Approximately 6.8% of the American population will meet full criteria for posttraumatic stress disorder (PTSD) at some point in their lives (Kessler, Chiu, Demler, & Walters, Reference Kessler, Chiu, Demler and Walters1995); however, experiencing some posttraumatic stress (PTS) symptoms in the acute aftermath of a traumatic event is normative, with the majority of symptoms remitting within 1–3 months (Bryant, Reference Bryant2003). A bias for attending to threat information (i.e., attentional bias to threat [ABT]) is one factor that has been implicated in the maintenance and exacerbation of PTSD. It has been suggested that trauma exposure may result in increased attention towards trauma- and threat-related stimuli for the large majority of trauma-exposed individuals (Aupperle, Melrose, Stein, & Paulus, Reference Aupperle, Melrose, Stein and Paulus2012). Thus, the differentiating factor between those who experience mild PTS symptoms in the acute aftermath of a traumatic event and those who go on to develop more severe, chronic PTS symptomatology may be related to relative deficits in top-down cognitive processes that limit the ability of the individual to disengage attention from trauma- and threat-related stimuli (Aupperle et al., Reference Aupperle, Melrose, Stein and Paulus2012). Because prolonged attentional engagement with perceived threat maintains negative affective states (Bardeen & Read, Reference Bardeen and Read2010; Compton, Reference Compton2000), difficulty disengaging attention from threat stimuli may result in greater demands on cognitive resources, thus leaving fewer resources available for emotional processing of threat-related information and increasing the likelihood that PTS symptoms will develop and/or be maintained.
Among top-down cognitive processes, attentional control (AC) has received considerable attention as a potential regulatory mechanism for reducing trauma-related distress. Research has shown that trauma-exposed individuals with higher levels of AC are better able to attenuate distress associated with retelling their trauma histories (Bardeen & Read, Reference Bardeen and Read2010), and higher levels of AC, measured prior to a traumatic event, predict relatively lower levels of PTS symptoms in the acute aftermath of that event (Bardeen, Fergus, & Orcutt, Reference Bardeen, Fergus and Orcutt2015). The top-down attentional processes of AC consist of the three primary components: (a) the inhibition of dominant, automatic responses; (b) shifting back and forth between multiple task demands; and (c) updating working memory (Eysenck, Derakshan, Santos, & Calvo, Reference Eysenck, Derakshan, Santos and Calvo2007; Miyake, Friedman, Emerson, Witzki, & Howerter, Reference Miyake, Friedman, Emerson, Witzki and Howerter2000). Although emotional distress has been shown to impair two primary functions of AC (i.e., inhibition and shifting; Graydon & Eysenck, Reference Graydon and Eysenck1989; Lavie, Hirst, de Fockert, & Viding, Reference Lavie, Hirst, de Fockert and Viding2004), some evidence indicates that AC can be used to reduce engagement with threat information and decrease distress, even among those with relatively higher levels of PTS symptoms (Bardeen & Orcutt, Reference Bardeen and Orcutt2011), as well as among those with higher levels of anxiety (Derryberry & Read, Reference Derryberry and Reed2002). These individuals may be more likely to recover quicker and/or experience less functional impairment related to their symptoms relative to their counterparts who cannot use AC to effectively regulate their distress.
The reliance on self-report measures of AC is one potential limitation in this line of research. It might be especially difficult for individuals to report on cognitive processes that can occur so rapidly as to go unnoticed on a moment-by-moment basis. Indeed, the modulation of ABT by AC has been evidenced as early as 100 (Peers & Lawrence, Reference Peers and Lawrence2009) and 150 ms (Bardeen & Orcutt, Reference Bardeen and Orcutt2011). Therefore, the use of behavioural measures of the top-down cognitive processes associated with AC will be important in future research for ensuring that the noted modulatory effects are due to one's actual cognitive abilities rather than one's perception of these abilities.
Another potential limitation in this area of research is the use of traditional attentional bias scores calculated from response times on commonly used behavioural measures of attentional bias (e.g., Stroop and dot-probe tasks). The use of such scores may be obscuring potentially important effects in examinations of ABT. Specifically, reaction times are used to calculate ABT as a static signal (i.e., bias toward or away from threat at a constant rate over time). As described by Zvielli, Bernstein, and Koster (Reference Zvielli, Bernstein and Koster2014), this method fails to account for the temporal dynamics of ABT. The traditional static method of calculating ABT may be responsible for (a) the consistently poor reliability of these scores (Schmukle, Reference Schmukle2005), (b) inconsistency across studies in replicating study findings (see Kimble, Frueh, & Marks, Reference Kimble, Frueh and Marks2009), (c) small to moderate effects when information processing biases are observed (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzandoorn, Reference Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg and van IJzandoorn2007), and (d) a general lack of clarity regarding whether ABT represents a form of faster threat detection or difficulty in disengaging from threat stimuli (Weierich, Treat, & Hollingworth, Reference Weierich, Treat and Hollingworth2008). Methods for calculating attention bias variability (ABV; i.e., within-subject variability of ABT, both toward and away from threat stimuli) have been put forth to remedy these issues.
Recently, Zvielli et al. (Reference Zvielli, Bernstein and Koster2014) used a method of calculating ABV that accounts for the temporal dynamics of ABT (i.e., a rapid succession of shifts toward and away from threat stimuli). As predicted, greater ABV was observed for spider phobics compared to control participants. This effect was statistically significant even after accounting for the effects of traditional attentional bias scores and variability observed on trials for which only neutral stimuli were present. Results were consistent with the hypothesis that the same participants exhibit attentional biases both toward and away from threat, thus supporting the use of methods for examining attentional bias that account for these temporal dynamics. Moreover, in contrast to the notoriously low level of reliability observed for traditional attentional bias scores, moderately sized significant correlations were observed for the split-half reliabilities of these ABV scores.
Given that hypervigilance for, and avoidance of, trauma-related stimuli are central to the symptom profile of PSTD, ABV may be particularly relevant for examining PTS-related ABT (Iacoviello et al., Reference Iacoviello, Wu, Abend, Murrough, Feder, Fruchter and . . .Charney2014). For example, using a dot-probe task with word stimuli and a stimulus presentation duration of 500 ms, Iacoviello et al. (Reference Iacoviello, Wu, Abend, Murrough, Feder, Fruchter and . . .Charney2014) found that participants with PTSD exhibited significantly greater ABV than both trauma control and healthy control participants. Iacoviello et al.’s (Reference Iacoviello, Wu, Abend, Murrough, Feder, Fruchter and . . .Charney2014) findings provide support for a general attentional dyscontrol among individuals with PTSD. However, as noted by Iacoviello et al. (Reference Iacoviello, Wu, Abend, Murrough, Feder, Fruchter and . . .Charney2014), it remains unclear whether this dyscontrol is general in nature or specific to threat stimuli, as they did not control for variability in responding in the absence of threat stimuli.
Another factor that should be considered in examinations of PTS-related ABT is threat saliency (i.e., the degree to which threat stimuli are fear inducing). A number of factors have been posited to influence threat saliency, including stimulus presentation duration, the saliency or intensity of threat (stimulus valence), and competition with other stimuli for processing resources (Bishop, Reference Bishop2008). At shorter presentation durations, threat stimuli may have less threat saliency because perceptual load is higher; participants have to use more cognitive resources to respond to task-relevant information. In contrast, threat saliency should be higher at relatively longer presentation durations for which participants have more time, and thus, more available resources to attend to task-irrelevant threat stimuli. As such, we would expect ABV to be greater, especially among participants with higher levels of PTS symptoms, at longer presentation durations.
The present study sought to explicate the time-course of PTS-related ABT by examining ABV using a dot-probe task and four presentation durations representing both early (i.e., subliminal, orienting) and later stages of information processing. We predicted that participants with PTSD versus trauma-exposed participants without PTSD would exhibit greater ABV to threat stimuli when threat salience was higher (i.e., at later stages of information processing). Importantly, we (a) accounted for variability on trials for which only neutral stimuli were present to ensure that the observed effects were specific to the presence of threat stimuli and not merely a function of general variability in response times, and (b) included traditionally computed attentional bias scores in our primary analyses to examine the degree to which ABV provides incremental utility, above and beyond the effects of a static measure, in examinations of ABT. As described, evidence suggests that self-reported AC can be used to reduce preferential processing of threat stimuli, even among those with relatively higher levels of PTS symptoms (Bardeen & Orcutt, Reference Bardeen and Orcutt2011). Given the noted limitations of measuring AC via self-report, we used a behavioural measure of the three primary components of AC (i.e., inhibition, switching, and updating working memory) to examine AC as a moderator of the hypothesised relation between PTSD and ABV at later stages of information processing. We predicted that relative deficits in AC would exacerbate ABV among participants with PTSD. That is, participants with PTSD and relatively worse AC abilities would exhibit significantly greater ABV than trauma control participants with relatively worse AC abilities.
Method
Participants
Participants for the current study included 29 (20 women) trauma-exposed adults (PTSD = 11, trauma control = 18) residing in a large urban area in the southern United States. Participants ranged in age from 18 to 64 years (M = 35.4, SD = 13.1) and 51.7% self-identified as African American, 41.4% as White, 3.4% as American Indian or Alaskan Native, and 3.4% endorsed ‘other’. Additionally, 3.4% of the sample reported being of Hispanic ethnicity. With regard to educational attainment, 93.2% of participants had received their high school diploma or GED, with 83.8% reporting the completion of at least some higher education. The majority of participants were single (68.8%), with a household income of less than $30,000 (55.1%), and were either currently unemployed (41.4%) or full-time students (17.2%).
Equipment
Participants completed self-report measures and stimulus-response tasks (i.e., dot-probe, task switching) on a 17-inch Dell Inspiron laptop computer (60 Hz). Participants were seated approximately 60 cm from the computer monitor. A computer keyboard was used to respond to the tasks. Qualtrics (http://www.qualtrics.com/) was used to present self-report measures and E-prime 2.0 software (Psychology Software Tools, Pittsburgh, PA) was used to present stimuli and record responses during the dot-probe task. The attentional control task was presented and scored via an executive functioning assessment website (http://www.Wiltonlogic.com/).
Dot-Probe Task
Pictorial stimuli were used because word stimuli require greater semantic processing (Pineles, Shipherd, Mostoufi, Abramovitz, & Yovel, Reference Pineles, Shipherd, Mostoufi, Abramovitz and Yovel2009) and are prone to greater subjective familiarity and frequency of use (Bradley et al., Reference Bradley, Mogg, Millar, Bonham-Carter, Fergusson and Parr1997). Forty general threat (e.g., man with gun, poisonous snake, plane crash) and 80 neutral images (e.g., ceiling fan, umbrella, mushroom caps) were presented twice over the course of the task (International Affective Picture System, IAPS; Lang, Bradley, & Cuthbert, Reference Lang, Bradley and Cuthbert1999). General threat (negative valence and high arousal) and neutral (neither negative nor positive valence and low arousal) stimuli were identified based on ratings of valence (M = 2.17 and 5.12, respectively) and arousal (M = 6.52 and 2.96, respectively; IAPS; Lang et al., Reference Lang, Bradley and Cuthbert1999).
Participants were provided with a standard set of instructions prior to starting the dot-probe task. Each trial started with a fixation cross presented in the centre of the screen for 1000 ms. Next, two images appeared side by side on the screen (i.e., neutral-neutral or threat-neutral) for one of four stimulus presentation durations (i.e., 15 ms, 85 ms, 150 ms, 500 ms). Next, a dot appeared on the screen in place of one of the images. Participants used one of two arrow keys to indicate the relative position of the dot on the screen. Participants completed 10 practice trials and then a continuous block of 120 trials (40 neutral-neutral and 80 neutral-threat stimulus pairs). Neutral-neutral image pairs were used to (a) reduce expectations that a threat image would be seen in each trial, and (b) to calculate neutral response time variability parameters to be used for control variables.
Four presentation durations were chosen to provide temporal snapshots of points on the continuum from early (i.e., subliminal processing, reflexive orienting) to later stage threat processing. The presentation duration of 500 ms provides ample time for one to shift attention multiple times (Mogg & Bradley, Reference Mogg and Bradley1998). In addition, 150 ms was used because this presentation duration provides enough time for top-down executive processes to influence preferential processing (e.g., Bardeen & Orcutt, Reference Bardeen and Orcutt2011). ERP and fMRI research suggest that attentional orienting occurs in the range of 70–100 ms (Boehler, Schoenfeld, Heinze, & Hopf, Reference Boehler, Schoenfeld, Heinze and Hopf2008; Heinze et al., Reference Heinze, Mangun, Burchet, Hinrichs, Scholz, Munte and Hillyard1994; Hopfinger, Luck, & Hillyard, Reference Hopfinger, Luck, Hillyard and Gazzaniga2004; Martinez et al., Reference Martinez, DiRusso, Anllo-Vento, Sereno, Buxton and Hillyard2001); thus, we used a presentation duration of 85 ms to capture initial orienting of attention. A subliminal presentation duration was identified based on the work of Bardeen (Reference Bardeen2015). Specifically, Bardeen (Reference Bardeen2015) used a discrimination task to determine an objective threshold (i.e., the threshold at which a stimulus cannot be identified at better than chance levels; Snodgrass & Shevrin, Reference Snodgrass and Shevrin2006). In that pilot study, 10 neutral-threat image pairings and 10 neutral-neutral image pairings (IAPS image pairings used in the present study) were presented to 10 participants in eight blocks for each of eight evenly spaced durations (i.e., 5–40 ms) and participants had to make a forced-choice decision as to whether the image pairing that was previously presented contained neutral or threat information (Snodgrass & Shevrin, Reference Snodgrass and Shevrin2006). Mean percentage accuracy was calculated for each presentation duration (Williams et al., Reference Williams, Liddell, Rathjen, Brown, Gray, Phillips and . . .Gordon2004). Results of eight one-sample chi-square tests (for each presentation duration) indicated that performance did not differ significantly from chance at presentation durations of 10 ms and 15 ms, but presentation durations of 20 ms and above provided participants with enough time to identify the content of the IAPS images at greater than chance levels. Thus, the 15 ms presentation duration was used to capture a pre-attentive stage of information processing in which one is not consciously aware of the stimuli being viewed (Luecken, Tartaro, & Applehans, Reference Luecken, Tartaro and Appelhans2004). All presentation conditions were presented for an equal number of trials; the order of conditions was randomised across participants (i.e., timing, image type). Image valence and arousal ratings were balanced across timing conditions.
Attentional Control
A computer-based assessment of AC, based on the work of Monsell (Reference Monsell2003), was used to assess ability to flexibly shift attention between task demands. For each trial, one of four stimuli (i.e., white circle, black circle, white square, black square) were displayed in the centre of the computer screen (see Figure 1). These stimuli appeared inside of a larger circle that had four equally sized outer segments. Participants were instructed to classify each stimuli based on either colour (black/white) or shape (circle/square), depending on a specific rule for each trial. The rule was indicated by the segment of the circle that was highlighted. For instance, if either of the top two segments was highlighted, the rule was to classify based on shape. If either of the bottom two segments were highlighted, the rule was to classify based on colour. The highlighted segment moved in a clockwise fashion (i.e., top right, top left, bottom left, bottom right). Participants were instructed to press one of two keys that corresponded to colour and shape, respectively. Reaction times and the number of errors were recorded. Failure to respond within 2 seconds resulted in moving on to the next trial. These trials were omitted from final score calculations. Participants completed 32 practice trials and then a continuous block of 84 trials. Switch cost ability, or the amount of disruption produced when the rule is changed, is calculated as the difference between trials for which the rule remains the same (42 trials) and trials for which the rule switches (42 trials). To efficiently switch between task demands one must inhibit the previous task-set rule and shift to the new rule while maintaining these rules in working memory (Koch, Gade, Schuch, & Philipp, Reference Koch, Gade, Schuch and Philipp2010; Monsell, Reference Monsell2003). As such, this computerised task measures an aggregate of the three primary components of AC (i.e., inhibition of dominant repsonse tendencies, shifting between task demands, and updating of working memory; Eysenck et al., Reference Eysenck, Derakshan, Santos and Calvo2007).

FIGURE 1 Attentional control task.
Self-Report and Interview Measures
Screening measures
The Structured Clinical Interview for DSM-IV (APA, 2000) Axis I disorders (SCID-I; First, Spitzer, Gibbon, & Williams, Reference First, Spitzer, Gibbon and Williams1996) is a commonly used structured interview for assessing Axis I psychopathology. The trauma screening questions from the PTSD module were used in the present study to ensure that participants met the eligibility requirement of having experienced a DSM-IV defined potentially traumatic event. In addition, the psychosis screener from the SCID-I was used to ensure that participants did not currently have a psychotic disorder. Finally, the Mini Mental Status Exam (MMSE) was used to ensure that participants were not cognitively impaired (a score of ≥24 on the MMSE; Folstein, Folstein, & McHugh, Reference Folstein, Folstein and McHugh1975).
Life Events Checklists (LEC)
The LEC is a psychometrically sound self-report measure that assesses lifetime exposure to potentially traumatic events (Blake et al., Reference Blake, Weathers, Nagy, Kaloupek, Klauminzer, Charney and Keane1990; Gray, Litz, Hsu, & Lombardo, Reference Gray, Litz, Hsu and Lombardo2004). Participants are provided with a list of 17 potentially traumatic events (e.g., sexual assault, motor vehicle accident, physical assault). For each event, respondents are asked to indicate whether the event happened to them, or they witnessed it, or they learned about it. From the events reported, participants are asked to identify the one event that currently bothers them the most and reference this event during the PTSD Symptom Scale Interview (Foa, Riggs, Dancu, & Rothbaum, Reference Foa, Riggs, Dancu and Rothbaum1993).
PTSD Symptom Scale Interview (PSS-I)
The PSS-I (Foa et al., Reference Foa, Riggs, Dancu and Rothbaum1993) is a semi-structured interview designed to assess the frequency and intensity of the 17 DSM-IV PTSD symptoms (i.e., Criteria B, C, and D). The clinical interviewer rates the frequency and/or severity of each symptom in the past month (0 = not at all to 3 = 5 or more times per week/very much) in relation to the potentially traumatic event that the participant identified as most distressing on the LEC. PTSD diagnosis was determined by counting the number of symptoms endorsed in each symptom cluster. Specifically, as per the PSS-I Manual, a rating ≥1 on any item indicated the presence of that particular symptom. The PSS-I has demonstrated adequate psychometric properties, including internal consistency, interviewer–rater reliability, and convergent and discriminant validity (Foa & Tolin, Reference Foa and Tolin2000).
Procedure
All methods were approved by the institution's Institutional Review Board. Participants were recruited via flyers posted in public areas of health care facilities (e.g., local hospitals, mental health clinics), as well as in other public areas (e.g., local coffee shops, grocery stores). Flyers targeted individuals who had ‘experienced one or more stressful events’. To be eligible, participants were required to: (1) be between the ages of 18–64, (2) show no evidence of cognitive impairment (a score of ≥24 on the MMSE; Folstein et al., Reference Folstein, Folstein and McHugh1975), (3) have no current psychotic disorder (as determined by the psychosis screener from the SCID-I), (4) have no visual impairment, and (5) be right-hand dominant (Rapp et al., Reference Rapp, Mutschler, Wild, Erb, Lengsfeld, Saur and Grodd2010; Razumnikova & Volf, Reference Razumnikova and Volf2011). In addition, participants had to report experiencing at least one potentially traumatic event as defined by Criterion A1 of the criteria for PTSD in the DSM-IV-TR (APA, 2000). To be included in the PTSD group, participants had to endorse the presence of one re-experiencing symptom, three avoidance symptoms, and two arousal symptoms. Participants were also assessed by the clinical interviewer (the first author, a doctoral-level clinical psychologist) to determine whether participants met Criterion E (i.e., symptoms lasting for at least one month) and Criterion F for a diagnosis of PTSD (i.e., clinically significant distress and/or impairment). Individuals in the PTSD group met DSM-IV criteria for a diagnosis of PTSD, while those in the trauma control group did not. Following the provision of informed consent, participants spent the remainder of the session completing self-report and interview measures and several computer tasks. Before leaving, participants were debriefed and given a gift card worth $20 to a local store for their participation.
Results
Preparation of Stimulus-Response Data
Trials with error responses were discarded (0.49% of trials). To reduce the effect of anticipatory responding and outliers, response times less than 200 ms or greater than 1500 ms (.52% of trials) were discarded (Salemink, van den Hout, & Kindt, Reference Salemink, van den Hout and Kindt2007). In addition, response times greater than three standard deviations above a participant's mean (2.61% of trials) were considered extreme outliers, and were thus replaced with a number three standard deviations above the participant's mean response time (Ratcliff, Reference Ratcliff1993). Approximately 3.6% of all responses were either incorrect or fell outside of the above timing guidelines.
A static total attentional bias score was calculated by subtracting mean latencies on trials where the probe replaced a threat image from mean latencies on trials where the probe replaced a neutral image in neutral-threat pairings (Frewen, Dozois, Joanisse, & Neufeld, Reference Frewen, Dozois, Joanisse and Neufeld2008; MacLeod, Mathews, & Tata, Reference MacLeod, Mathews and Tata1986). Negative scores indicated attention to neutral simuli and positive scores indicated attention to threat stimuli. Next, four ABV scores were calculated using 20 trials (10 congruent and 10 incongruent) for each of the four presentation durations. For each of the scores, response times (RTs) of temporally contiguous (+/- 5 trials) congruent trials (neutral-threat trials where the probe replaced the threat image) were subtracted from incongruent trails (neutral-threat trials where the probe replaced the neutral image; Zvielli et al., Reference Zvielli, Bernstein and Koster2014). More specifically, for each participant, incongruent trials were paired with the nearest congruent trial of the same presentation duration; if the nearest congruent trial fell beyond five trials, that incongruent trial was omitted and no ABV score was computed for that trial. Approximately 2.8% of responses were omitted based on Zvielli et al.’s (Reference Zvielli, Bernstein and Koster2014) five-trial threshold. If the nearest congruent trial fell within five trials, the response time of the congruent trial was subtracted by the response time of the incongruent trial. This method was repeated to pair congurent trials with temporally contiguous incongruent trials. ABV was calculated by finding the absolute distance between these scores, summing all of the resultant values, and dividing this sum by the total number of scores. Accordingly, participants whose scores did not change throughout the task had relatively low ABV, but participants whose scores fluctuated substantially throughout the task had relatively high ABV. As a control parameter for use in regression analysis, the absolute difference between temporally contiguous response times on neutral-neutral trials was calculated in the same manner as described above.
Descriptive Statistics and Zero-Order Correlations
Analysis of variance (ANOVA) or chi-square procedures for continuous or categorical variables were used to examine between group (PTSD, trauma control) differences on demographic variables (i.e., age, sex, and race/ethnicity). Race and ethnicity were collapsed into a single dummy coded variable, coded as Hispanic and/or non-White (n = 19, 65.5%) versus non-Hispanic White (n = 10, 34.5%). No significant differences were found between the groups on age, F(1, 27) = 1.40, p = .25, gender, χ2 = 1.37, p = .24, and race/ethnicity, χ2 = 2.09, p = .15. As such, these demographic variables were not included in subsequent analyses.
Descriptive statistics and zero-order correlations for primary variables of interest are presented in Table 1. Of the four ABV scores, ABV 500 ms was the only one that was significantly associated with PTSD status (r = .42, p < .05). The relation was such that individuals with PTSD, versus without, had significantly greater ABV at 500 ms. Additionally, the ABV scores for all of the supraliminal presentation durations (i.e., 85 ms, 150 ms, 500 ms) were significantly associated with AC (rs = .47, .42, .47, respectively, ps < .05). That is, relatively worse AC (i.e., greater scores indicate worse AC) was associated with greater ABV at these presentation durations. As expected, all of the ABV scores were significantly associated (ps < .01). Of note, none of the static attentional bias scores (i.e., 15 ms, 85 ms, 150 ms, and 500 ms) was significantly correlated with PTSD status (rs from .02 to .28, ps from .14 to .91) and AC (rs from .08 to -.27, ps from .16 to .68).
TABLE 1 Descriptive Statistics and Correlations for Study Variables

Note: N = 29. ABV = attention bias variability; PTSD = posttraumatic stress disorder status (0 = trauma control, 1 = PTSD).
* p < .05. **p < .01. ***p < .001.
Participants reported experiencing an average of 8.86 (SD = 4.90, range = 2–16) potentially traumatic events. There was not a significant difference in the total number of potentially traumatic events endorsed between those in the PTSD and trauma control groups, t(27) = 0.04, p = .97. The only statistically significant difference between groups in the types of potentially traumatic events reported was that individuals in the PTSD group reported higher incidence of rape, t(27) = -2.15, p = .04; n = 5 (45.5%) PTSD group, n = 2 (11.1%) control group. In addition, participants in the PTSD group reported significantly more PTSD symptoms than those in the trauma control group, M = 12.27 (SD = 2.76), M = 2.94 (SD = 2.26), respectively, t(27) = -9.44, p < .001. Participant endorsement of the most distressing potentially traumatic event experienced is presented in Table 2.
TABLE 2 Frequency of Most Distressing Events
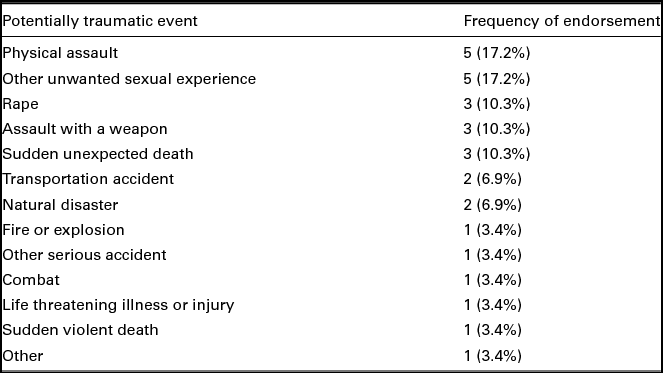
Note: N = 29. Participant endorsement of the most distressing potentially traumatic event that they have experienced in their lifetime.
Regression Analyses
Four hierarchical regressions were conducted to test the hypothesised interaction effects. Consistent with Aiken and West (Reference Aiken and West1991), the covariates (i.e., static attentional bias score and neutral ABV for each presentation duration) and the predictor variables of interest (PTSD status and AC) were mean-centred and entered into the first step of each model. An interaction term, calculated as the product of the two centred predictor variables (PTSD status and AC), was entered into the second step of each model. Each of the ABV scores (15 ms, 85 ms, 150 ms, and 500 ms) served as outcome variables in their respective models. Significant interaction effects were further examined via simple slopes analysis (Aiken & West, Reference Aiken and West1991). Simple slopes analysis consists of constructing two simple regression equations in which the relationship between the predictor variable (i.e., PTSD status) and the outcome variable is tested at both high (+1 SD) and low (-1 SD) levels of the moderating variable (i.e., AC).
Results for each of the four regressions are presented in Table 3. None of the predictor variables significantly predicted ABV 15 ms. The only variable that significantly predicted both ABV 85 ms and ABV 150 ms was AC (ps < .05), with relatively worse AC predicting greater ABV at these presentation durations. In the fourth and final model, both PTSD status and AC predicted ABV 500 ms (ps < .05). Relatively worse AC and PTSD, versus trauma control, predicted greater ABV at this presentation duration. In the second step of the model, the interaction term significantly predicted ABV 500 ms (p < .05). Simple slopes analysis revealed a significant positive association between PTSD status (coded as 0 for trauma control and 1 for PTSD) and ABV 500 ms for participants with relatively worse (β = .74, p < .01), but not better (β = .01, p = .97), AC (see Figure 2). The interaction effect was medium to large in size (Cohen's f2 = .20; Aiken & West, Reference Aiken and West1991).
TABLE 3 Regression Analyses With Attention Bias Variability (ABV) at Each Presentation Duration as Outcome Variables
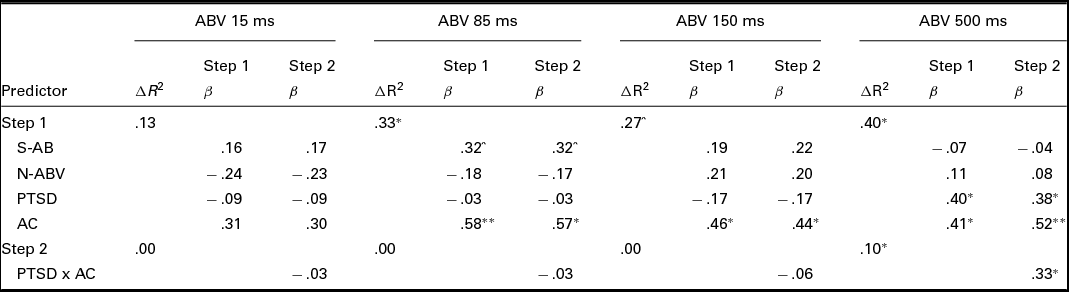
Note: N = 29. S-AB = static attentional bias score; N-ABV = neutral ABV; PSTD = posttraumatic stress disorder status (0 = trauma control, 1 = PTSD); AC = attentional control.
^p < .10. *p < .05. **p < .01.
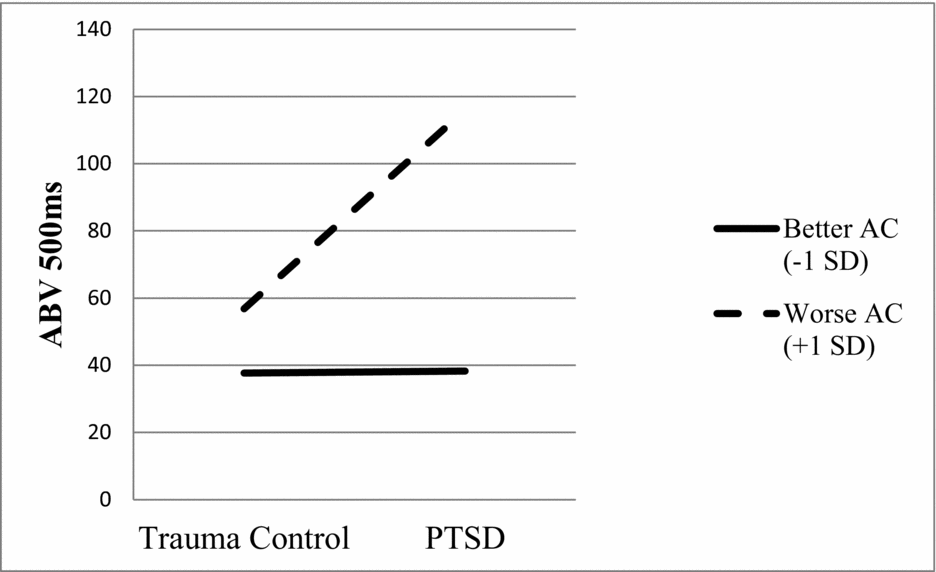
FIGURE 2 The interaction effect (PTSD by attentional control [AC]) was a significant predictor of attention bias variability at 500 ms (ABV 500ms, β = .33, p < .05).
Discussion
As predicted, participants with PTSD, versus trauma control participants, exhibited greater ABV to threat stimuli. This finding is consistent with previous research indicating a general attention-regulation impairment in PTSD (Aupperle et al., Reference Aupperle, Melrose, Stein and Paulus2012), as well as greater ABV in the presence of threat stimuli (Iacoviello et al., Reference Iacoviello, Wu, Abend, Murrough, Feder, Fruchter and . . .Charney2014). Importantly, by accounting for variability on trials for which only neutral stimuli were presented, we were able to ensure that PTSD-related ABV was not merely a byproduct of intra-individual differences in response time variability, but showed specificity for threat-related information. Further, the present results suggest that PTSD-related ABV occurs at relatively later stages of supraliminal threat processing; differences in ABV were not observed at subliminal and early supraliminal presentation durations (i.e., 15, 85, and 150 ms), but at a later supraliminal stage of processing (i.e., 500 ms). As suggested by Aupperle et al. (Reference Aupperle, Melrose, Stein and Paulus2012), threat-related stimuli may take on higher stimulus value among the majority of trauma-exposed individuals, and thus, differences in more automatic threat processing between those with and without PTSD may not be observed. Instead, the present results suggest that threat-related attentional dyscontrol (rapid fluctuations between attending toward and away from threat stimuli) may be a function of a combination of increased stimulus value for perceived threat and cognitive impairments in AC processes (inhibition, shifting, and updating working memory). Results of the moderation analysis support this proposition, as greater ABV was only observed among participants with PTSD who also had relative deficits in our behavioural measure of AC.
As described, difficulty attending to goal relevant pursuits in the presence of perceived threat may have detrimental effects on one's ability to cope with the rigours of daily living and may maintain negative affective states and PTS symptomatology. As such, individuals with PTSD and relative deficits in AC may experience a more chronic course of PTS symptoms and greater functional impairment than individuals with PTSD and relatively better AC. Individuals with PTSD and better AC may also fare better in exposure therapy by more effectively regulating negative affective states during treatment sessions. These individuals may be more likely to continue attending exposure sessions rather than exhibiting escape and avoidance behaviours. In the future, prospective research and treatment efficacy studies will be important in testing these hypotheses.
Findings from this present preliminary study suggest that PTSD-related ABV is not limited solely to trauma-specific stimuli, as threat stimuli were general rather than trauma-specific. This distinction is important because it suggests that the cognitive and behavioural phenomena associated with PTSD may be evoked by stimuli that are not directly related to one's traumatic experience, thus increasing distress, maintaining PTSD symptoms, and decreasing functioning across contexts. This is consistent with the fear generalisation that is observed in clinical settings. The present findings suggest the possibility that threat stimuli that are seemingly unrelated to one's traumatic experience may elicit activation of the trauma-related fear network; thus, specificity of the stimuli used during exposure therapy may be of less importance in the therapeutic context than previously thought (Foa & Kozak, Reference Foa and Kozak1986).
Also of note, traditional static attentional bias scores were not significantly associated with PTSD status and AC. Moreover, associations between our variables of interest remained significant when accounting for these traditional static scores. These findings are not particularly surprising given the noted psychometric problems related to these scores. However, these findings do highlight the importance of using methods of assessing ABT that account for the temporal dynamics of this phenomena. Use of the traditional static method of computing attentional bias scores may obscure potentially important results, as would be the case in the present study had we not assessed ABV.
Study limitations must be acknowledged. First, the relatively small sample size increases the risk for both Type I and Type II error; thus, these findings should be considered preliminary and in need of further investigation and replication in future research. Although the limitations of retrospective power analysis have been well documented (e.g., Faul, Erdfelder, Lang, & Buchner, Reference Faul, Erdfelder, Lang and Buchner2007), providing such information may be helpful for informing future research in this area. Based on data from the present study, power analysis indicates that a sample size >34 will provide sufficient power (i.e., 0.80) to detect the interaction effect of primary interest when alpha is set at .05. It is also important to note that several of the symptoms of PTSD have been reconfigured and new symptoms have been added to the definition of PTSD in the most recent iteration of the DSM (i.e., Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [DSM-5]; APA, 2013). As such, it will be important to examine hypotheses from the present study in the context of the updated symptom profile outlined in the DSM-5 in future research. Further, future research should: (a) include participants without a history of trauma exposure, (b) provide more information regarding trauma exposure (e.g., time since trauma), and (c) account for psychopathology that commonly co-occurs with PTSD (e.g., comparing individuals with PTSD to individuals with PTSD and a co-occurring substance use disorder). An examination of a broader range of psychopathology will provide an understanding of the degree to which the pattern of threat-related ABV observed in the present study is specific to PTSD, or is exhibited in anxiety pathology more generally.
Because participants did not rate the images that were used in the study, the degree to which these stimuli were unrelated to the individual traumatic experiences is unknown. Given that we sought to use general threat stimuli, it would be methodologically ideal to choose images that were completely unrelated to one's most distressing event. However, given that participants had experienced multiple traumatic events on average, this methodology would have been extremely difficult to implement. In addition, the results of the study would have likely been confounded had we asked participants to rate stimuli prior to completing the dot-probe task. That is, repeated exposure to the stimuli may have resulted in reduced image-related arousal during the dot-probe task. Therefore, we identified negatively valenced and highly arousing threat images of a wide variety that had been pretested elsewhere (IAPS; Lang et al., Reference Lang, Bradley and Cuthbert1999). IAPS images, such as those in the present study, have been used to measure PTS-related attentional bias (Bardeen & Orcutt, Reference Bardeen and Orcutt2011) and have been shown to produce negative affective states in multiple studies (e.g., Erk et al., Reference Erk, Kiefer, Grothe, Wunderlich, Spitzer and Walter2003; Pretz, Totz, & Kaufman, Reference Pretz, Totz and Kaufman2010). We intended to reduce trauma-specific responding by choosing threat images with a variety of themes.
Although our use of a computer monitor with a frame rate of 60 Hz is not uncommon in similar studies in the extant literature, it is important to note that timing precision regarding stimulus presentation duration can be increased by using computer monitors with a higher frame rate (e.g., 120 Hz). Additionally, although some evidence suggests that the number of dot-probe trials may be far less influential in determining stability of bias scores than previously thought, with relatively few trials being considered adequate when other design features are accounted for (Price et al., Reference Price, Kuckertz, Siegle, Ladouceur, Silk and Amir2015), the use of more than 20 trials per score would be preferable for alleviating concerns regarding score stability. As such, we recommend reducing the number of duration conditions used in future studies in favour of increasing the number of trials used to calculate ABV scores. We also suggest using equivalent numbers of threat and neutral stimuli in future studies to ensure that stimulus frequency does not influence study results. Finally, it may be important in future studies to disentangle relations among the variables of interest and negative valence and high arousal. The observed effects may be specific to one of these factors, or may be the result of an aggregate of the two.
Despite its limitations, the present preliminary findings suggest AC as a buffering mechanism against threat-related attentional dyscontrol. Importantly, AC abilities can be significantly improved through clinical intervention (Jha, Krompinger, & Baime, Reference Jha, Krompinger and Baime2007) and mindfulness training techniques (Bherer et al., Reference Bherer, Kramer, Peterson, Colcombe, Erickson and Becic2008; Zylowska et al., Reference Zylowska, Ackerman, Yang, Futrell, Horton, Hale, Pataki and Smally2008). Preliminary evidence has shown some support for the effectiveness of attention-training programs in treating multiple forms of anxiety pathology (for a review, see MacLeod & Clarke, Reference MacLeod and Clarke2015). Many of these training programs are designed to train attention away from threat. Given evidence from the present study, as well as previous studies (Iacoviello et al., Reference Iacoviello, Wu, Abend, Murrough, Feder, Fruchter and . . .Charney2014), that individuals with PTSD exhibit attentional dyscontrol in the presence of perceived threat (rapid fluctuations in attention both toward and away from threat), larger treatment effects may be observed if attention-training programs for PTSD are designed to reduce threat-related attentional dyscontrol by balancing these fluctuations rather than training individuals to either attend toward or away from threat stimuli (e.g., Badura-Brack et al., Reference Badura-Brack, Naim, Ryan, Levy, Abend, Khanna and Bar-Haim2015). Treatment techniques that target AC may be beneficial in reducing this threat-related attentional dyscontrol, thus potentially enhancing the effects of empirically supported treatments for PTSD.
Declaration of interest
Dr Evenden is the founder of WiltonLogic, a company that develops and markets computerised neuropsychological assessment measures. The attentional control task used in the present study was developed by Dr Evenden and presented via WiltonLogic's website.







