analysis. Reference Wium-Andersen, Ørsted and Nordestgaard25 Participants filled in a questionnaire, which was reviewed together with an investigator on the day of attendance. Furthermore, all participants had a physical examination performed and had blood samples drawn for CRP measurement and DNA extraction. Because all individuals in Denmark have a unique identification number, we used the national Danish Civil Registration System to register emigration or death for all participants. After description of the study to the participants, written informed consent was obtained. This study was approved by Herlev Hospital and Danish ethical committees (KF-100.2039/91 and H-KF-01-144/01).
Biochemical analyses
Plasma levels of CRP were measured at the Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital. Reference Wium-Andersen, Ørsted, Nielsen and Nordestgaard4 CRP was measured with a high-sensitivity assay using latex-enhanced turbidimetry (Dako, Glostrup, Denmark) or nephelometry (Dade Behring, Deerfield, Illinois, USA), masked to bipolar disorder status; results were similar for the two assays and therefore all statistical analyses were combined, but with adjustment for assay type and study. Furthermore, we genotyped four polymorphisms in the CRP gene: rs1130864, rs1205, rs3091244 and rs3093077 using TaqMan, ABI Prism 7900HT Sequence Detection System (Applied BiosystemsInc, Foster City, California, USA). Each genotyping run included a known non-carrier, a heterozygous and a homozygous control. Genotyping was verified by Sanger DNA sequencing in >30 participants with each genotype. The call-rate was >99.8% after two rounds of reruns. All genotypes were in Hardy–Weinberg equilibrium. Minor allele frequencies, genomic location and linkage disequilibrium between the four genetic variants are shown in Fig. DS1 in an online supplement to this paper.
Bipolar disorder, major depression, schizophrenia and anxiety
Diagnoses of bipolar disorder and dates of diagnoses were obtained from the national Danish Patient Registry and the national Danish Causes of Death Registry. The national Danish Patient Registry has information on all hospital discharge diagnoses of psychiatric diseases from psychiatric and somatic hospitals since January 1977, including out-patient and emergency wards since 1995. The national Danish Causes of Death Registry has information on causes of death on all individuals in Denmark since 1970, including diagnoses at time of death. Bipolar disorder was classified according to ICD-8 codes 296.19 and 296.39 until 1994 and ICD-10 codes F30 and F31 from 1994 through 2011. Major depression was ICD-8 codes 296.0, 296.2, 298.0 and 300.4, and ICD-10 codes F32 and F33. Schizophrenia was ICD-8 codes 295.0-9, and ICD-10 codes F20.0-9. Anxiety was ICD-8 codes 300.0-9, and ICD-10 codes F40 and F41.
Covariates
Participants reported on alcohol consumption (drinks/week; 1 drink ∼12 g), smoking status (never; former; current), physical activity (0–2 h light activity/week; 2–4 h light activity/week; >4 h light activity or 2–4 h vigorous activity/week; >4 h vigorous activity/week), level of education after primary and lower secondary school (no education; shorter education less than 3 years; basic vocational training 1–3 years; higher education ⩾3 years; university education) and level of income (lowest; middle; highest). Body mass index was measured weight in kilograms divided by measured height in meters squared. Chronic disease was ascertained by collecting information on diagnosis and date of diagnosis from the national Danish Patient Registry, the national Danish Cancer Registry, and the national Danish Causes of Death Registry on ischemic heart disease, myocardial infarction, stroke, diabetes, hypertension, cancer, pneumonia, chronic obstructive pulmonary disease, asthma, deep venous thrombosis and pulmonary embolism.
Statistical analyses
Stata version 12.1 on Windows was used for all statistical analyses.
First, we examined differences in plasma levels of CRP between all participants with and without a diagnosis of late-onset bipolar disorder, that is, diagnoses before or after study entry combined for maximal statistical power. To reduce the influence of covariates on plasma CRP levels we examined logarithmically transformed CRP levels in three ways: (a) unadjusted; (b) adjusted for age and gender; and (c) adjusted multifactorially for age, gender, alcohol consumption, smoking status, physical activity, level of education, level of income, BMI and chronic disease. Differences between CRP levels were determined using multiple regression.
Second, we tested the association between elevated CRP and admission to hospital/death with late-onset bipolar disorder cross-sectionally using clinical categories of CRP as well as CRP quintiles. We used multifactorially adjusted logistic regression models to calculate odds ratios (ORs) with 95% confidence intervals (CIs), and used three different models of adjustment: (a) unadjusted; (b) age- and gender-adjusted; and (c) multi-factorially adjusted for age, gender, alcohol consumption, smoking status, physical activity, level of education, level of income, BMI, and chronic disease.
Third, we tested the association between elevated CRP and admission to hospital/death with late-onset bipolar disorder prospectively using a Cox proportional hazards regression model with age as the underlying time scale (which means that age is automatically adjusted for), and left truncation (= delayed entry) in 1991–1994, 2001–2003, or 2003–2011 as appropriate, to calculate hazard ratios (HRs) with 95% CIs. Participants with previous or current bipolar disorder at baseline (n = 38) were excluded. Follow-up began at blood sampling and participants were censored at admission to hospital/death with bipolar disorder (n = 55), death (n = 6484), emigration (n = 379), or end of follow-up June 2011, whichever came first. Models were adjusted similarly to the logistic regression models. For the unadjusted model we calculated HRs with follow-up time as underlying time scale. We tested the proportional hazards assumption graphically by plotting -log[-log (survival)] v. log(age) and by using Schoenfeld residuals; no violations were detected. We further used logarithmically transformed CRP values to calculate HRs for a doubling of CRP stratified on age, gender, and birth year. Each stratum was adjusted for all other variables than the one stratified on. Interaction between the different variables and CRP levels was tested for by introducing a two-factor interaction term in the model, and subsequently comparing the two models using a likelihood ratio test.
Because 4317 individuals had CRP measured in both the 1991–94 and in 2001–2003 examinations of the Copenhagen City Heart Study, we were able to calculate a regression dilution ratio of 0.82 for the clinical CRP categories and a ratio of 0.85 for quintiles using the non-parametic method Reference Clarke, Shipley, Lewington, Youngman, Collins and Marmot26 (Table DS1, online data supplement), and to correct ORs, HRs and CIs for regression dilution bias to avoid underestimation of the association; importantly however, significance levels are not influenced by this correction. It is sufficient to calculate a regression dilution ratio in a subsample and then apply this ratio to the entire study population. Reference Clarke, Shipley, Lewington, Youngman, Collins and Marmot26
Fourth, we used Fine-Grey curves to plot cumulative incidence of late-onset bipolar disorder as a function of age; this analysis allows for competing risk of death and emigration. For this analysis, we divided participants into clinical categories of CRP but combined the two groups with a CRP above 3 mg/L to maximise statistical power. We used log-rank tests to examine differences between groups.
Finally, we used a Mendelian randomisation design to test for a possible causal relationship between elevated CRP and late-onset bipolar disorder, using logarithmically transformed CRP values because CRP was not normally distributed. Reference Lawlor, Harbord, Sterne, Timpson and Davey27 First, we tested whether each of the four CRP SNPs were associated with plasma levels of CRP. For this analysis we generated all possible genotype combinations and ranked the nine most common combinations based on their CRP levels. Second, we used logarithmically transformed CRP values to examine the association between a doubling of CRP and late-onset bipolar disorder using a logistic regression model in three ways: (a) unadjusted; (b) for age and gender; and (c) multifactorially for age, gender, alcohol consumption, smoking status, physical activity, level of education, level of income, BMI and chronic disease. Third, we performed an instrumental variable (IV) analysis with a two-stage regression model using each SNP and the genotype combination as instruments to estimate the causal effect of a doubling in CRP on risk of late-onset bipolar disorder. Reference Palmer, Sterne, Harbord, Lawlor, Sheehan and Meng28 The first stage was a linear regression additive model of each of the CRP SNPs on CRP levels. F-statistics were used to evaluate the strength of the instruments, with F>10 indicate sufficient statistical strength to carry out valid IV analyses. The second stage was a logistic regression of genetically predicted values of CRP (generated in the first stage) on late-onset bipolar disorder to calculate causal ORs. Reference Palmer, Sterne, Harbord, Lawlor, Sheehan and Meng28 Finally, the unadjusted observational and causal models were compared using a Wald test.
We had 98% complete data on alcohol consumption, smoking status, physical activity, education, income and BMI. All missing values were imputed based on age and gender before multifactorial adjustment.
In sensitivity analyses, we repeated analyses excluding participants with a co-diagnosis of schizophrenia or anxiety. Also, we compared ORs for the association between a doubling in CRP levels and admission to hospital/death with major depression, schizophrenia, and anxiety with the results for admission to hospital/death with late-onset bipolar disorder.
Results
Baseline characteristics of the 78 809 participants based on clinical categories of CRP are listed in Table 1, and by end-point and genotype in online Tables DS2 and DS3. Collectively, these data illustrate that CRP levels and late-onset bipolar disorder are associated with several covariates and thus potential confounders, while the CRP genotype combination is not associated with any, illustrating that the genotype combination can be used as an unconfounded instrument to study a possible causal relationship between genetically elevated CRP levels and risk of late-onset bipolar disorder (online Fig. DS2).
Table 1 Baseline characteristics of 78 809 individuals from the general population by C-reactive protein levels

| C-reactive protein |
P-value bipolar disorder** |
P-trend genotype combination*** |
|||||
|---|---|---|---|---|---|---|---|
| ⩽1.00 | 1.01–3.00 | 3.01–10.00 | >10.00 | P-trend | |||
| n (%) | 14 832 (19) | 48 230 (61) | 13 008 (17) | 2739 (3) | 78 809 | 78 809 | 76 479 a |
| Age, years: median (interquartile range) | 54 (45–64) | 57 (47–66) | 61 (51–70) | 63 (51–72) | <1*10−300 | 4*10−4 | 0.81 |
| Women, n (%) | 7952 (54) | 266 672 (55) | 7587 (58) | 1554 (57) | 3*10−13 | 0.48 | 0.60 |
| Alcohol consumption,
drinks/ week: median (interquartile range) |
8 (3–15) | 8 (3–15) | 7 (2–15) | 7 (2–14) | 4*10−26 | 0.09 | 0.29 |
| Never smokers, n (%) | 6124 (41) | 18 112 (38) | 3915 (30) | 801 (29) | 5*10−94 | 0.03NS | 0.81 |
| High leisure time
physical activity, more than 2–4 h light/day, n (%) |
8075 (54) | 23 646 (49) | 4722 (36) | 899 (33) | 7*10−246 | 0.01NS | 0.02NS |
| Less than 3 years of education,* n (%) | 7897 (54) | 27 528 (58) | 9252 (72) | 1965 (73) | 6*10−241 | 0.09 | 0.10 |
| Low income, n (%) | 2047 (14) | 7665 (16) | 3531 (27) | 894 (33) | 8*10−264 | 7*10−7 | 0.51 |
| Body mass index,
kg/m2: median (interquartile range) |
24 (22–26) | 26 (23–28) | 28 (25–31) | 27 (24–31) | <1*10−300 | 0.84 | 0.02NS |
| Chronic disease, n (%) | 4171 (28) | 16389 (34) | 6015 (46) | 1493 (55) | <1*10−300 | 4*10−10 | 0.22 |
Baseline characteristics for participants in the Copenhagen General Population Study and the Copenhagen City Heart Study combined.
a. Individuals with rare genotype combinations were excluded.
NS, non-significant when corrected for 9 multiple comparisons (required P-value for significance 0.05/9 = 0.006).
* Education after primary and secondary lower school.
** From Table DS2.
*** From Table DS3.
In total, 93 participants had a diagnosis of bipolar disorder (55 after study entry; of these one was registered only in the national Causes of Death Registry). Diagnoses of bipolar disorder are shown in online Table DS4. The median follow-up to a diagnosis of bipolar disorder was 5.9 years (interquartile range: 4.4–7.6). The mean age at a diagnosis of bipolar disorder was 67 years (range: 43–87) for participants with a diagnosis after study entry and 54 years (range: 29–70) for participants with a diagnosis before study entry.
Plasma CRP levels in participants with and without late-onset bipolar disorder
Mean CRP levels were 55% higher for participants with late-onset bipolar disorder compared with those without (P = 2*10−7; Fig. 1). Furthermore, plasma CRP levels remained 45% (P = 3*10−6) and 29% (P = 0.001) higher for participants with late-onset bipolar disorder compared with those without, when we adjusted for age and gender, and multifactorially for age, gender, alcohol consumption, smoking status, physical activity, level of education, level of income, BMI and chronic disease respectively.

Fig. 1 Plasma C-reactive protein levels in participants with and without bipolar disorder before or after CRP measurement.
Based on 78 809 participants from the Copenhagen General Population Study and Copenhagen City Heart Study combined. Multifactorially adjusted was for age, gender, alcohol consumption, smoking status, physical activity, level of education, level of income, body mass index and chronic disease. CRP, C-reactive protein. Plasma CRP is shown as mean+standard error.
Cross-sectional analyses
In cross-sectional analyses, multifactorially adjusted ORs were 1.40 (95% CI 0.61–3.26) for participants with a plasma CRP level of 1.01–3.00 mg/L, 2.44 (0.97–6.16) for participants with a plasma CRP level of 3.01–10.00 mg/L and 3.48 (1.11–10.9) for participants with a plasma CRP level >10.00 mg/L compared to participants with a plasma CRP level >1.00 mg/L (online Fig. DS3, top). These risk estimates were higher in unadjusted models and when only adjusted for age and gender. When participants were divided into quintiles by plasma CRP levels, ORs were 1.21 (95% CI = 0.50–2.91) for participants in the second quintile, 0.76 (95% CI = 0.29–1.97) for participants in the third quintile, 1.13 (95% CI = 0.48–2.67) for participants in the fourth quintile and 1.99 (95% CI = 0.90–4.40) for participants in the fifth quintile compared with participants in the first quintile (Fig. DS3, top). These risk estimates were higher in unadjusted models and when only adjusted for age and gender.
Prospective analyses
In prospective analyses, multifactorially adjusted HRs were 3.03 (95% CI = 0.84–11.0) for participants with a plasma CRP level of 1.01–3.00 mg/L, 6.09 (1.53–24.1) for participants with a plasma CRP level of 3.01–10.00 mg/L and 4.10 (0.63–26.6) for participants with a plasma CRP level >10.00 mg/L compared with participants with a plasma CRP level >1.00 mg/L (Fig. DS3, bottom). These risk estimates were slightly decreased in unadjusted models and when only adjusted for age and gender. When participants were divided into quintiles by plasma CRP, HRs were 1.29 (95% CI = 0.35–4.66) for participants in the second quintile, 1.60 (95% CI = 0.53–6.18) for participants in the third quintile, 2.49 (95% CI = 0.77–8.06) for participants in the fourth quintile and 3.65 (95% CI = 1.18–11.3) for participants in the fifth quintile compared with participants in the first quintile (Fig. DS3, bottom). These risk estimates were slightly decreased in unadjusted models and when only adjusted for age and gender.
When CRP levels were on a continuous scale, a doubling in plasma CRP was associated with a HR of 1.38 (95% CI = 1.10–1.74) after multifactorial adjustment (Fig. 2). There were no statistically significant interactions between a doubling in CRP and age, gender, or birth year, even though the association seemed to be more pronounced in women compared with men.
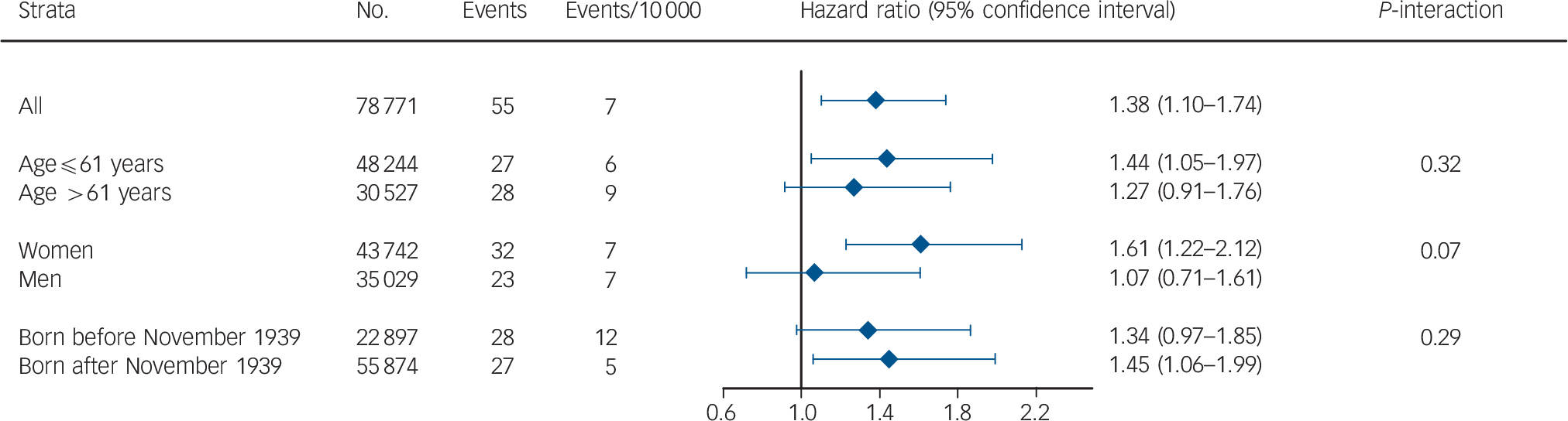
Fig. 2 Prospective risk of bipolar disorder stratified for age, gender, and birth year when C-reactive protein levels double.
Based on 78 771 participants from the Copenhagen General Population Study and the Copenhagen City Heart Study combined. All hazard ratios were adjusted for age, gender, alcohol consumption, smoking status, physical activity, level of education, level of income, body mass index and chronic disease.
Cumulative incidence
The cumulative incidence of late-onset bipolar disorder was increased in participants with increasing plasma CRP levels compared with participants with a plasma CRP >1.00 mg/L (log-rank, P-trend = 0.02; Fig. 3).

Fig. 3 Cumulative incidence of bipolar disorder as a function of age by clinical categories of C-reactive protein (CRP).
Participants with a CRP of 3–10 mg/L and those with a CRP above 10 mg/L were combined to maximise power. Based on 78 809 participants from the Copenhagen General Population Study and the Copenhagen City Heart Study combined, followed for up to 20 years. Participants with previous or current bipolar disorder at baseline (n = 38) were excluded.
Mendelian randomisation
Observational estimates (based on measured plasma CRP levels)
When CRP levels were on a continuous scale, a doubling in plasma CRP was associated with an unadjusted OR of 1.46 (1.25–1.71), an OR of 1.41 (1.20–1.65) after age and gender adjustment and an OR of 1.28 (1.08–1.52) after multifactorial adjustment (Fig. 4). The difference from 1.28 in this figure to 1.38 in Fig. 2 is because the two numbers come from cross-sectional analysis using logistic regression and prospective analysis using Cox regression respectively.
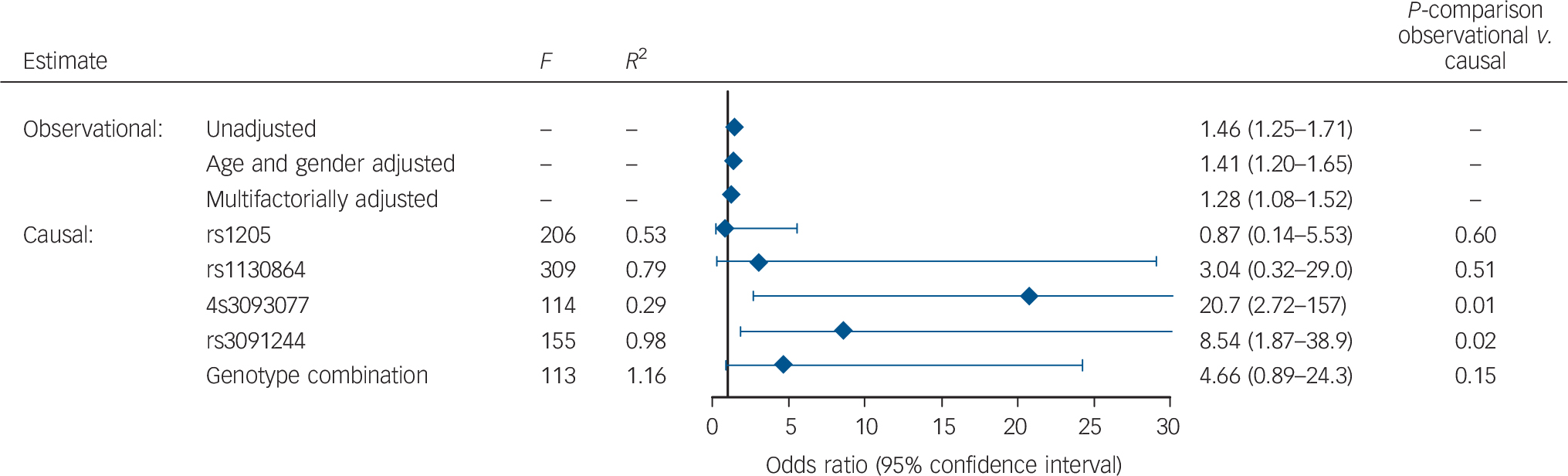
Fig. 4 Observational and causal risk of bipolar disorder when C-reactive protein doubles.
Based on 78 809 participants from the Copenhagen General Population Study and the Copenhagen City Heart Study combined. Multifactorially adjusted was for age, gender, alcohol consumption, smoking status, physical activity, level of education, level of income, body mass index, and chronic disease. P-comparison was calculated by a Wald test and tested the difference between the unadjusted observational model and each of the causal models. F-values are from F-statistics with F>10 indicating sufficient statistical strength to carry out valid instrumental variable analyses. R 2 is from the linear regression of each of the single nucleotide polymorphisms or the genotype combination on C-reactive protein levels.
Causal estimates (based on genetically determined plasma CRP levels)
A doubling in genetically determined CRP was associated with a causal OR of 0.87 (0.14–5.53) estimated from the rs1205 SNP (observational v. causal model: P = 0.60), a causal OR of 3.04 (0.32–29.0) estimated from the rs1130864 SNP (P = 0.51), a causal OR of 20.7 (2.72–157) estimated from the rs309377 SNP (P = 0.01), a causal OR of 8.54 (1.87–38.9) estimated from the rs3091244 SNP (P = 0.02) and finally a causal OR of 4.66 (0.89–24.3) estimated from the combined genotype combinations (P = 0.15; Fig. 4).
Sensitivity analyses
Of the 93 participants with late-onset bipolar disorder, 3 also had a diagnosis of schizophrenia, and 10 also had a diagnosis of anxiety. In total, 60 participants with late-onset bipolar disorder did not have a diagnosis of depression, schizophrenia or anxiety. When participants with major depression, schizophrenia or anxiety were excluded, results were similar (data not shown).
When we compared ORs for the association between CRP and admission to hospital/death with major depression, schizophrenia and anxiety with the results for admission to hospital/death with late-onset bipolar disorder, a doubling in plasma CRP was associated with a multifactorially adjusted OR of 1.28 (95% CI = 1.08–1.52) for late-onset bipolar disorder, of 1.11 (1.05–1.17) for major depression, of 1.31 (1.08–1.58) for schizophrenia, and of 1.17 (1.06–1.28) for anxiety (Fig. 5). All ORs were slightly higher when adjusting only for age and gender.

Fig. 5 Cross-sectional risk of bipolar disorder, major depression, schizophrenia, and anxiety when C-reactive protein levels double.
Based on 78 809 participants from the Copenhagen General Population Study and the Copenhagen City Heart Study combined. Multifactorially adjusted was for age, gender, alcohol consumption, smoking status, physical activity, level of education, level of income, body mass index and chronic disease.
Discussion
The main finding of this study is that elevated plasma levels of CRP were associated cross-sectionally and prospectively with late-onset bipolar disorder in 78 809 men and women from the general population. These findings remained significant after multifactorial adjustment for lifestyle factors, socioeconomic status and chronic disease. Consistent with results from previous case–control studies, Reference Cunha, Andreazza, Gomes, Frey, da Silveira and Gonçalves11–Reference Dickerson, Stallings, Origoni, Boronow and Yolken13,Reference Huang and Lin16,Reference Tsai, Chung, Wu, Kuo, Lee and Huang18 we also found higher plasma CRP levels in participants with a diagnosis of late-onset bipolar disorder compared with participants without a diagnosis of late-onset bipolar disorder, and most studies without a significant association have still found non-significantly elevated CRP levels among patients with bipolar disorder compared with healthy controls. Reference Dickerson, Stallings, Origoni, Vaughan, Khushalani and Yang14,Reference Hope, Dieset, Agartz, Steen, Ueland and Melle15,Reference Hung, Hsieh, Chen, Pei, Kuo and Shen17,Reference Wadee, Kuschke, Wood, Berk, Ichim and Maes20 The lack of a significant association could be due to low power. Finally, based on our genetic analyses we cannot exclude that elevated CRP levels per se may be causally associated with late-onset bipolar disorder as two of four genetic variants support a causal association; however, this latter question needs to be examined in independent studies.
Examining the association between elevated CRP (and also elevated levels of most other inflammatory markers) and bipolar disorder, most studies to date have used a cross-sectional case–control design, and previously no prospective studies have examined the association between elevated CRP and bipolar disorder. The lack of longitudinal studies means that the nature and direction of this association is currently unclear. Chronic low-grade inflammation caused by for example obesity or smoking might cause bipolar disorder, but bipolar disorder might also cause low-grade inflammation, e.g. through weight gain Reference Goldstein, Kemp, Soczynska and McIntyre5 as patients with bipolar disorder have increased risk of overweight or obesity. Reference McElroy and Keck29 Also, other studies suggest that elevated CRP is associated with severity of symptoms in bipolar disorder. Reference Dickerson, Stallings, Origoni, Boronow and Yolken13,Reference Lee, Chen, Chang, Chen, Huang and Tzeng30 Our prospective study suggests that low-grade inflammation (indicated by elevated CRP) increases risk of late-onset bipolar disorder, but the association could be bi-directional or explained by unmeasured confounders such as early life stress which has been associated with both a higher mood disorder prevalence and increased levels of inflammatory markers such as CRP. Danese et al studied a birth cohort of almost 1000 children and found that individuals experiencing childhood maltreatment in the first decade had increased CRP levels at age 32 and estimated that more than 10% of cases of low-grade inflammation in the population, as indexed by high levels of CRP, may be attributable to childhood maltreatment. Reference Danese, Pariante, Caspi, Taylor and Poulton31 Not only might this explain our results, but also offers a potential model linking the findings of increased inflammation to development of mood disorders.
The biological mechanism linking inflammation, CRP and bipolar disorder is not fully understood. Contrary to smaller molecules, CPR does not cross the blood–brain barrier and is thus prevented from affecting the brain directly. However, some studies indicate that CRP may increase the permeability of the blood–brain barrier to molecules such as proinflammatory cytokines Reference Hsuchou, Kastin, Mishra and Pan32 and hereby CRP levels could possibly be an indicator of the inflammatory status in the brain. Increased levels of proinflammatory cytokines in the brain may promote multiple abnormalities including activation of the enzyme indolamine 2,3-dioxygenase leading to decreased production of serotonin and increased production of kynurenine and quinolinic acid. Reference Miller, Maletic and Raison33 This may in turn impact glutamate signalling, including the stimulation of extra synaptic N-methyl-d-aspartate (NMDA) receptors, which leads to the decreased production of brain-derived neurotrophic factor (BDNF), a potent inducer of neurogenesis, which is thought to contribute to the pathogenesis of bipolar disorder. Reference Miller, Haroon, Raison and Felger34–Reference Raison and Miller36 Furthermore, proinflammatory cytokines can affect neurotransmitter signalling in the brain by increasing the number and function of the reuptake pumps for serotonin, norepinephrine, and dopamine, reducing levels of these neurotransmitters within the synaptic cleft. Reference Raison and Miller36 Finally, treatment with the cytokine interferon-α has been shown to increase cortisol and glucocorticoid resistance, which correlated with increases in depressive symptoms. Reference Raison, Borisov, Woolwine, Massung, Vogt and Miller37
Strengths of our study include the large number of participants from the general population with up to 20 years of follow-up. Due to the completeness of the national Danish Patient Registry, the national Danish Causes of Death Registry and the national Danish Civil Registration System, we had 100% complete data on admission to hospital with bipolar disorder, on emigrations, and on deaths. Thus, we had no losses to follow-up. Finally, to our knowledge the present study is the first to examine the association between elevated CRP levels and late-onset bipolar disorder prospectively, and to examine a possible causal association between elevated CRP levels and late-onset bipolar disorder using CRP genotypes.
A potential limitation is that our only way to identify participants with bipolar disorder was to use hospital or death certificate diagnoses of bipolar disorder. When we combined those with a diagnosis of bipolar disorder from the national Danish Patient Registry and the national Danish Causes of Death Registry with data from the national Danish Medicinal Product Statistics, we found that 57% of those with a diagnosis of bipolar disorder also had a previous prescription with lithium – a medication often used for treating bipolar disorder in Denmark. One study of the diagnostic validity and stability of the diagnoses of bipolar disorder from the Danish registries concluded that only 56% patients with bipolar disorder were given the diagnosis at first hospital contact and this number was lower for patients younger than 40 years. Furthermore, 30% of participants with a diagnosis of bipolar disorder will later change diagnosis. Reference Kessing38 This is probably also the reason why mean age at time of diagnosis among cases was higher (67 years for cases with a diagnosis after study entry) than expected. Due to this high age of the cases, we can only make conclusions for late-onset bipolar disorder. Also, as many individuals with bipolar disorder are never admitted to hospital but rather treated by private psychiatrists, some participants among those without an event may have had a previous depressive/manic episode treated outside a hospital setting; however, such misclassification among controls will only bias the results towards the null hypothesis and therefore cannot explain all our results. At baseline, only 38 individuals were excluded due to bipolar disorder, but it is likely that many others at the same time were misclassified as non-affected individuals. Since bipolar disorder often debuts with a depressive episode the low prevalence of bipolar disorder might also be explained by misdiagnosed unipolar depressed patients. Some of these may have been admitted to hospital and thus diagnosed with bipolar disorder later when the disease worsened, which limits the interpretation of CRP levels as predictor of development of bipolar disorder. Another explanation for the low number of individuals with bipolar disorder is that individuals with bipolar disorder may not participate in a population study as often as individuals without a psychiatric disease (non-responder bias). When we examined individuals who were invited to the CCHS but chose not to attend (non-responders), the incidence of an admission to hospital with bipolar disorder was higher in this group compared with responders (online Fig. DS4). We also did not have information on severity of bipolar disorder because we included discharge diagnoses from both somatic and psychiatric hospitals and furthermore, the information on type of episode (maniac or depressive) was too limited to use, as more than 50% of participants had a diagnosis of ‘unspecified bipolar disorder’. Another potential limitation of our study is that we studied White patients only, and therefore our results may not necessarily apply to other races. On the other hand, we are not aware of data to suggest that our results should not be applicable to individuals of all races. Finally, the Mendelian randomisation approach may have some limitations. Mendelian randomisation can be influenced by linkage disequilibrium, pleiotropy of CRP SNPs that influence other biomarkers, gene–gene interactions (false-negative conclusions due to failure to account for a second gene that modifies CRP levels) and canalisation (compensatory changes in other systems counterbalance genetic elevations and CRP levels). Reference Lawlor, Harbord, Sterne, Timpson and Davey27,Reference Smith and Ebrahim39 To examine pleiotropic effects of each of the SNPs, we searched the public database SCAN that includes information on whether SNPs have effects on expression of mRNA or proteins. We found only few and no consistent effects on other proteins for three of the SNPs and no pleiotropic effects of the important triallelic SNP which has the largest influence on CRP levels (online Table DS5).
In conclusion, we found that elevated plasma levels of CRP were associated cross-sectionally and prospectively with late-onset bipolar disorder in the general population after multifactorial adjustment for lifestyle factors, socioeconomic status and chronic disease. We cannot exclude that this association may be causal. However, as this is the first study to examine the prospective and possible causal associations between elevated CRP and late-onset bipolar disorder in the general population, further studies are needed to confirm or refute our results.
Funding
This study was supported by Herlev Hospital, Copenhagen University Hospital, and The Danish Council for Independent Research, Medical Sciences.
Acknowledgements
The funding source had no role in the design and conduct of the study, the collection and management of data, analysis and interpretation of the data, or the preparation of the manuscript. We thank participants and staff of the Copenhagen General Population Study and the Copenhagen City Heart Study for their important contributions.









eLetters
No eLetters have been published for this article.