Introduction
Ultrasound guidance has become an important part of performing peripheral nerve blocks within the emergency department. Real-time ultrasound guidance during nerve blockade decreases the risks associated with improper needle placement.Reference Chan 1 , Reference Marhofer, Greher and Kapral 2 Nerve block simulation training before direct patient practice has demonstrated a reduction in procedural length and improvement of outcomes.Reference Niazi, Haldipur and Prasad 3 , Reference Choy and Okrainec 4 However, most low-fidelity nerve block models, although able to simulate the visual appearance of a nerve, are unable to mimic the sonographic findings of the injection of an anesthetic agent. High-fidelity models have demonstrated improved functionality but at significantly increased cost.Reference Kessler, Moriggl and Grau 5
We sought to create an inexpensive model that could be easily reproduced while still allowing for a realistic sonographic appearance of human nerve anatomy, the performance of the nerve block, and the subsequent hydrodissection. The use of pork or lamb to simulate human tissue in nerve block models has been described previously for nerve block models.Reference Koscielniak-Nielsen, Rasmussen and Hesselbjerg 6 , Reference Sparks, Evans and Byars 7 The use of chicken breast to simulate human tissue has been described previously in the use of vascular access models,Reference Rippey, Blanco and Carr 8 but, to our knowledge, there have been no publications related to its use for nerve–block-related teaching.
Methods
The femoral neurovascular bundle was used as a roadmap for construction of the model. A long, tubular balloon (28.5 cm×0.9 cm in dimensions, commonly used for the construction of balloon animals) was filled with water to simulate the femoral vein. For the femoral artery, we put a paper straw (19.5 cm×0.5 cm) inside a water-filled balloon and allowed it to sit for 30 minutes to allow the water to absorb through the paper. This made a compressible, hypoechoic vein and a less compressible artery. The third balloon, representing the femoral nerve, was filled with water and five pieces of regular-thickness flour spaghetti noodles that created a “honeycomb” appearance typical of the sonographic appearance of large nerves, with the pasta representing the nerve itself and the balloon representing the overlying fascia. (Figure 1) All three were then placed between two chicken breasts in their normal anatomic positions. (Figure 2) A syringe filled with vegetable oil and an 18-gauge needle were used for performance of the nerve block. We found that oil proved better than water at staying within the balloon itself to create the appearance of hydrodissection around the nerve.

Figure 1 Deconstructed model showing balloons with internal components. Blue balloon for vein, red balloon and paper straw for artery, yellow balloon and 5 pieces of spaghetti for nerve, one bottle of canola oil, one bottle of water, an 18-gauge needle, 2 syringes (10cc and 50cc), and 2 chicken breasts.

Figure 2 Constructed model oriented to represent the left femoral neurovascular bundle.
Discussion
We found that the model was able to accurately demonstrate femoral neurovascular anatomy and reliably simulated the performance of the nerve block, (Figure 3, 4) from needle visualization (Figure 5) through hydrodissection around the nerve bundle. (Figure 6)The model was able to stand up to multiple attempts, although the balloon structures being cheap and easy to assemble were easily switched out when needed. All of the materials used were available at a local supermarket, and the overall cost was less than fifteen dollars.
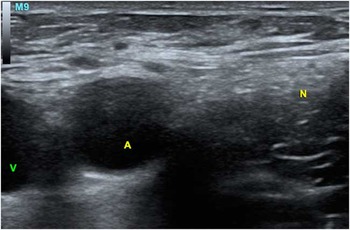
Figure 3 Ultrasound image of human femoral neurovascular bundle. M9=probe marker, V=vein, A=artery, N=nerve.
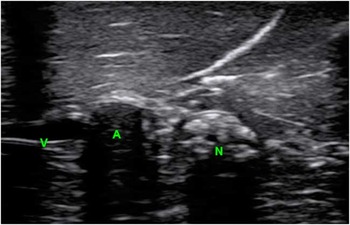
Figure 4 Model under ultrasound. V=vein, A=artery, N=nerve.

Figure 5 Model with needle in long axis showing hydrodissection. V=vein, A=artery, N=nerve, HD=hydrodissection. Needle with reverberation artifact.

Figure 6 Model post injection showing hydrodissection. V=vein, A=artery, N=nerve, HD=hydrodissection.
The limitations of this model include the need to frequently exchange the nerve itself, because, after about three to four successful blockades, we lost the ability to reliably visualize hydrodissection. The use of the paper straw in the artery led to a more realistic lack of compression but also resulted in more shadowing than would be expected in a human artery, which we considered to be an acceptable trade-off. Finally, we did not use any plastic wrap or other barriers to prevent exposure of the machine itself to the bacteria present in raw chicken. Ultrasound-safe antibiotic wipes were considered acceptable from our standpoint for infection control, although this may vary, depending on disinfection methods used at a particular institution.
In conclusion, our chicken–breast-based ultrasound-guided nerve block model was able to simulate both the sonographic appearance of the femoral neurovascular bundle and the ultrasound findings that occur during the injection of anesthetic. Given the affordability of the materials, this model has broad utility, especially in situations where higher fidelity simulators would be cost prohibitive.
Competing interests: None declared.








