Patients with bipolar disorder have a three times increased risk of type 2 diabetes compared with the general population. Reference Cassidy, Ahearn and Carroll1–Reference McIntyre, Konarski, Misener and Kennedy3 In addition to increasing risk for cardiovascular disease (the leading cause of death in patients with bipolar disorder Reference Osby, Brandt, Correia, Ekbom and Sparen4 ), comorbid type 2 diabetes heralds greater psychiatric symptom severity. In a cross-sectional study, we found that patients with bipolar disorder and type 2 diabetes had higher rates of rapid cycling, a more chronic course of illness, lower scores on the Global Assessment of Functioning (GAF) scale and had received disability benefits more often for bipolar disorder compared with those without type 2 diabetes. Reference Ruzickova, Slaney, Garnham and Alda5 No studies, however, have examined the possible relationship between type 2 diabetes and bipolar treatment response. Further, little is known about early stages of metabolic dysregulation, namely, insulin resistance (or ‘pre-diabetes’) in relation to bipolar disorder. In a previous study, we examined response to lithium, the principal treatment for bipolar disorder, in relation to obesity; a risk factor for insulin resistance and type 2 diabetes. Patients with lower body mass index (BMI), in the healthy range, achieved complete remission of symptoms on lithium, compared with those in the obese range (BMI ≥30), who had no clinical response to lithium. Reference Calkin, Van de Velde, Ruzickova, Slaney, Garnham and Hajek6 Kemp et al found that for every unit increase in BMI, the likelihood of response to any bipolar treatment decreased by 7.5% and the likelihood of remission decreased by 7.3%. Reference Kemp, Gao, Chan, Ganocy, Findling and Calabrese7 Yet no previous study has explored the relationship between laboratory-established insulin resistance and outcomes in bipolar disorder, including treatment response. This is important because insulin resistance cannot be diagnosed using fasting glucose measurements alone and requires specific testing for which there are no clinical recommendations. The relevance of studying insulin resistance is that it may be an overlooked, modifiable factor contributing to the outcome of bipolar disorder. The goal of the present study was to explore the relationship between laboratory measures of insulin resistance and type 2 diabetes and clinical course and treatment response in bipolar disorder. We hypothesised that patients with bipolar disorder and insulin resistance or type 2 diabetes would have a chronic course of bipolar disorder, more rapid cycling and poorer response to prophylactic treatment with lithium than patients who were euglycaemic (glucose tolerant). We expected patients with insulin resistance to have intermediate findings between those of patients who were euglycaemic and those with type 2 diabetes.
Method
We recruited 121 patients with bipolar disorder type I or II from the Mood Disorders Program at Dalhousie University and from the Maritime Bipolar Registry in the Atlantic Provinces of Canada. Reference Ruzickova, Slaney, Garnham and Alda5 Patients in our Program and Registry are followed prospectively. Consecutive patients seen in the Mood Disorders Program and patients in the Registry were invited to participate in this study by the attending psychiatrist or by a research nurse; over 98% agreed and provided written informed consent. Patients at least 18 years of age with a diagnosis of bipolar disorder type I or II were included. The study was approved by the Research Ethics Board of the Capital District Health Authority, Nova Scotia.
The initial diagnosis of bipolar disorder type I or II was established using the Schedule for Affective Disorders and Schizophrenia, lifetime version (SADS-L). Reference Endicott and Spitzer8 The final diagnosis was reached by consensus of at least two experienced psychiatrists using Research Diagnostic Criteria (RDC), Reference Spitzer, Endicott and Robins9 and DSM-IV-TR 10 criteria and who were masked to the blood test results, treatment response and other non-diagnostic characteristics. Further collected data include details of history of illness, detailed symptom profile using operational criteria (OPCRIT), Reference Rucker, Newman, Gray, Gunasinghe, Broadbent and Brittain11 clinical course (age at onset, polarity of first episode, history of rapid cycling, lifetime number of episodes, history of psychosis with mood episodes), treatment history and response, psychiatric and medical comorbidity, ethnicity, gender, smoking history, level of functioning and history of long-term disability. Patients were asked whether they had a known history of type 2 diabetes, hypertension, dyslipidaemia, cardiovascular and cerebrovascular diseases or thyroid disorders.
The National Institute of Mental Health (NIMH) Life Chart Method Reference Denicoff, Leverich, Nolen, Rush, McElroy and Keck12,Reference Denicoff, Ali, Sollinger, Smith-Jackson, Leverich and Post13 was used to document clinical course and treatment history, including exposure to mood stabilisers and antipsychotic medication. Clinical course was defined as a binary variable (chronic v. episodic) according to the presence/absence of remission of symptoms between episodes. Using modified SADS-L and DSM-IV-TR course specifier criteria, we defined a chronic course as continuous, fluctuating or residual symptoms without full remissions of a minimum of 2 months’ duration. An episodic course was defined by clear episodes of illness with full remissions of at least 2 months’ duration, without residual symptoms. Of the 121 patients in the study, 80 had received an adequate trial of lithium (at least 6 months’ duration at therapeutic blood levels) and these patients were used in the analysis of insulin resistance or type 2 diabetes and response to lithium. We did not test response to other mood stabilising treatments, as these medications were used less frequently as monotherapy. In order to assess response to prophylactic lithium treatment, we used the Retrospective Criteria of Long-term Treatment Response in Bipolar Disorder (Alda) scale. Reference Grof, Duffy, Cavazzoni, Grof, Garnham and MacDougall14 Patients were scored on a scale of 0-10, with a score of ≥7 indicating complete response and ≤3, complete lack of response. A score of 4-6 indicates a partial response. This is a valid measure with an interrater reliability of 0.54-0.75 in assessing long-term response to treatment. Reference Garnham, Munro, Slaney, MacDougall, Passmore and Duffy15,Reference Manchia, Adli, Akula, Ardau, Aubry and Backlund16 We used the Global Assessment of Functioning (GAF) scale Reference Jones, Thornicroft, Coffey and Dunn17 to measure current functional impairment. We systematically enquired regarding family history of psychiatric disorders and type 2 diabetes in first-degree relatives. In 55 participants we corroborated the family history by interviewing additional family members using the SADS-L and Family History-RDC. Reference Andreasen, Endicott, Spitzer and Winokur18 Family history was recorded as a dichotomous measure (positive or negative). Obesity was determined by BMI. In addition, we measured waist circumference and blood pressure.
Laboratory analyses
The presence of insulin resistance or type 2 diabetes was determined by laboratory testing after a minimum 8 h fast. In patients who did not have a pre-existing diagnosis of type 2 diabetes with documented treatment, type 2 diabetes was diagnosed by a fasting plasma glucose (FPG) ≥7.0 mmol/L, confirmed by repeated FPG on another day, or by a 2 h glucose level of >11.1 mmol/L after a 2 h oral glucose tolerance test. In non-diabetic patients, insulin resistance was estimated by FPG and concurrent fasting serum insulin (FSI) levels, using the homeostatic model assessment – insulin resistance equation (HOMA-IR): Reference Katsuki, Sumida, Gabazza, Murashima, Furuta and Araki-Sasaki19,Reference Wallace, Levy and Matthews20 HOMA-IR = FPG (mmol/L) × FSI(μU/mL)/22.5.
These tests were analysed in a single laboratory with the same assay to eliminate variability. The HOMA-IR strongly correlates with estimates using the euglycaemic clamp method Reference Katsuki, Sumida, Gabazza, Murashima, Furuta and Araki-Sasaki19,Reference Wallace, Levy and Matthews20 and is a well-accepted measure of insulin resistance. Values of 1.8 or 2.0 or more using HOMA-IR have been previously suggested as indicating insulin resistance, based on the point at which risk for metabolic syndrome significantly increases. Reference Esteghamati, Ashraf, Khalilzadeh, Zandieh, Nakhjavani and Rashidi21 We used the more conservative cut-off and accepted HOMA-IR value of ≥2.0 to establish insulin resistance. Reference Katsuki, Sumida, Gabazza, Murashima, Furuta and Araki-Sasaki19,Reference Wongwananuruk, Rattanachaiyanont, Leerasiri, Indhavivadhana, Techatraisak and Angsuwathana22,Reference Matthews, Hosker, Rudenski, Naylor, Treacher and Turner23 By this method, we were able to distinguish three groups of patients: those who were ‘euglycaemic’ (normal FPG and no insulin resistance), those with insulin resistance and those with type 2 diabetes.
Statistical analyses
We used χ2 linear × linear association to test for relationships between abnormal glucose metabolism and specific clinical characteristics (bipolar subtype: bipolar disorder types I and II, polarity at onset, presence of psychosis with mood episodes, gender, medical comorbidity (hypertension, dyslipidaemia, cardiovascular or cerebrovascular disease, thyroid disorder), history of suicidal behaviour, smoking, long-term disability, family history of psychosis or type 2 diabetes). We set the level of significance at P<0.05.
Clinical course was defined as a binary variable (chronic v. episodic). We used logistic regression to test the relationships between insulin resistance and type 2 diabetes and chronic course of bipolar disorder, history of rapid cycling, lifetime number of episodes and GAF score. Given the strong association of type 2 diabetes and insulin resistance with age, we controlled for age and gender in all analyses. In all logistic regression models, the outcome of interest (chronic course, rapid cycling, response to lithium) was the dependent variable; insulin resistance, type 2 diabetes, age and gender were independent variables. Additionally, we carried out sensitivity analyses controlling for present and lifetime use of antipsychotic medication and BMI, entered as covariates in addition to age and gender.
Response to prophylactic lithium treatment was classified as complete, partial or absent/ineffective, and recorded as a three-level ordinal variable. We used ordered logistic regression to test the effect of insulin resistance and type 2 diabetes on lithium response, controlling for age and gender. In sensitivity analyses, we also controlled for present and lifetime use of antipsychotic medication and BMI.
Results
Demographic and clinical characteristics
Our patients were most commonly of Irish, Scottish or French Acadian descent and ranged in age between 19 and 85 years. The male: female ratio in our sample was 1: 2. Patients with the diagnosis of type I bipolar disorder comprised 69.9%; patients with type II bipolar disorder were 30.1% of our sample. Mean age at study entry was 48.1 years (s.d. = 13.9) and mean age at diagnosis of bipolar disorder was 22.6 years (s.d. = 8.0). Mean age at onset for type 2 diabetes was 47.8 years (s.d. = 12.8). In terms of current functioning, the mean GAF score was 65.5 (s.d. = 13.4), indicating mild impairment. Mean BMI was 30.0 (obese range) and mean HOMA-IR for all participants not diagnosed with diabetes was 2.02, just exceeding the cut-off for insulin resistance. As expected, there was a positive correlation between BMI and HOMA-IR (r = 0.35, P = 0.001).
Results of glucose and insulin testing
Glucose and insulin testing revealed that more than half of the patients had insulin resistance or type 2 diabetes. The proportion of patients with insulin resistance was 32.2% (n = 39), whereas 21.5% (n = 26) had type 2 diabetes. Of those diagnosed with type 2 diabetes, 38.5% (n = 10) were unaware that they had type 2 diabetes.
Association between glucose metabolism and clinical characteristics
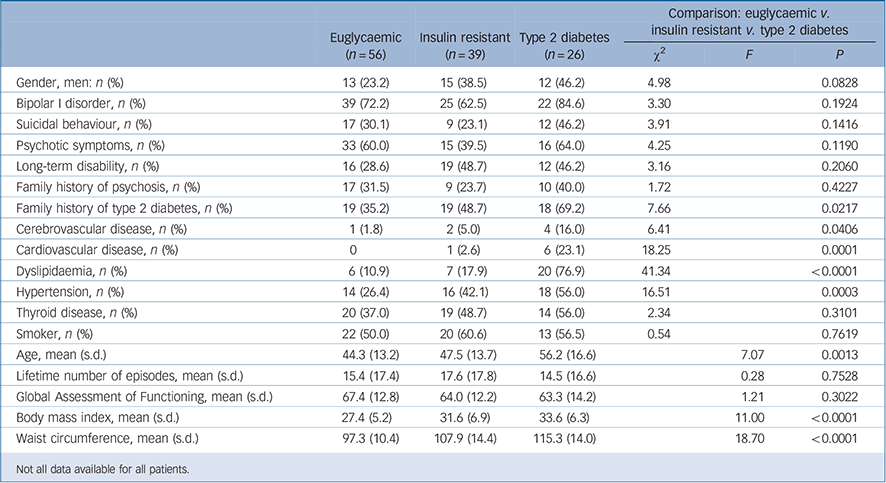
Table 1 shows clinical descriptors for groups defined by glucose metabolism status. Insulin resistance and type 2 diabetes were strongly positively associated with a history of hypertension, dyslipidaemia, cardiovascular disease, cerebrovascular disease, higher BMI, greater waist circumference and family history of type 2 diabetes. We found no association between abnormalities in glucose metabolism and polarity at onset, age at onset of bipolar
Table 1 Comparison of clinical variables associated with abnormal glucose metabolism
| Euglycaemic (n = 56) |
Insulin resistant (n = 39) |
Type
2 diabetes (n = 26) |
Comparison: euglycaemic
v.
insulin resistant v. type 2 diabetes |
|||
|---|---|---|---|---|---|---|
| χ2 | F | P | ||||
| Gender, men: n (%) | 13 (23.2) | 15 (38.5) | 12 (46.2) | 4.98 | 0.0828 | |
| Bipolar I disorder, n (%) | 39 (72.2) | 25 (62.5) | 22 (84.6) | 3.30 | 0.1924 | |
| Suicidal behaviour, n (%) | 17 (30.1) | 9 (23.1) | 12 (46.2) | 3.91 | 0.1416 | |
| Psychotic symptoms, n (%) | 33 (60.0) | 15 (39.5) | 16 (64.0) | 4.25 | 0.1190 | |
| Long-term disability, n (%) | 16 (28.6) | 19 (48.7) | 12 (46.2) | 3.16 | 0.2060 | |
| Family history of psychosis, n (%) | 17 (31.5) | 9 (23.7) | 10 (40.0) | 1.72 | 0.4227 | |
| Family history of type 2 diabetes, n (%) | 19 (35.2) | 19 (48.7) | 18 (69.2) | 7.66 | 0.0217 | |
| Cerebrovascular disease, n (%) | 1 (1.8) | 2 (5.0) | 4 (16.0) | 6.41 | 0.0406 | |
| Cardiovascular disease, n (%) | 0 | 1 (2.6) | 6 (23.1) | 18.25 | 0.0001 | |
| Dyslipidaemia, n (%) | 6 (10.9) | 7 (17.9) | 20 (76.9) | 41.34 | <0.0001 | |
| Hypertension, n (%) | 14 (26.4) | 16 (42.1) | 18 (56.0) | 16.51 | 0.0003 | |
| Thyroid disease, n (%) | 20 (37.0) | 19 (48.7) | 14 (56.0) | 2.34 | 0.3101 | |
| Smoker, n (%) | 22 (50.0) | 20 (60.6) | 13 (56.5) | 0.54 | 0.7619 | |
| Age, mean (s.d.) | 44.3 (13.2) | 47.5 (13.7) | 56.2 (16.6) | 7.07 | 0.0013 | |
| Lifetime number of episodes, mean (s.d.) | 15.4 (17.4) | 17.6 (17.8) | 14.5 (16.6) | 0.28 | 0.7528 | |
| Global Assessment of Functioning, mean (s.d.) | 67.4 (12.8) | 64.0 (12.2) | 63.3 (14.2) | 1.21 | 0.3022 | |
| Body mass index, mean (s.d.) | 27.4 (5.2) | 31.6 (6.9) | 33.6 (6.3) | 11.00 | <0.0001 | |
| Waist circumference, mean (s.d.) | 97.3 (10.4) | 107.9 (14.4) | 115.3 (14.0) | 18.70 | <0.0001 | |
Not all data available for all patients.
disorder, bipolar diagnostic subcategories (bipolar disorder type I v. II), number of lifetime episodes, history of long-term disability, thyroid disorders, suicidal behaviour, family history of psychosis or personal history of psychosis during mood episodes. Male gender was associated with insulin resistance and type 2 diabetes in univariate analysis, but this was accounted for by men in our sample being older and the effect disappeared when age was included as a covariate.
Association between glucose metabolism and course of illness
Course of illness differed by glucose metabolic status (euglycaemic v. insulin resistant or type 2 diabetes) with 50% (n = 13) of patients with bipolar disorder and type 2 diabetes and 48.7% (n = 19) with insulin resistance having a chronic course compared with 27.3% (n = 15) of euglycaemic patients (OR = 3.07, 95% CI 1.35-6.95, P = 0.0007, controlling for age and gender, Fig. 1). The relationship between impaired glucose metabolism and
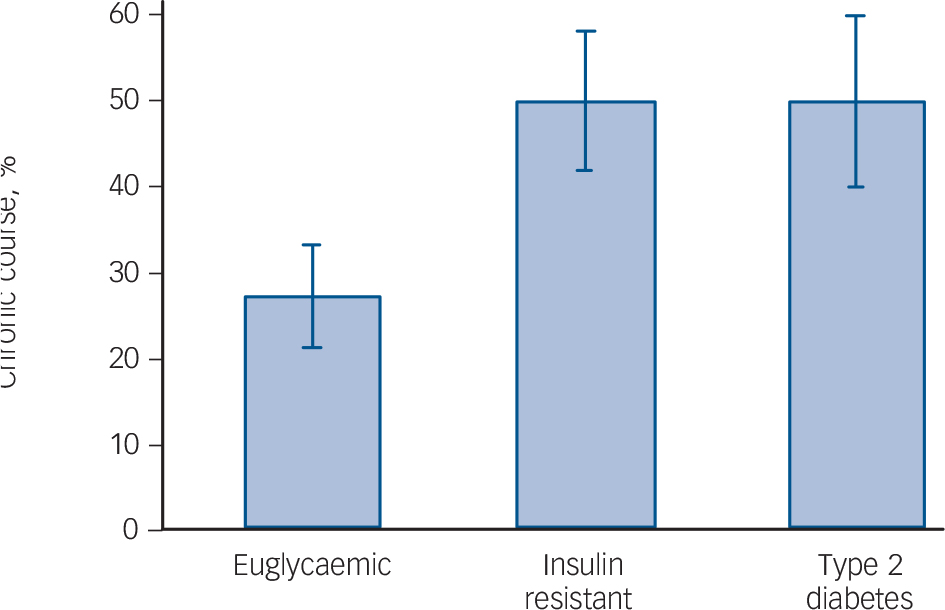
Fig. 1 Chronic course of bipolar disorder in individuals with euglycaemia, insulin resistance and type 2 diabetes.
chronic course of bipolar disorder remained significant in a sensitivity analysis controlling for lifetime and current use of antipsychotic medication in addition to age and gender (OR = 2.57, 95% CI 1.09–6.04, P = 0.031). Body mass index was a weaker predictor of unfavourable course of illness than glucose metabolic status, not reaching a level of statistical significance (OR = 1.04, 95% CI 1.00–1.10, P= 0.072). Insulin resistance or type 2 diabetes remained strong predictors of an unfavourable course, even after controlling for BMI (OR = 2.19, 95% CI 1.30–3.69, P = 0.0031).
Association between glucose metabolism and rapid cycling
Patients with type 2 diabetes or insulin resistance had significantly higher rates of rapid cycling (38.5% (n = 10) and 39.5% (n = 15), respectively) than euglycaemic patients (18.2%, n = 10), resulting in a significant difference in the rate of rapid cycling by glucose metabolism status (OR = 3.13, 95% CI 1.28–7.63, P = 0.012), controlling for age and gender. The relationship between glucose metabolism and rapid cycling course of illness remained significant in a sensitivity analysis controlling for current and lifetime use of antipsychotic medication in addition to age and gender (OR = 2.65, 95% CI 1.05–6.67, P = 0.039). Body mass index also significantly predicted rapid cycling (OR = 1.08, 95% CI 1.02–1.15, P = 0.013). However, when both insulin resistance/type 2 diabetes and BMI were entered into a logistic regression, insulin resistance or type 2 diabetes significantly predicted rapid cycling, even after controlling for BMI (OR = 3.36, 95% CI 1.19–9.52, P = 0.022) whereas the effect of BMI was no longer significant (OR = 1.04, 95% CI 0.97–1.11, P = 0.271).
Association between glucose metabolism and response to lithium treatment
Glucose metabolic status was strongly related to therapeutic response to prophylactic lithium treatment (Figs 2 and 3). Only 3.2% of patients (n = 1) who were euglycaemic had no response to lithium whereas 36.7% (n = 11) of patients with insulin
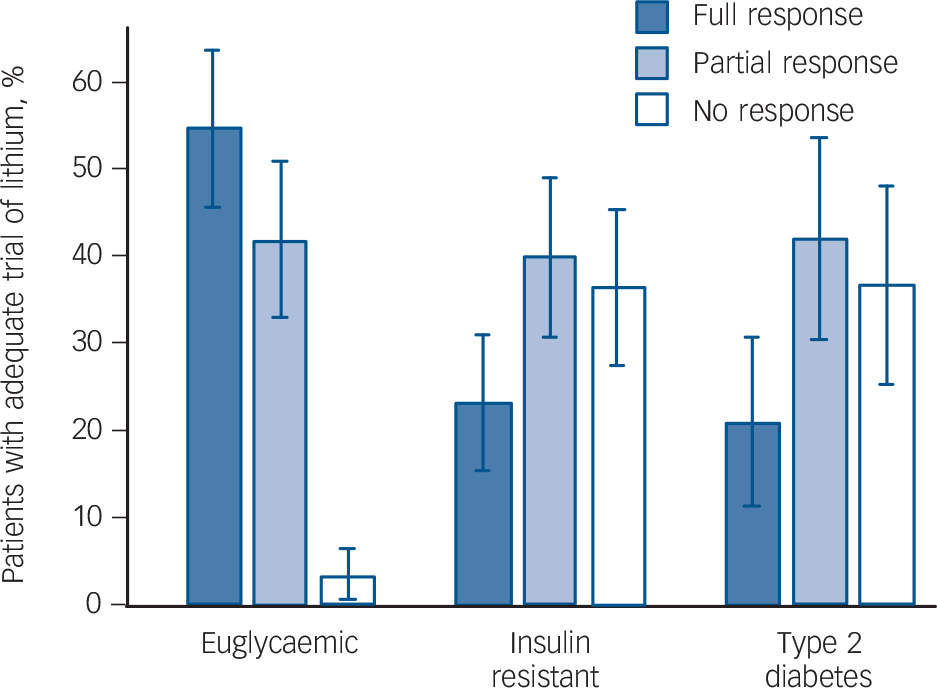
Fig. 2 Response to prophylactic lithium in individuals with euglycaemia, insulin resistance and type 2 diabetes.
resistance and 36.8% of patients (n = 7) with type 2 diabetes had no response. A significantly greater proportion of patients who were euglycaemic achieved remission on lithium (54.8%, n = 17) compared with those with insulin resistance (23.3%, n = 7) or type 2 diabetes (21.1%, n = 4; OR = 8.40, 95% CI 3.03–23.3, P<0.0001, controlling for age and gender, Fig. 2). The association between impaired glucose metabolism and poor response to prophylactic treatment with lithium remained significant in a sensitivity analysis controlling for current and lifetime use of antipsychotic medication in addition to age and gender (OR = 7.86, 95% CI 2.54–24.36, P = 0.0004). Insulin resistance or type 2 diabetes was strongly associated with non-response to lithium even after controlling for BMI in addition to age and gender (OR = 3.85, 95% CI 1.89–7.34, P= 0.0002) and after additionally controlling for past or current antipsychotic use (OR = 3.04, 95% CI 1.44–6.45, P = 0.0036). Insulin resistance or type 2 diabetes was associated with lithium non-response even among the 42 non-obese participants with BMI <30 (OR = 3.87, 95% CI 1.43–10.45, P = 0.008).
In addition, we found that response to lithium was negatively associated with a continuous measure of insulin resistance, as estimated by HOMA-IR (OR = 1.39, 95% CI 1.07–1.80, P = 0.013, controlling for age and gender, Fig. 3). This relationship
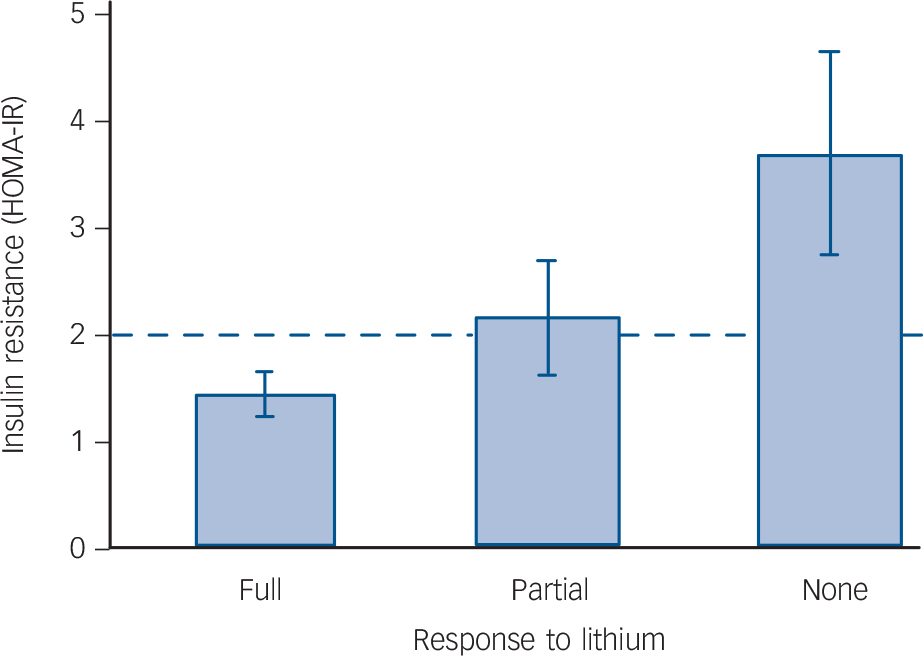
Fig. 3 Relationship between insulin resistance (as estimated by the homeostatic model assessment – insulin resistance (HOMA-IR)) and response to lithium.
Patients with type 2 diabetes were excluded.
remained significant in a sensitivity analysis controlling for current and lifetime use of antipsychotic medication in addition to age and gender (OR = 1.34, 95% CI 1.02–1.75, P = 0.035). It also remained significant when controlling for BMI (OR = 1.37, 95% CI 1.03–1.81, P = 0.028), whereas the effect of BMI was not significant (OR = 1.04, 95% CI 0.94–1.15, P = 0.4179).
The clinical relevance of identifying insulin resistance
In clinical practice, patients with insulin resistance are typically not identified as having abnormal glucose metabolism, as they have normal FPG. To estimate the value of measuring insulin resistance clinically, we compared variance in clinical outcomes explained under two scenarios, one where insulin resistance was identified and the other in which it was not. This comparison revealed a pseudo R 2 of 0.132 (OR = 8.4, P<0.0001) for the euglycaemic v. insulin resistant/type 2 diabetes model (insulin resistance identified), and a pseudo R 2 of 0.055 (OR = 3.7, P = 0.016) for the euglycaemic/insulin resistant v. type 2 diabetes model (insulin resistance not identified) after controlling for age and gender. When we also controlled for past or present use of antipsychotic medication, the values of pseudo R 2 were 0.247 (OR = 7.9, P = 0.0004) and 0.175 (OR = 2.3, P = 0.145), respectively. Thus, models with identified insulin resistance explained 7-8% of the variance in outcomes more than models that only identified type 2 diabetes. According to simulations of clinical significance in major depressive disorder, this difference in prediction would have been clinically relevant. Reference Uher, Tansey, Malki and Perlis24
Discussion
Main findings
We have identified high rates of insulin resistance and undiagnosed type 2 diabetes in a clinical sample of individuals with bipolar disorder. Of all patients, 8% were unaware that they had type 2 diabetes until we tested FPG levels and a further 32.2% were unaware that they had insulin resistance until we tested concurrent FSI. Together, this represents 40% of all patients in our sample who have previously unidentified metabolic disturbance, that may be affecting clinical outcome. Although insulin resistance is not screened for clinically, this occult metabolic disturbance is associated with an unfavourable course of bipolar illness and poor response to lithium.
The development of type 2 diabetes typically follows a progression of metabolic disturbance from a euglycaemic state with no insulin resistance, to euglycaemic state with insulin resistance, then to glucose intolerance and eventually type 2 diabetes. Thus, one might expect to find that patients with insulin resistance would have intermediate findings between those for patients with euglycaemia and type 2 diabetes, but in fact, patients with insulin resistance were indistinguishable from those with type 2 diabetes in respect to psychiatric outcomes. This is important, as there are no recommendations to screen for insulin resistance, even in patients whose illness is treatment refractory, even though insulin resistance appears to be as clinically significant as type 2 diabetes in patients with bipolar disorder. The newly identified features of glucose metabolic status are associated with these important outcomes with an effect size that is likely to be clinically significant. Reference Uher, Tansey, Malki and Perlis24 Therefore, glucose metabolic status is a measurable factor that may meaningfully contribute to clinical decision-making.
The implications of the present results depend on the representativeness of our sample and role of potential confounders, including antipsychotic medication use and obesity. Our sample was partly derived from a tertiary referral centre and it might represent a sicker population of patients with more severe illness who might have higher rates of type 2 diabetes. However, 37.8% of the patients were followed in the community by either general psychiatrists or family physicians and had higher rates of type 2 diabetes than those in the specialised Mood Disorders Program. Therefore, it is likely that these results will generalise to both primary care and specialist care patients. The age at onset of type 2 diabetes was close to the average age of the sample, likely reflecting a large number of type 2 diabetes diagnoses made at study entry.
In a previous study, we determined that obesity was associated with worse illness trajectory and poor response to lithium. Reference Calkin, Van de Velde, Ruzickova, Slaney, Garnham and Hajek6 We wondered whether poor outcome might be due to underlying insulin resistance, for which obesity is a risk factor. To determine whether poor outcome was better explained by BMI or insulin resistance, we controlled for BMI in our statistical analyses in addition to age and gender and found no significant relationship with course of illness or response to lithium. Insulin resistance and type 2 diabetes were strongly associated with outcome even after controlling for BMI, and this remained the case even when we restricted the analyses to non-obese participants.
Possible contributory factors to impaired glucose metabolism
Although metabolic adverse effects of antipsychotics are well documented in the literature, Reference Yood, DeLorenze, Quesenberry, Oliveria, Tsai and Willey25 antipsychotic use does not explain our results. There was no significant relationship between past use of antipsychotics for at least 6 months and the presence of insulin resistance or type 2 diabetes (P = 0.116). Current use of anti-psychotics was significantly related to insulin resistance or type 2 diabetes (P = 0.044); however, when we controlled for current and lifetime antipsychotic use in addition to age and gender in our statistical analyses, the relationships between glucose metabolic status and clinical outcome changed little and all associations remained significant, with similar effect sizes.
If antipsychotic use does not fully explain the presence of insulin resistance/type 2 diabetes in patients with bipolar disorder and poor response to lithium, what else might be contributing? Many patients with insulin resistance and type 2 diabetes are also obese, potentially affecting drug distribution volume; however, lithium is titrated to therapeutic blood levels and is not fat soluble. Further, obesity was only marginally associated with the course of illness and response to lithium.
One possible explanation is that insulin resistance may have a direct effect on the brain, influencing outcome. Chronic peripheral hyperinsulinaemia is known to downregulate blood-brain barrier insulin receptors, consequently limiting insulin transport into the brain, Reference Wallum, Taborsky, Porte, Figlewicz, Jacobson and Beard26 and creating central insulin resistance that may contribute, via a chronic proinflammatory effect (among other mechanisms) to neurodegeneration and progression of disease. Reference Craft and Watson27 Therefore, impaired glucose metabolism may be a complicating factor affecting the course of illness and may be responsible for the quality of remission, at least in a subset of patients with bipolar disorder.
The effects of insulin resistance/type 2 diabetes on the brain may also account for some of the brain changes seen in patients with bipolar disorder. Our recent magnetic resonance neuroimaging studies are the first to show that some of the neurochemical and neuroanatomical changes found in bipolar disorder may be associated with impaired glucose metabolism. Patients with bipolar disorder and insulin resistance or type 2 diabetes showed lower prefrontal N-acetyl aspartate (NAA) levels compared with euglycaemic patients with bipolar disorder, who had comparable NAA levels to euglycaemic, non-psychiatric controls. Reference Hajek, Calkin, Blagdon, Slaney and Alda28 The NAA levels were positively associated with total creatine (an energy metabolite), suggesting that the NAA changes are related to impaired energy metabolism, a hallmark of insulin resistance and type 2 diabetes. Patients with bipolar disorder and insulin resistance or type 2 diabetes also had significantly smaller hippocampal volumes than euglycaemic patients with bipolar disorder or euglycaemic, non-psychiatric controls. Reference Hajek, Calkin, Blagdon, Slaney, Uher and Alda29 Therefore, the variable neuroimaging results in patients with bipolar disorder reported in the literature may be a result of comorbid impaired glucose metabolism and not bipolar disorder itself, per se.
Implications
It is not surprising that we see bipolar disorder and metabolic disturbance as co-occurring disorders. An emerging stream of evidence suggests that insulin resistance and type 2 diabetes have shared pathophysiological features with bipolar disorder, including hypothalamic–pituitary–adrenal and mitochondrial dysfunction, neuroinflammation, common genetic links and epigenetic interactions. Reference McIntyre, Konarski, Misener and Kennedy3,Reference McIntyre, Danilewitz, Liauw, Kemp, Nguyen and Kahn30–Reference Calkin, Gardner, Ransom and Alda32 Theoretically, targeting insulin resistance in bipolar disorder may yield a new approach to treatment refractory illness, via a more direct mechanism. With the broad availability of insulin-sensitising treatments, impaired glucose metabolism (both insulin resistance and type 2 diabetes) may potentially represent a modifiable factor for the most severe form of bipolar disorder. Clinical observations in specific cases suggest that modification of glucose metabolism may influence the course of bipolar illness; however, systematic evidence for this is lacking. Experimental studies will be required to determine whether normalisation of glucose metabolism will affect course and treatment outcomes among patients with bipolar disorder. We may find that unless we identify and treat underlying insulin resistance in patients with refractory bipolar disorder, these patients may remain unwell. The use of dietary modification, exercise and insulin-sensitising drugs may prove to be effective augmentation strategies for achieving complete remission. Further, early intervention to treat insulin resistance could delay progression to type 2 diabetes in this population at high risk, decreasing morbidity and mortality, psychiatric healthcare costs and also medical costs inherent to the treatment of type 2 diabetes and its complications.
In conclusion, insulin resistance and type 2 diabetes are common among patients with bipolar disorder and are associated with an unfavourable clinical course and poor treatment outcomes. This study is limited by its cross-sectional design. Although our results revealed a significant association, we are not able to draw conclusions regarding a causal relationship between comorbid insulin resistance and type 2 diabetes and outcome in bipolar disorder. Prospective and experimental studies are needed to establish whether glucose metabolism represents a modifiable risk factor for poor outcomes in people with bipolar disorder.







eLetters
No eLetters have been published for this article.