Introduction
The integration of soil-residual herbicides in glyphosate-resistant crops is a common recommendation to improve consistency of weed management systems (Bond et al. Reference Bond, Dodds, Eubank and Reynolds2011; Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Fisvold, Powles, Burgos, Witt and Barrett2012). Research has shown that programs containing a soil-residual herbicide in glyphosate-resistant cotton maximizes weed control and lint yield (Burke et al. Reference Burke, Troxler, Askew, Wilcut and Smith2005; Clewis et al. Reference Clewis, Miller, Koger, Baughman, Price, Porterfield and Wilcut2008; Culpepper Reference Culpepper2006; Grichar et al. Reference Grichar, Besler, Brewer and Minton2004; Price et al. Reference Price, Koger, Wilcut, Miller and Van Santen2008; Scroggs et al. Reference Scroggs, Miller, Griffin, Wilcut, Blouin, Stewart and Vidrine2007). Everman et al. (Reference Everman, Clewis, York and Wilcut2009) and Scroggs et al. (Reference Scroggs, Miller, Griffin, Wilcut, Blouin, Stewart and Vidrine2007) reported increased weed control with the addition of PRE soil-residual herbicides in weed control programs for glufosinate- and glyphosate-resistant cotton. Using soil-residual herbicides not only can eliminate or reduce early-season competition from weeds to help secure maximum crop yield, using them also allows grower flexibility in timing of POST applications if needed (Ellis and Griffin Reference Ellis and Griffin2002). Soil-residual herbicides are commonly used now to control glyphosate-resistant weeds in various crops.
In susceptible plants, inhibition of protoporphyrinogen oxidase (PPO), an enzyme in the chlorophyll biosynthesis pathway, causes accumulation of porphyrins and increases peroxidation of membrane lipids, which leads to irreversible damage of the membrane function and structure (Duke et al. Reference Duke, Lydon, Becerril, Sherman, Lehnen and Matsumoto1991; Grossman et al. Reference Grossmann, Niggeweg, Christiansen, Looser and Ehrhardt2010, Reference Grossmann, Hutzler, Caspar, Kwiatkowski and Brommer2011). Increasing infestations of glyphosate- and acetolactate synthase (ALS)-resistant Palmer amaranth (Amaranthus palmeri S. Watson) in cotton has forced producers to use herbicides with alternative modes of action in their management systems (Sosnoskie and Culpepper Reference Sosnoskie and Culpepper2014). Of particular interest is the increased use of PPO herbicides and glufosinate for the management of glyphosate-resistant Palmer amaranth. Palmer amaranth can be controlled in systems using glufosinate and PPO herbicides such as flumioxazin and fomesafen (Everman et al. Reference Everman, Clewis, York and Wilcut2009; Gardner et al. Reference Gardner, York, Jordon and Monks2006; Whitaker et al. Reference Whitaker, York, Jordan and Culpepper2011a, Reference Whitaker, York, Jordon, Culpepper and Sosnoskie2011b). Of the PPO herbicides typically applied PRE or preplant (PP), both fomesafen and flumioxazin have been two of the most effective, providing 74% to 100% Palmer amaranth control 20 d after planting (DAP) (Whitaker et al. Reference Whitaker, York, Jordon, Culpepper and Sosnoskie2011b). The use of PPO-inhibiting herbicides across all crops has increased 25% (by value) globally since 2009 (Phillips McDougall 2014). The increase in use has been in response to the occurrence of glyphosate-resistant weeds in the United States, especially Amaranthus species.
Trifludimoxazin [1,5-dimethyl-6-sulfanylidene-3-(2,2,7-trifluoro-3-oxo-4-prop-2-ynyl-1,4-benzoxazin-6-yl)-1,3,5-triazinane-2,4-dione] is the first PPO-inhibiting herbicide containing a triazinone heterocycle (Armel et al. Reference Armel, Hanzlik, Witschel, Hennigh, Bowe, Simon, Libel and Mankin2017), has a water solubility of 1.78 mg L−1, is a nonionic molecule, and has a soil adsorption coefficient (Koc) of 315–692 mL g−1 (Table 1) (APVMA 2020). Trifludimoxazin is active when applied PRE or POST on broadleaf weeds, including known PPO target–based resistant Amaranthus biotypes possessing the δ glycine deletion or R128 substitution mutation, which are not controlled by currently registered PPO inhibitors (Armel et al. Reference Armel, Hanzlik, Witschel, Hennigh, Bowe, Simon, Libel and Mankin2017).
Table 1. Attributes of various herbicides and details to experimental conductance.

Flumioxazin is a dicarboxamide herbicide developed by Valent (Senseman Reference Senseman2007), has a water solubility of 1.79 mg L−1 with no apparent pH effect on water solubility, is a nonionic molecule, and has a Koc of 557 mL g−1 (Table 1) (Mueller et al. Reference Mueller, Boswell, Mueller and Steckel2014). Flumioxazin absorption to soil was highly correlated with organic matter, although it can become readily available in soil solution with an increase in soil water content (Ferrell et al. Reference Ferrell, Vencill, Xia and Grey2005). Flumioxazin is used in a wide range of crops, including soybean [Glycine max (L.) Merr] and cotton (Anonymous 2016).
Saflufenacil is a pyrimidinedione herbicide developed by BASF (Grossman et al. Reference Grossmann, Niggeweg, Christiansen, Looser and Ehrhardt2010). It has a water solubility of 210 mg L−1 at pH 7, which is directly related to pH, is an ionic molecule, and has a Koc of 27 mL/g. Observed low sorption to soil and rapid dissipation suggest that saflufenacil would be readily available for degradation or plant uptake in the plant root zone (Table 1) (Mueller et al. Reference Mueller, Boswell, Mueller and Steckel2014). The primary use of saflufenacil is PP burndown () in several crops including corn (Zea mays L.), soybean, and cotton (Anonymous 2017).
Soils differ across cropping regions and within fields in the United States, often resulting in herbicide-rate adjustment to obtain desired efficacy and crop safety. Many soil-residual herbicides have use restrictions or limitations related to soil properties that affect their behavior in the soil. The use of fomesafen, another PPO herbicide used in cotton, is limited to coarse-textured soils (eg, sandy loam, loamy sand, sandy clay loam) when applied PRE (Anonymous 2019)
West Texas cotton-production soils range in texture from fine to coarse, generally have low soil organic matter content and typically a high pH. Evaluating application rates and timings of soil-applied residual herbicides as influenced by various soil parameters is critical for determining effective rates for weed control, crop selectivity, and recropping intervals (Gannon et al. Reference Gannon, Hixson, Keller, Weber, Knezevic and Yelverton2014). As new soil-residual herbicides are developed for commercialization, testing under a wide range of soil conditions is required to determine their utility in weed management programs in West Texas. With the development and spread of glyphosate-resistant Palmer amaranth, multiple control options, including the use of soil-residual herbicides, will be needed to effectively manage this weed and other troublesome weeds in cotton. The objective of this research was to develop a use pattern for trifludimoxazin in West Texas cotton. Vertical mobility and greenhouse cotton tolerance trials were performed using three West Texas soils, and the results were compared with two commercially used PPO herbicides in cotton.
Materials and Methods
Bulk Soils
Bulk samples were collected from the top 15 cm of the soil profile (Acuff loam [Cotton Center, TX; 33.56°N, 102.0°W]; Olton loam [Halfway, TX; 34.10°N, 101.56°W]; Amarillo loamy sand [Seagraves, TX; 32.58°N, 102.39°W]). To prepare the soils, each was air dried at room temperature and passed through a 2-mm sieve. Samples were sent to Midwest Laboratories, Inc., (Omaha, NE) for soil-property analysis (Table 2).
Table 2. Properties of soil samples (0–15 cm deep) for each West Texas soil.

a Abbreviations: C, clay; CEC, cation exchange capacity; L, loam; LS, loamy sand; OM, organic matter; Tex, soil texture.
b Soil textural classification.
c Organic matter was determined by a loss on ignition method (Dean Reference Dean1974).
d Particle analysis was determined using the hydrometer method (Gee and Orr Reference Gee, Orr, Dane and Tropp2002).
e CEC was determined using the summation of exchangeable cations procedure (Mehlich Reference Mehlich1984).
f pH was determined using a 1:1 soil-to-distilled water ratio (Peech Reference Peech and Black1965).
Herbicide Vertical Mobility
Comparative vertical mobility of trifludimoxazin, saflufenacil, and flumioxazin was evaluated within each of the three West Texas soils using a bioassay soil-column technique (Nelson and Penner Reference Nelson and Penner2007). A polyvinyl chloride (PVC) soil column 15.24 cm tall by 7.62 cm diam was filled with 626.6 cm3 of each soil (Acuff, 755 g; Amarillo, 845 g; Olton, 741 g). Soil was placed in each column, one-third of the total amount at a time, and hand-packed between fillings. After filling and packing, a 15-mm headspace remained at the top of each PVC soil column. The packed soil columns were irrigated with a rainfall simulator to bring each soil to field capacity (Acuff, 198 mL; Amarillo, 125 mL; Olton, 211 mL). Columns were allowed to drain and dry for 48 h.
Using a bulb pipette, 5 mL of stock herbicide solution providing the 1× field rate of each herbicide (Table 1) was applied to the surface of each soil column. Rates represent the current commercial use rate for flumioxazin and saflufenacil and the targeted use rate for trifludimoxazin (Table 1). After 2 h, columns were placed in the rainfall simulator and 2.54 cm of rainfall (116 mL of water applied to each soil column) was applied over 40 min. Columns were set aside for 24 h to drain before being split vertically into two halves. Two rows of DeKalb DKL 52-41 Roundup Ready® (Monsanto, St. Louis, MO) canola (Brassica napus L.) was seeded (0.64 cm deep; 1.0 teaspoon of seed column−1) lengthwise into the soil column as the herbicide-susceptible indicator species. Columns were placed in the greenhouse (constant temperature of 28 C, 14 h day-length with supplemental lighting triggered when ambient light reached less than 2,000 watts m−2, constant 50% relative humidity) and watered twice daily. Vertical mobility from the soil surface, indicated by the depth at which the indicator species exhibited a visual response was measured 7 and 10 d after treatment (DAT).
Greenhouse Cotton Tolerance
Plastic pots, 10.16 by 10.16 cm, were filled with each of the three West Texas soils. Pots were transferred to the greenhouse (same conditions as described in the vertical mobility study), fully watered, and allowed to drain to field capacity. A single Stoneville 4946 GlyTol® LibertyLink® Genuity® Bollgard II® (Bayer Crop Science, Research Triangle Park, NC) cotton seed was planted at a depth of 2.54 cm in each pot immediately before PRE treatments and 14 d after application for the PP treatments. This depth is within the recommended planting depth of 0.6 to 3.8 cm to optimize emergence (Cotton Foundation 2020) and the current label restriction for flumioxazin used PP in strip-till/no-till cotton (Anonymous 2016). Each of the herbicides was applied using a spray chamber (TeeJet® XR 80015; 140 L ha−1, 275 kPa, 4.8 km h−1) using the 1× field rate shown in Table 1. Pots were transferred to the greenhouse and allowed to dry for 2 h prior to receiving an overhead watering of 2.54 cm to activate the herbicide. Pots were watered twice daily for the duration of the experiment. Visual plant response was recorded at 14 and 28 DAP.
Aboveground cotton fresh weight was recorded 28 DAP by cutting each plant at the soil surface. Plants were placed in an oven dryer at 32 C for 12 h and dry weights recorded. Dry weight as compared with the nontreated control (NTC) within each soil type was calculated for each plant.
Statistical Analysis
The herbicide vertical mobility experiment used a completely randomized design with three replications for each soil by herbicide combination and the experiment was conducted twice. The greenhouse cotton tolerance experiment was arranged in a completely randomized design with 3three pots repetition−1 and 3 repetitions run−1, and was conducted twice. Data were subjected to ANOVA and means separated by Fisher protected LSD at the 5% level (SAS 2013).
Results and Discussion
Herbicide Vertical Mobility
Differences in herbicide vertical mobility were observed among herbicide by soil combinations. No differences were seen with observation time (7 DAT vs. 10 DAT); thus, only the 10 DAT data are reported. All flumioxazin soil combinations had similar vertical mobility at 10 DAT (Table 3). Trifludimoxazin mobility at 10 DAT in the Acuff and Olton soils was similar to flumioxazin (range, 1.7–2.5 cm), but trifludimoxazin exhibited 79% greater vertical mobility than flumioxazin in the Amarillo soil (Table 3). Vertical mobility of saflufenacil was greater than all other respective herbicide by soil combinations 10 DAT (6.2–13.5 cm) and was greatest in the Amarillo soil 10 DAT (13.5 cm) (Table 3). The Amarillo soil was the most coarse-textured soil (loamy sand) and contained the least amount of organic matter (0.3%) of the three West Texas soils tested. These findings are consistent with previous studies that showed percent organic matter had the greatest impact on herbicide bioavailability (Parochetti Reference Parochetti1973; Rahman and Matthews Reference Rahman and Matthews1979; Sheets et al. Reference Sheets, Crafts and Drever1962; Stevenson Reference Stevenson1972; Weber et al. Reference Weber, Tucker and Isaac1987).
Table 3. Vertical mobility of three protoporphyrinogen oxidase–inhibiting herbicides in three West Texas soils.
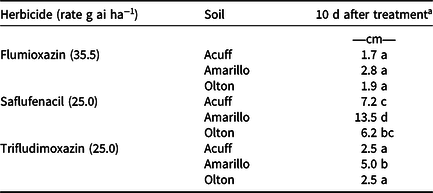
a Means within a column followed by the same letter are not different according to Fisher protected LSD test at P = 0.05.
Greenhouse Cotton Tolerance
At the PP application timing, trifludimoxazin and flumioxazin by soil combinations caused similar cotton response 14 and 28 DAP (0% to 13%) (Table 4). The PP saflufenacil treatment caused more cotton response than all trifludimoxazin or flumioxazin PP by soil combinations except for the Amarillo soil 14 DAP, when all three herbicides produced similar cotton response (Table 4). All PRE herbicide by soil combination treatments caused greater levels of cotton response than the PP herbicide by soil combinations at 14 and 28 DAP, except for flumioxazin in the Olton soil (7% and 8%, respectively). Trifludimoxazin and saflufenacil PRE treatments had similar levels of cotton response across all soil combinations at 28 DAP (65% to 79%) and were greater than any flumioxazin by soil PRE treatment (8% to 36%). The only PRE herbicide by soil treatment with similar cotton response to the PP herbicide by soil combinations at 28 DAP was flumioxazin in the Olton soil (8%) (Table 4).
Table 4. Cotton response from PRE and preplant applications of three protoporphyrinogen oxidase–inhibiting herbicides in three West Texas soils.
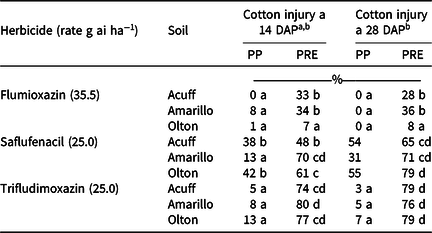
a Abbreviations: DAP, days after planting; PP, preplant.
b Means within a column followed by the same letter are not different according to Fisher protected LSD test at P = 0.05.
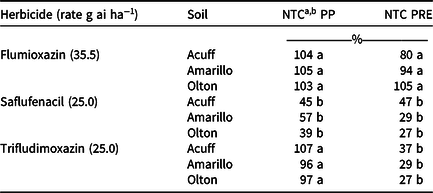
Cotton fresh-weight comparisons to the NTC (data not shown) were similar to the dry weight comparisons. Within each soil, cotton dry weight after the trifludimoxazin and flumioxazin PP treatments was similar to the NTC and ranged from 96% to 107% and 103% to 105%, respectively (Table 5). This corresponds to the visual cotton response recorded at 28 DAP, which indicated that these treatments were similar (Table 4). Preplant saflufenacil cotton dry weight within each soil was less than the NTC and lower than trifludimoxazin and flumioxazin (Table 5). This also corresponded to the cotton response observed at 28 DAP (Table 4). Across soils, cotton dry weight after the trifludimoxazin and saflufenacil PRE treatments were similar and lower than the NTC (29% to 37% and 27% to 47%, respectively) (Table 5). Flumioxazin-induced cotton dry weight was similar to the NTC after the PRE treatments across all three West Texas soils (80% to 105%) (Table 5).
Table 5. Percentage of the nontreated control cotton dry weight from preplant and PRE applications of three protoporphyrinogen oxidase–inhibiting herbicides in three West Texas soils.
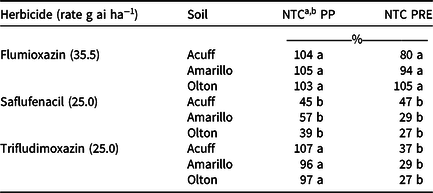
a Abbreviations: NTC, nontreated control; PP, preplant.
b Means within a column followed by the same letter are not different according to Fisher protected LSD test at P = 0.05.
PRE herbicide treatments resulted in greater levels of cotton response than did the PP treatments within all soils. PRE treatments of trifludimoxazin and saflufenacil resulted in similar high levels of cotton response across soils at 28 DAT (78% and 72%, respectively), which were greater than flumioxazin (24%). Cotton dry weight as a percent of the NTC within each soil was similar for the flumioxazin PRE treatments. All PP treatments of trifludimoxazin and flumioxazin, when averaged across soils, had similar levels of cotton response to the NTC at 14 and 28 DAT. Cotton dry weight as a percent of the NTC within each soil was similar for all trifludimoxazin and flumioxazin PP treatments. All saflufenacil PP treatments resulted in greater cotton response and reduced dry weights when compared with the NTC.
Soil vertical mobility of saflufenacil does not offer the opportunity for selectivity based on placement for a PRE or 14 d PP applications in cotton. This supports the current label use restriction of 42 d PP before cotton planting (Anonymous 2017). For flumioxazin, planting cotton at a depth of 2 cm or deeper would create an opportunity for selectivity based on placement, at least in the Acuff and Olton soils, because the herbicide only moved to a depth of 1.7–1.9 cm in those soils. This work supports the results reported by Berger et al. (Reference Berger, Ferrell, Brecke, Faircloth and Rowland2012), indicating the current labelled PP application window (Anonymous 2016) of 14 to 21 d could be shortened with little crop response after flumioxazin PP. Along with a PP interval, placement selectivity with flumioxazin with planting depths of 2 cm or deeper might be achieved. To achieve selectivity in cotton with trifludimoxazin, a PP application window must be implemented. Selectivity based on placement will not be possible, because the vertical mobility of trifludimoxazin was 2.5 cm or greater in all three West Texas soils. To refine the use pattern for trifludimoxazin in cotton, more work is needed to define the PP application window and if a use-rate range can be created for soils with differing soil properties.
Acknowledgments
Appreciation is extended to BASF Corporation for providing laboratory and greenhouse facilities for this research. BASF and several authors on this paper are involved with obtaining registration and developing a use pattern for trifludimoxazin in cotton and various other crops. No conflicts of interest have been declared.