Postpartum depression (PPD) is a disorder characterised by symptoms of major depression occurring after delivery.Reference Gelaye, Rondon, Araya and Williams1 The DSM-5 uses a peripartum specifier for affective symptoms occurring within 4 weeks after childbirth,2 but longer time frames of up to 1 year postpartum are also used in clinical and research settings.Reference Fonseca, Ganho-Avila, Lambregtse-van den Berg, Lupattelli, Rodriguez-Munoz and Ferreira3–5 This differentiation in terms of onset timing may account for the considerable phenomenological heterogeneity of PPD, which is increasingly gaining attention.6 Additionally prevalence rates of PPD vary worldwide with estimates ranging between 10 and 25%.Reference Gelaye, Rondon, Araya and Williams1 These rates suggest that an epidemiologically relevant proportion of women in the peripartum phase suffer from PPD. Therefore, PPD introduces globally a major public health problem with adverse sequelae for maternal and offspring well-being.Reference Srinivasan, Pearson, Johnson, Lewis and Lewis7–Reference Netsi, Pearson, Murray, Cooper, Craske and Stein9 Specifically, PPD has been associated with maternal and familiar distress,Reference Gelaye, Rondon, Araya and Williams1 suicidal risk, impaired child development and behaviour outcomes.Reference Netsi, Pearson, Murray, Cooper, Craske and Stein9,Reference Duan, Wang, Tao, Bower, Yu and Wang10 Awareness regarding screening, detecting and treating PPD has increased in past years.Reference O'Connor, Rossom, Henninger, Groom and Burda11,Reference Kuehn12 Nevertheless, the understanding of related mechanisms and associated risk factors remains poor.Reference Gelaye, Rondon, Araya and Williams1,Reference Fonseca, Ganho-Avila, Lambregtse-van den Berg, Lupattelli, Rodriguez-Munoz and Ferreira3 Across risk factors of PPD, research has yielded evidence for several predictors. A substantial body of literature has contributed data on various types of PPD risk factors mainly using case–control and cohort design studies,Reference Hutchens and Kearney13 which are the most appropriate type of study to answer such epidemiological questions.Reference Frieden14 History of affective disorders and life stress are among the early identified major risk factors for PPD.Reference Beck15 Moreover, consistent data suggests higher risk of PPD in women having experienced violence.Reference Alvarez-Segura, Garcia-Esteve, Torres, Plaza, Imaz and Hermida-Barros16,Reference Zhang, Wang, Yang, Chen, Qiu and Wang17 Different types of violence, such as child and adult physical, sexual and psychological abuse, have been strongly associated with PPD symptoms.Reference Gelaye, Rondon, Araya and Williams1 Further, lower socioeconomic status and social resources have been also investigated in the context of PPD, implying a clear role for lack of social support when facing obstetrical complications.Reference Dibaba, Fantahun and Hindin18,Reference Azad, Fahmi, Shrestha, Joshi, Hasan and Khan19 The effects of complications during pregnancy and delivery have been addressed in an interdisciplinary fashion. Specifically, the effects of conditions such as gestational diabetes (GDM), preeclampsia, vitamin D deficiency and anaemia, as well as exercise and dietary habits, on the prevalence of affective symptoms in the peripartum have been investigated.Reference Nakamura, van der Waerden, Melchior, Bolze, El-Khoury and Pryor20–Reference Teo, Chia, Colega, Chen, Fok and Pang24 Parallel to complications during pregnancy, aggravating or protective factors related to delivery, such as preterm birth, use of labour analgesia and Caesarean section, have also been assessed.Reference de Paula Eduardo, de Rezende, Menezes and Del-Ben25–Reference Kountanis, Vahabzadeh, Bauer, Muzik, Cassidy and Aman27 Although data are increasingly available, methodological controversies introduce limited generalisability, and hamper a global understanding of risk factors and a pragmatic risk quantification.Reference Hutchens and Kearney13,Reference Solmi, Correll, Carvalho and Ioannidis28 Moreover, as quality indicators directly affect the certainty of risk estimates, formal syntheses of the credibility of the exponentially emerging fragmented meta-analyses are required.Reference Solmi, Correll, Carvalho and Ioannidis28 This umbrella review aimed to identify, quantify and measure the degree of credibility of the association of PPD with different risk factors, including peripheral markers, obstetric complications and psychological factors.
Method
We used the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines to guide this umbrella review (Supplementary Table 1, available at https://doi.org/10.1192/bjp.2021.222).Reference Stroup, Berlin, Morton, Olkin, Williamson and Rennie29
The review protocol was registered with International Prospective Register of Systematic Reviews (PROSPERO; registration number CRD42020168468).
Study design
We summarised the evidence from multiple research syntheses, performing an umbrella review,Reference Solmi, Correll, Carvalho and Ioannidis28 which is a form of review of previously conducted systematic reviews and meta-analyses. It consists in repeating the meta-analyses adopting a uniform approach for available factors to enable their comparison.Reference Fusar-Poli and Radua30 Considering the growing number of systematic reviews and meta-analyses available, this approach allows us to compare and contrast the findings of separate reviews related to a topic of interest.Reference Aromataris, Fernandez, Godfrey, Holly, Khalil and Tungpunkom31 The conduct of an umbrella review also provides a comprehensive overview of healthcare areas, to highlight whether the evidence is consistent or contradictory, and to explore possible heterogeneity sources for existing evidence.Reference Aromataris, Fernandez, Godfrey, Holly, Khalil and Tungpunkom31,Reference Ioannidis32 This type of review is considered among the highest level of evidence,Reference Grant and Booth33 and is particularly useful to inform clinical practice and policies.Reference Fusar-Poli, Hijazi, Stahl and Steyerberg34 Further details are described in the Supplementary Methods.
Two reviewers (C.G. and G.S.) independently conducted the literature search; the screening of the titles, abstracts and full-text papers; and the data extraction. Details of the process are described in the Supplementary Methods.
Eligible reviews were exclusively systematic reviews with a meta-analysis. We considered systematic reviews of observational studies (prospective or retrospective cohort and case–control studies) that investigated the association between exposure to any risk factor and the risk of developing PPD. We excluded systematic reviews that did not present study-level data, such as odds ratios or relative risks with 95% confidence intervals. When more meta-analyses on the same research topic were available, the meta-analysis with the largest number of included studies, providing effect sizes at a study level, was considered for inclusion, as previously described.Reference Radua, Ramella-Cravaro, Ioannidis, Reichenberg, Phiphopthatsanee and Amir35–Reference Theodoratou, Tzoulaki, Zgaga and Ioannidis37 From each included systematic review, two investigators (C.G. and G.S.) independently extracted information on first author, publication year, outcomes of interest, number of studies per meta-analysis and summary meta-analytic estimates. Primary studies included in all systematic reviews were retrieved and inspected by two reviewers (C.G. and G.S.). Details of the selection and extraction process are described in the Supplementary Methods.
Reporting quality of included systematic reviews and meta-analyses
Two reviewers (C.G. and G.S.) independently assessed the quality of each systematic review, using A Measurement Tool to Assess Systematic Reviews (AMSTAR-2), a 16-point assessment tool of the methodological quality of systematic reviews. AMSTAR-2 has good interrater agreement, test–retest reliability and content validity (details in the Supplementary Methods).Reference Shea, Reeves, Wells, Thuku, Hamel and Moran38
It assesses the methodological strength of reviews, through 16 domains, which include the research question and design, literature search, data extraction, explicit reports of each step and choice made by reviewers to allow transparency (e.g. presence of a list of excluded studies, with reason for exclusion), quality and statistical assessments. Each item allows for the following response options: yes, partial yes or no. Of these 16 domains, seven are considered critical domains, as they can particularly affect the validity of the review and its conclusion. The AMSTAR-2 is a qualitative tool, not designed to be scored. AMSTAR-2 offers a scheme for interpreting weaknesses identified in critical and non-critical items: reviews with no or only one non-critical weakness are considered ‘high quality’; reviews with more than one non-critical weakness but no critical flaws are considered ‘moderate quality’; reviews with only one critical flaw with or without non-critical weaknesses are considered ‘low quality’; finally, reviews with more than one critical flaw with or without non-critical weaknesses are considered ‘critically low quality’ (see Supplementary Methods and Supplementary Box 1 for further details).Reference Shea, Reeves, Wells, Thuku, Hamel and Moran38,Reference AMSTAR39
Statistical analysis and umbrella review criteria
We extracted effect sizes of individual studies included in each meta-analysis for every association, and afterwards we re-estimated the summary effect sizes with 95% confidence intervals, using random-effects models as we expected large heterogeneity.Reference DerSimonian and Laird40 Additionally, we calculated the 95% prediction intervals for the summary random effect sizes, which account for heterogeneity between studies and specify the uncertainty for the effect that would be expected in a new study examining that same research question.Reference Riley, Higgins and Deeks41 We evaluated heterogeneity with Cochran's Q-statistic (statistical significance set at P-value <0.10) and estimated with the I 2 metric.Reference Cochran42 The I 2 ranges from 0 to 100%, and it is considered very large, large, moderate and low for values ≥75%, 50–74%, 25–49% and <25%, respectively.Reference Higgins, Thompson, Deeks and Altman43 Potential publication and small-study effects biases were evaluated with Egger's test.Reference Sterne, Sutton, Ioannidis, Terrin, Jones and Lau44,Reference Egger, Smith, Schneider and Minder45 Specifically, small-study effects bias was considered to be present when a more conservative effect in the largest study and a P-value ≤0.10 in the regression asymmetry test were found. Further, we assessed the excess significance, which is a test that examines if the observed number of studies (O) with statistically significant results (i.e. with P < 0.05) in the meta-analysis is higher than the expected number (E).Reference Ioannidis and Trikalinos46 For each meta-analysis, E was calculated as the sum of the statistical power estimates for every study in the meta-analysis. The power of each study was calculated assuming a non-central t distribution.Reference Lubin and Gail47 The estimated power depends on the plausible effect size. As the true effect size for any meta-analysis is unknown, this approach assumes that the most plausible effect is the one provided by the largest study included. Excess significance for all meta-analyses was set at P-value ≤0.10.Reference Radua, Ramella-Cravaro, Ioannidis, Reichenberg, Phiphopthatsanee and Amir35,Reference Ioannidis and Trikalinos46,Reference Bortolato, Köhler, Evangelou, León-Caballero, Solmi and Stubbs48,Reference Dragioti, Evangelou, Larsson and Gerdle49 Statistical analyses were performed with RSTUDIO version 1.3.1056 for Windows (RStudio, Boston MA, US; see https://www.rstudio.com/products/rstudio/download/).
Based on the above calculations, we assessed the umbrella review criteria (URC) to classify the strength of associations as ‘convincing’ (class I), ‘highly suggestive’ (class II), ‘suggestive’ (class III) or ‘weak’ (class IV) (details in Supplementary Box 2).Reference Solmi, Correll, Carvalho and Ioannidis28,Reference Bellou, Belbasis, Tzoulaki, Evangelou and Ioannidis50–Reference Barbui, Purgato, Abdulmalik, Acarturk, Eaton and Gastaldon55 Precisely, meta-analyses were considered without bias (class I) if they met the following criteria: ≥1000 cases, random-effects P-value ≤10−6 of the meta-analysis, low or moderate between-study heterogeneity (I 2 ≤ 50%), 95% prediction intervals that excluded the null value, and absence of both small-study effects and excess significance. Associations were considered highly suggestive (class II) when the following criteria were met: ≥1000 cases, highly significant summary associations (P-value ≤10−6 in the random-effects estimate) and 95% prediction intervals not including the null value. Suggestive evidence (class III) criteria required ≥1000 cases and P-value ≤0.001 in the random-effects model. Weak association (class IV) criteria required only P-value ≤0.05. Associations were not considered significant if the P-value in the random-effects model was ≥0.05. All P-values were two tailed.
Grading of Recommendations, Assessment, Development and Evaluations
Additionally, the overall certainty in the estimates was qualitatively assessed by two reviewers (G.S. and C.G.) – with one author (C.B.) adjudicating the decision in case of discrepancies – using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) method.Reference Guyatt, Oxman, Vist, Kunz, Falck-Ytter and Alonso-Coello56
GRADE allows to rate the certainty of estimate for each outcome and gives an overview of findings easily understandable for patients, clinicians, researchers, guideline developers and policy makers.Reference Guyatt, Thorlund, Oxman, Walter, Patrick and Furukawa57 The following factors were considered for each outcome of interest, according to the GRADE method: study design, risk of bias, inconsistency, imprecision, indirectness, presence of large effect, dose–response gradient and publication bias.Reference Egger, Smith, Schneider and Minder45
Based on GRADE assessments, the certainty of estimates was classified as high, moderate, low or very low (further details in Supplementary Box 3).Reference Balshem, Helfand, Schünemann, Oxman, Kunz and Brozek58 In the case of observational studies, the certainty of evidence is low when there are no reasons to downgrade the certainty of evidence, and very low when at least one reason to downgrade the certainty of evidence is found. The only case in which evidence from observational studies could be ‘moderate’ is when some reason to upgrade the certainty of evidence is found (e.g. strong association), with no downgrades on the other items.
Tables of summary of findings were developed with the GRADEProGDT app (GRADEpro Guideline Development Tool [Software], McMaster University and Evidence Prime, Canada; see gradepro.org).
Overall evaluation
Risk factors were quantified together with a formal assessment of the certainty of estimates, using quantitative URC and GRADE. We employed both methods because they are complementary. The URC quantitatively evaluate the strength of the associations, and GRADE qualitatively assesses the certainty of evidence. We ranked all risk factors based first on the strength of each association (URC), then on the certainty of evidence (GRADE), and finally, on the quality of the systematic review (AMSTAR-2).
Sensitivity analyses
We performed three sensitivity analyses to assess whether the credibility of the evidence and the strength of the association varied when the following studies were retained in the analysis: cohort studies; studies in which the PPD diagnosis/symptoms were assessed with standardised criteria (e.g. ICD or DSM diagnosis, The Mini-International Neuropsychiatric Interview (M.I.N.I.), Structured Clinical Interview for DSM Disorders (SCID) or Edinburgh Postnatal Depression Scale (EPDS) score with a cut-off score of ≥13), excluding non-validated or self-assessed screening tools; and studies assessing mood symptoms at least 7 days after delivery (further details in Supplementary Methods).
Results
Description of studies included in the meta-analyses
The systematic search yielded 703 records. After duplicate removal, title and abstract screening, 73 full-text articles were retrieved and checked for inclusion. Eleven systematic reviews were included, including 12 meta-analyses, with 185 primary studies and 3 272 093 participants (Fig. 1 and Supplementary Table 2). The excluded articles and the reasons for their exclusion are provided in the Supplementary Material.

Fig. 1 PRISMA flow chart. Flow chart of included and excluded systematic reviews and meta-analyses.
The 12 risk factors reported in the 11 systematic reviews were anaemia (during pregnancy and postpartum), GDM, Caesarean section, preterm delivery, intra-labour epidural analgesia, medically assisted conception, violent experiences, premenstrual syndrome (PMS), vitamin D deficiency and unintended pregnancy, whereas one review provided data on a protective factor, the presence of the serotonin-transporter-linked polymorphic region (5-HTTLPR) polymorphism. Supplementary Table 2 summarises the main review and individual study characteristics. The number of studies per meta-analysis ranged between 4 and 33. Seven meta-analyses included ≥1000 cases (range 1074–15 758), and five had <1000 cases. Of the 185 studies, 123 (66.5%) were cohort studies and 62 (33.5%) adopted a case–control or cross-sectional design. Study participants were pregnant females, exposed to one or multiple risk factors. PPD was identified with an EPDS score of ≥13 or with ICD-9 or 10 or DSM-IV criteria in 81 studies; the remaining studies used self-reported or other scales or operational criteria. Assessments of mood symptoms were conducted within the first postpartum week in 31 studies.
Quality assessment of the systematic reviews
The PMS review was of moderate quality according to the AMSTAR-2 scoring system,Reference Cao, Jones, Tooth and Mishra59 the systematic review on medically assisted conception was of low quality,Reference Gressier, Letranchant, Cazas, Sutter-Dallay, Falissard and Hardy60 and the remaining nine were of critically low quality (Table 1).Reference Zhang, Wang, Yang, Chen, Qiu and Wang17,Reference Wang, Liu, Sun, Chen, Zhao and Zhang21,Reference de Paula Eduardo, de Rezende, Menezes and Del-Ben25–Reference Kountanis, Vahabzadeh, Bauer, Muzik, Cassidy and Aman27,Reference Azami, Badfar, Khalighi, Qasemi, Shohani and Soleymani61–Reference Xu, Ding, Ma, Xin and Zhang64 The most common weakness was that all the systematic reviews did not contain an explicit statement that the review methods were established before the conduct of the review (Table 1, question 2), with the exception of the review on preterm delivery.Reference de Paula Eduardo, de Rezende, Menezes and Del-Ben25 The nine reviews rated as critically low also did not provide a list of excluded studies with reasons to justify the exclusion (Table 1, question 7).Reference Zhang, Wang, Yang, Chen, Qiu and Wang17,Reference Wang, Liu, Sun, Chen, Zhao and Zhang21,Reference de Paula Eduardo, de Rezende, Menezes and Del-Ben25–Reference Kountanis, Vahabzadeh, Bauer, Muzik, Cassidy and Aman27,Reference Azami, Badfar, Khalighi, Qasemi, Shohani and Soleymani61–Reference Li, Chen, Xiang, Xiang, Tang and Tang64 Other critical flaws were that authors did not consider the risk of bias when interpreting results (Table 1, question 13), and the absence of adequate investigation of publication bias or small study bias and their impact on the results (Table 1, question 15).
Table 1 AMSTAR-2 assessment of the included meta-analyses
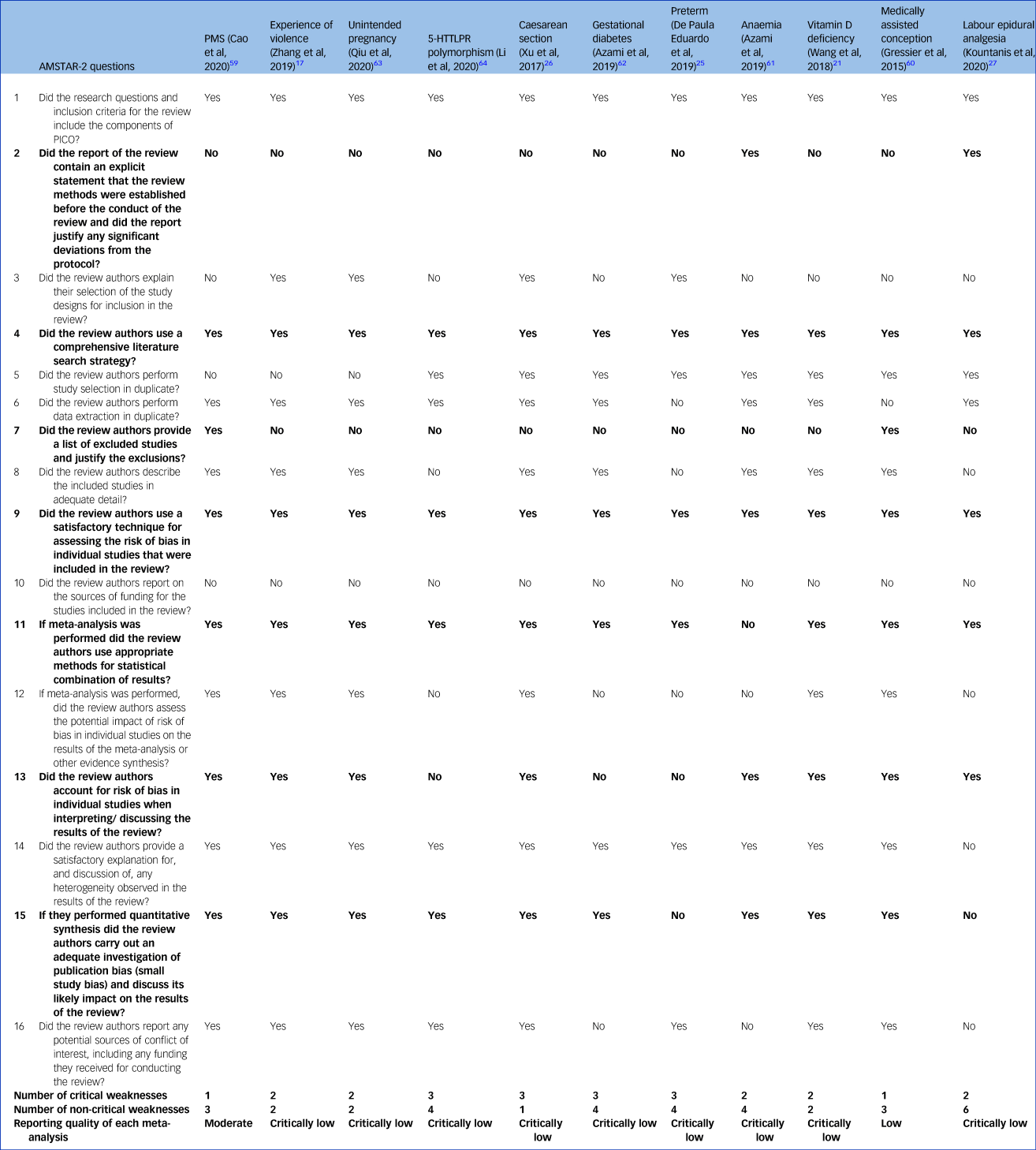
Bolding indicates critical questions. Further details can be found in the Supplementary Material. AMSTAR-2, A Measurement Tool to Assess Systematic Reviews; PMS, premenstrual syndrome; 5-HTTLPR, serotonin-transporter-linked polymorphic region; PICO: population, intervention, comparator, outcome.
Summary of associations and URC
Ten meta-analyses showed significant summary random-effects estimates; exposure to PMS, violent experiences, unintended pregnancy, Caesarean section, preterm delivery, GDM, anaemia during pregnancy, vitamin D deficiency and postpartum anaemia increased the risk of PPD (Table 2 and Fig. 2).Reference Zhang, Wang, Yang, Chen, Qiu and Wang17,Reference Wang, Liu, Sun, Chen, Zhao and Zhang21,Reference Caropreso, de Azevedo Cardoso, Eltayebani and Frey23,Reference de Paula Eduardo, de Rezende, Menezes and Del-Ben25,Reference Xu, Ding, Ma, Xin and Zhang26,Reference Cao, Jones, Tooth and Mishra59,Reference Azami, Badfar, Khalighi, Qasemi, Shohani and Soleymani61–Reference Qiu, Zhang, Sun, Li and Wang63 Conversely, the 5-HTTLPR polymorphism was associated with a lower PPD risk.Reference Li, Chen, Xiang, Xiang, Tang and Tang64 Associations between PPD and medically assisted conception or intra-labour analgesia were not significant.Reference Kountanis, Vahabzadeh, Bauer, Muzik, Cassidy and Aman27,Reference Gressier, Letranchant, Cazas, Sutter-Dallay, Falissard and Hardy60
Table 2 Strength of reported associations according to umbrella review criteria
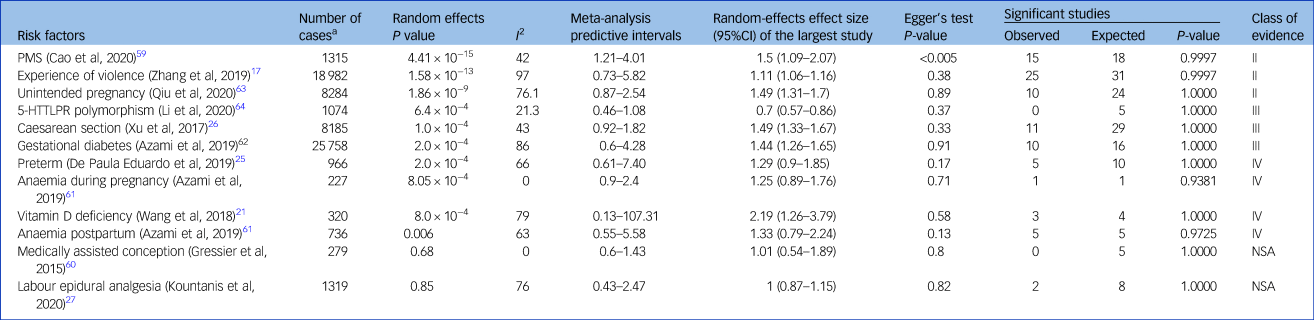
Convincing evidence (class I): ≥1000 cases, significant summary associations (P < 10−6) per random-effects calculations, no evidence of small-study effects, no evidence of excess of significance bias, prediction intervals not including the null value, largest study nominally significant (P < 0.05) and not large heterogeneity (i.e. I 2 < 50%). Highly suggestive evidence (class II): ≥1000 cases, significant summary associations (P < 10−6) per random-effects calculation and largest study nominally significant (P < 0.05). Suggestive evidence (class III): ≥1000 cases and significant summary associations (P < 10−3) per random-effects calculations. Weak evidence (class IV): all other associations with P < 0.05. Non-significant associations: all associations with P > 0.05. PMS, premenstrual syndrome; 5-HTTLPR, serotonin-transporter-linked polymorphic region; NSA, non-significant associations.
a. Number of women with postpartum depression.

Fig. 2 Ranking of associations on the risk of post-partum depression with exposure to risk factors.
5-HTTLPR, serotonin-transporter-linked polymorphic region; AMSTAR-2, A Measurement Tool to Assess Systematic Reviews; GRADE, Grading of Recommendations, Assessment, Development and Evaluations; PMS, premenstrual syndrome.
According to the URC, three associations (between PPD and PMS, violent experiences and unintended pregnancy) were highly suggestive (class II); three associations (between PPD and Caesarean section, GDM and 5-HTTLPR polymorphism) were suggestive (class III); four associations (between PPD and preterm delivery, anaemia during pregnancy, vitamin D deficiency and postpartum anaemia) were weak (Table 2); and non-significant associations were reported for medically assisted conception and labour epidural analgesia.
Certainty of evidence according to GRADE
For Caesarean section, preterm delivery, anaemia during pregnancy and 5-HTTLPR polymorphism, the certainty was rated as low (Table 3), based on the a priori GRADE baseline assumption of low certainty for observational studies. We found no reasons to upgrade this baseline evaluation. For postpartum anaemia, PMS, violent experiences, GDM, labour epidural analgesia and unintended pregnancy, the certainty was very low, mainly because of inconsistency.
Table 3 Grading of Recommendations, Assessment, Development and Evaluations appraisal
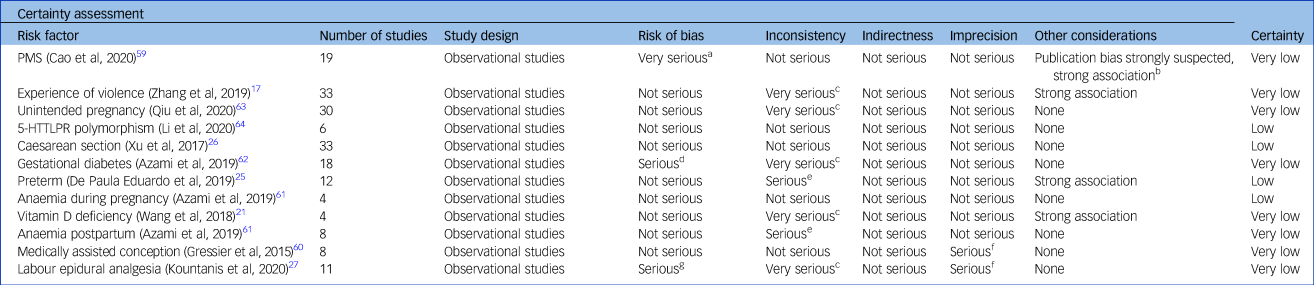
Grading: high certainty: we are very confident that the true effect lies close to the estimate of the effect; moderate certainty: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; low certainty: the true effect may be substantially different from the estimate of the effect; very low certainty: the true effect is likely to be substantially different from the estimate of effect. PMS, premenstrual syndrome; 5-HTTLPR, serotonin-transporter-linked polymorphic region.
a. Eleven out of nineteen studies with serious risk of bias.
b. Funnel plot inspections showed asymmetry and Egger's test confirmed.
c. Very high heterogeneity.
d. Not assessed.
e. High heterogeneity.
f. Confidence interval includes no difference.
g. More than half of the studies with low quality.
Overall ranking
Figure 2 presents a ranking of associations based on URC, GRADE and AMSTAR-2. The association between PPD and PMS was the most reliable, followed by associations with violent experiences and unintended pregnancy. The association with Caesarean section, GDM and 5-HTTLPR was suggestive, and the association with preterm delivery, anaemia during pregnancy, vitamin D deficiency and postpartum anaemia was weak. No association was found for medically assisted conception or intra-labour epidural analgesia.
Sensitivity analyses
In cohort studies, the associations between PPD and violent experiences (class II), unintended pregnancy (class II), GDM (class III), preterm delivery (weak), vitamin D deficiency (weak), postpartum anaemia (weak), medically assisted conception (no association) and intra-labour analgesia (no association) remained at the same strength. Conversely, the association with PMS was downgraded from highly suggestive (class II) to weak because the criterion of ≥1000 cases was not met. Moreover, for PMS, the GRADE certainty assessed in the sensitivity analysis was in contrast with the URC, as it was upgraded from very low to low, as the risk of bias was rated as ‘not serious’ in this case. In the case of Caesarean section, the association was downgraded from suggestive (class III) to non-significant, and the GRADE certainty was downgraded from low to very low. In the remaining cases, the GRADE certainty did not change between the main and the sensitivity analysis (Supplementary Table 3). This sensitivity was not performed for two risk factors: in the case of anaemia during pregnancy, all included studies were cohort, and for the 5-HTTLPR, none of the studies investigated cohorts.
In studies with standardised criteria for the PPD diagnosis/symptoms, the association with PPD was upgraded from suggestive to highly suggestive for GDM, remained suggestive for Caesarean section, and was non-significant for labour epidural analgesia and medically assisted conception. The associations with PMS and unintended pregnancy were downgraded from highly suggestive to weak, violent experiences was downgraded from highly suggestive to suggestive, 5-HTTLPR polymorphism was downgraded from suggestive to weak, and preterm delivery and postpartum anaemia were downgraded from weak to non-significant. The GRADE certainty was upgraded only for anaemia and PMS, from very low to low, whereas the association with 5-HTTLPR polymorphism remained low. In all other cases, GRADE certainty was very low, as in the main analysis (Supplementary Table 4). For anaemia during pregnancy and vitamin D deficiency, sensitivity analysis was not performed, as not enough studies using standardised criteria were available (one and zero studies, respectively).
Excluding studies that assessed mood symptoms within the first postpartum week, the strength of association remained for violent experiences, Caesarean section, vitamin D deficiency, preterm delivery and unintended pregnancy, whereas the association with GDM was downgraded by one level and the association with PMS and 5-HTTLPR polymorphism was downgraded by two levels. For the latter, we no longer found a significant association (Supplementary Table 5). Intra-labour epidural analgesia and medically assisted conception remained non-significant. For both types of anaemia, it was not possible to perform analyses, as study evaluations occurred after the first postpartum week. No differences between the main and sensitivity analysis were found in GRADE, except for the 5-HTTLPR polymorphism, which was downgraded from low to very low (Supplementary Table 5).
Discussion
The most reliable association with PPD was found for women suffering from PMS, followed by violent experiences and unintended pregnancy. The risk of PPD was more than doubled in women with PMS or a violent experience, and was 50% higher in women with unintended pregnancy. Women suffering from PMS could have an affective vulnerability underpinned by hormonal fluctuations, which occur both during the premenstrual period and on a much larger scale during postpartum.Reference Amiel Castro, Pataky and Ehlert65 Experience of violence may be a less specific risk factor, as it has been implicated in different psychiatric disorders, including other affective and addiction disorders.Reference Köhler, Evangelou, Stubbs, Solmi, Veronese and Belbasis66,Reference Brown, Cosgrave, Killackey, Purcell, Buckby and Yung67 Unintended pregnancy has been previously suggested as a PPD risk factor, mainly because of increased stress levels, which can activate the hypothalamic-pituitary-adrenal axis, resulting in a release of glucocorticoids influencing psychological functions.Reference Brummelte and Galea68,Reference Meltzer-Brody69 Specifically, women who did not plan their pregnancy may be unprepared/worried about the health of the foetus, feel a potential conflict between continuing and terminating the pregnancy, and start the prenatal care later than women who planned the pregnancy.Reference Karaçam, Onel and Gerçek70–Reference Abbasi, Chuang, Dagher, Zhu and Kjerulff72
For these risk factors, the effect sizes were generally small (odds ratio < 3.5),Reference Chen, Cohen and Chen73 despite the URC showing class II associations. Therefore, evidence regarding such associations might only play a partial role with respect to depression onset, and confounding could not be ruled out because of the observational nature of studies, which was reflected by the GRADE certainty (very low).
At an intermediate credibility level, we found associations of PPD with 5-HTTLPR polymorphism, Caesarean section and GDM. The latter increased the risk of PPD by 60%. Conversely, women who underwent Caesarean section had a slightly increased risk of PPD. However, it is noteworthy that in the included meta-analysis,Reference Xu, Ding, Ma, Xin and Zhang26 Caesarean section was a significant risk factor for PPD when performed in an emergency situation, but not when elective, suggesting a central role of unexpected complications for PPD. The allelic model of 5-HTTLPR polymorphism was the only protective factor, which slightly reduced the PPD risk. The 5-HTT gene is a key factor that affects risk for depression and other mental disorders.Reference Robson, Quinlan and Blakely74 The transcriptional activity of the 5-HTT gene may be regulated by this polymorphism,Reference Hranilovic, Stefulj, Furac, Kubat, Balija and Jernej75,Reference Mushtaq, Shoib, Shah and Mushtaq76 resulting in different levels of serotonin transporters, increasing the susceptibility to affective disorders.Reference Ezaki, Nakamura, Sekine, Thanseem, Anitha and Iwata77,Reference Ming, Zhang, Yi, Wang, Zhu and Yao78
Sensitivity analyses in cohort studies confirmed all of the results, with the exception of the association between PPD and PMS, which was found to be less reliable, dropping from highly suggestive to weak because of the small number of cases.
The sensitivity analyses based on standardised criteria for PPD led to different results. Here, we discuss the most relevant differences. First, it is important to note that the evidence on GDM was upgraded to highly suggestive with a slightly higher odds ratio (from 1.44 to 1.57), indicating that GDM may be more strongly associated with clinical depression rather than subclinical depressive symptoms. Second, evidence on PMS, unintended pregnancy and 5-HTTLPR polymorphism was downgraded to weak, suggesting that these factors might be associated with subclinical depression symptoms.
Restricting the analyses to studies with an assessment after the first postpartum week, we found lower certainty for almost all associations, with the exception of the association of PPD with violent experiences, Caesarean section and unintended pregnancy. These associations remained at the same level of credibility indicating that apart from violent experiences, Caesarean section and unintended pregnancy, the other risk (or protective) factors might be associated more with the so-called ‘maternity blues’ or adjustments within the first postpartum week, rather than with depression diagnosed later. This result is notable if we consider that authors previously suggested that maternity blues could be a risk factor for developing depression later on in the postpartum period.Reference Hutchens and Kearney13,Reference Beck79,Reference Bloch, Rotenberg, Koren and Klein80
Moreover, the credibility of the association with violent experiences was maintained across all analyses. The increased risk was slightly lower in the two sensitivity analyses based on clinical criteria and assessment timing, suggesting that some bias could have inflated the main results. Nevertheless, violent experiences were found as one of the most reliable risk factors for developing PPD, in line also with literature on major depression.Reference Köhler, Evangelou, Stubbs, Solmi, Veronese and Belbasis66
Finally, the sensitivity analyses confirmed the non-differential results on medically assisted conception and intra-labour epidural analgesia, which neither increased nor decreased the risk of PPD, suggesting that the influence of these factors might be negligible. Some authors suggested that the quality of the intra-labour analgesia might be related with women's satisfaction, and therefore with reduced stress during delivery, becoming a possible protective factor for PPD.Reference Lim, Farrell, Facco, Gold and Wasan81 Further studies with less confounders are needed to confirm these hypotheses.
The American College of Obstetricians and Gynecologists recommends that obstetrician-gynaecologists and other obstetric care providers screen patients at least once during the perinatal period for depression and anxiety symptoms, using a standardised, validated tool.82 We suggest that, based on the results of this umbrella review, the risk factors listed in this tool might need to be revised and updated along with guidelines. Specifically, considering that currently PMS, Caesarean section and GDM are not included in the tool, we suggest that these risk factors may be at least considered. Moreover, the assessment of some risk factors, such as violent experience, that are only partially mentioned under the ‘Experiencing stressful life events’ category, could be more weighted. Finally, some risk factors already considered may be less strong than commonly considered, such as preterm delivery.
A number of limitations need to be considered when interpreting these results. First, although broadly employed in mental health and medicine,Reference Bellou, Belbasis, Tzoulaki, Evangelou and Ioannidis50,Reference Tsilidis, Kasimis, Lopez, Ntzani and Ioannidis51,Reference Papola, Ostuzzi, Thabane, Guyatt and Barbui53–Reference Barbui, Purgato, Abdulmalik, Acarturk, Eaton and Gastaldon55,Reference Köhler, Evangelou, Stubbs, Solmi, Veronese and Belbasis66 and corroborated by standard statistical tests,Reference Cochran42,Reference Sterne, Sutton, Ioannidis, Terrin, Jones and Lau44–Reference Ioannidis and Trikalinos46 the URC classification to classify strengths has been just recently introduced. The criteria of the ≥1000 cases might not be fully applicable for studies that target very specific samples, especially those with low incidence.
A second general cautionary note is the observational nature of the primary studies, which does not allow us to establish a causal association between risk factors and PPD. Moreover, this type of studies is vulnerable to bias because of unmeasured confounding and lower internal validity compared with randomised controlled trials.Reference Carlson and Morrison83,Reference Schoretsanitis and Deligiannidis84 In fact, it is unclear if some of the investigated factors were proxies for other factors or shared background risk. It should be noted, however, that this risk of bias is taken into consideration by GRADE, which suggests rating the certainty of estimates from observational studies as low quality instead of high quality, to acknowledge issues with internal validity.Reference Guyatt, Oxman, Vist, Kunz, Falck-Ytter and Alonso-Coello56 On the other hand, findings of meta-analyses of observational studies are more generalisable and pragmatic, as they have larger sample sizes and include real-world patients.Reference Song and Chung85,Reference Barnish and Turner86 Moreover, although qualitatively more reliable, a randomised design is not the best design to address risk factors.
Third, the investigated populations were highly heterogeneous, also because of the variations in the definitions of assessed risk factors. Despite attempts to adjust for relevant variables, heterogeneity lowered the reliability of the identified associations. As previously argued, genuine heterogeneity might operate in the field of depression research for several cultural reasons.Reference Köhler, Evangelou, Stubbs, Solmi, Veronese and Belbasis66 Future observational studies should attempt to better identify high-risk groups by adopting sophisticated measures of exposure to risk factors. On a secondary level, future meta-analyses should access individual-level patient data and apply harmonised inclusion criteria, covariate definitions and statistical approaches.Reference Rubio, Schoretsanitis, John, Tiihonen, Taipale and Guinart87
Fourth, none of the risk factors for PPD were supported by convincing evidence. This may be because of specific limitations in this research field. Pregnant women are a very specific and limited population, and PPD has a lower prevalence in the general population compared with other mental disorders. Moreover, the field of perinatal mental and physical health research faces several obstacles that traditionally relate to ethical challenges of conducting research in these vulnerable populations, as well as to the involvement of various disciplines, which requires multidisciplinary approaches.88,Reference Tachibana, Koizumi, Akanuma, Tarui, Ishii and Hoshina89 This fact may further explain the lower number of women enrolled in studies in the peripartum period compared with depression unrelated to pregnancy and childbirth or other disorders.Reference Kaye90 Hence, it is expected that the number of cases evaluated could be low (<1000), which is the first URC criterion to upgrade the class of evidence.Reference Solmi, Correll, Carvalho and Ioannidis28,Reference Fusar-Poli and Radua30
Finally, data for some potential or known risk factors for PPD may not have been meta-analysed yet, and thus were not included in our umbrella review, such as social support of the mother, history of mental disorders, income, maternity blues and obesity.Reference Molyneaux, Poston, Ashurst-Williams and Howard91 This limitation refers to the umbrella review methodology, as this approach is based on a statistical re-analysis of meta-analyses. Therefore, umbrella reviews only include statistical re-analyses with a meta-analysis (i.e. that employed a quantitative approach to the data presentation), whereas statistical re-analyses providing only qualitative descriptions of the included studies are excluded. However, typically the lack of a meta-analytical approach is driven by lack of sufficient and homogeneous data.Reference Fusar-Poli and Radua30
Despite these limitations, the main strengths of this work are still the comprehensiveness of the search and the quantitative and qualitative approaches employed to rate the credibility of evidence. To the best of our knowledge, this is the first umbrella review that systematically summarised data on the association between PPD and several risk and protective factors grading the certainty and strength of evidence by applying well-recognised criteria.Reference Solmi, Correll, Carvalho and Ioannidis28,Reference Fusar-Poli and Radua30,Reference Barbui, Purgato, Abdulmalik, Acarturk, Eaton and Gastaldon55 A previous overview of reviews on PPD risk factors was recently published; however, the authors did not perform any re-analysis and did not grade the credibility of evidence and strength of association by using any qualitative or quantitative criteria.Reference Hutchens and Kearney13 In contrast with our approach, Hutchens and KearneyReference Hutchens and Kearney13 narratively summarised systematic reviews on PPD risk factor, regardless of the presence of a meta-analysis. Moreover, previous umbrella reviews have assessed risk factors for other mental disorders, such as depression, anxiety and psychosis, but did not include PPD or postpartum depressive symptoms.Reference Bortolato, Köhler, Evangelou, León-Caballero, Solmi and Stubbs48,Reference Machado, Veronese, Sanches, Stubbs, Koyanagi and Thompson54,Reference Köhler, Evangelou, Stubbs, Solmi, Veronese and Belbasis66,Reference Solmi, Radua, Stubbs, Ricca, Moretti and Busatta92–Reference Belbasis, Köhler, Stefanis, Stubbs, van Os and Vieta94 Our approach led to the inclusion of an extremely large number of participants, at over 3 million. Additionally, the retrieved risk factors were either social/environmental stressors or medical/obstetric complications of the pregnancy and delivery. This highlights the central role of social environment in the future mothers’ mental health and well-being, as well as in the pathogenesis of mental disorders in the general population.Reference Hutchens and Kearney13,Reference Vasa, Eldeirawi, Kuriakose, Nair, Newsom and Bates95,Reference Campbell, Cohn, Flanagan, Popper and Meyers96
Furthermore, this work provides methodological directions for future studies aiming to improve our understanding of predictors of PPD. First, there is an urgent need to generate further multidisciplinary evidence to more effectively tackle mental (and physical) health challenges for women during pregnancy, as well as the postpartum phase.Reference Kaye90,Reference Weld, Bailey and Waitt97 Second, further replication of the evidence regarding biological and psychosocial factors in more sophisticated models is hoped to lay the groundwork for a comprehensive PPD screening tool. Moreover, timing of assessment of depression seems extremely relevant. Therefore, more well-designed, prospective observational studies on risk factors for PPD, collecting data pre/intrapartum and following up on women after the first week postpartum are needed to determine whether risk factors are associated with maternity blues or later depression.Reference Vasa, Eldeirawi, Kuriakose, Nair, Newsom and Bates95,Reference Goodman98 Ultimately, our data may enhance efforts to develop interdisciplinary prevention and care targeting patients at high risk for PPD.
Our results could be of help in updating postpartum screening tools employed to identify and screen women at risk of PPD, such as the one developed by the American College of Obstetricians and Gynecologists.82 The early recognition and management of these patients will improve treatment outcomes improving maternal health and new-born development.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1192/bjp.2021.222
Data availability
The data that support the findings of this study are available from the corresponding author, C.G., upon request.
Author contributions
C.G., G.S., C.B., M.S. and C.U.C. designed the study. C.G. and G.S. drafted the manuscript. C.G., G.S. and C.B. contributed to the database preparation and double-checked the data. C.G., G.S. and M.S. conducted data analyses. C.G. and G.S. assessed the quality of meta-analyses with AMSTAR-2. All authors critically revised the manuscript and approved the draft and final manuscripts.
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Declaration of interest
C.B. and C.G. have nothing to disclose. G.S. has served as a consultant for HLS Therapeutics. C.U.C. has been a consultant and/or advisor to, or has received honoraria from, Alkermes, Allergan, Angelini, Boehringer-Ingelheim, Gedeon Richter, Gerson Lehrman Group, Indivior, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Medscape, Merck, Mylan, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Servier, Sumitomo Dainippon, Sunovion, Supernus, Takeda and Teva. He has provided expert testimony for Bristol-Myers Squibb, Janssen and Otsuka. He served on a Data Safety Monitoring Board for Boehringer-Ingelheim, Lundbeck, Rovi, Supernus and Teva. He has received royalties from UpToDate and grant support from Janssen and Takeda. He is also a shareholder of LB Pharma. M.S. has been a consultant and/or advisor to, or has received honoraria, from Angelini and Lundbeck.









eLetters
No eLetters have been published for this article.