Introduction
The cognitive deficits in schizophrenia are now well established as core features of the disorder that do not resolve when psychotic symptoms remit (Asarnow & MacCrimmon, Reference Asarnow and MacCrimmon1978; Nuechterlein et al., Reference Nuechterlein, Dawson, Gitlin, Ventura, Goldstein, Snyder and Mintz1992) and that are key factors in limiting everyday functioning (Fett et al., Reference Fett, Viechtbauer, Dominguez, Penn, van Os and Krabbendam2011; Green, Reference Green1996; Green, Kern, & Heaton, Reference Green, Kern and Heaton2004). Antipsychotic medications have limited impact on the core cognitive deficits (Harvey & Keefe, Reference Harvey and Keefe2001), although their consistent use may allow systematic cognitive training (CT) to be more beneficial early in the course of illness (Nuechterlein et al., Reference Nuechterlein, Ventura, Subotnik, Gretchen-Doorly, Turner, Casaus and Medalia2022). While several potential cognitive-enhancing drugs have reached Phase III trials as adjuncts to antipsychotic medications, so far no cognitive enhancer has been sufficiently effective to achieve FDA approval for schizophrenia (Keefe, Reference Keefe2019; Keefe et al., Reference Keefe, Buchanan, Marder, Schooler, Dugar, Zivkov and Stewart2013).
Among the most promising interventions for the core cognitive deficits of schizophrenia at this point are systematic cognitive training and aerobic exercise. Systematic cognitive training, or cognitive remediation, has been well demonstrated in meta-analyses to have small to medium effect sizes on overall cognitive functioning (0.28 to 0.45) (Kambeitz-Ilankovic et al., Reference Kambeitz-Ilankovic, Betz, Dominke, Haas, Subramaniam, Fisher and Kambeitz2019; Vita et al., Reference Vita, Barlati, Ceraso, Nibbio, Ariu, Deste and Wykes2021; Wykes, Huddy, Cellard, McGurk, & Czobor, Reference Wykes, Huddy, Cellard, McGurk and Czobor2011) in multi-episode schizophrenia and might have some what larger effects in the initial phase of schizophrenia (Bowie, Grossman, Gupta, Oyewumi, & Harvey, Reference Bowie, Grossman, Gupta, Oyewumi and Harvey2014; Nuechterlein et al., Reference Nuechterlein, Ventura, Subotnik, Gretchen-Doorly, Turner, Casaus and Medalia2022). The impact of cognitive training on cognition and functional outcome is enhanced when it is completed in the context of an active psychosocial rehabilitation program (Vita et al., Reference Vita, Barlati, Ceraso, Nibbio, Ariu, Deste and Wykes2021; Wykes et al., Reference Wykes, Huddy, Cellard, McGurk and Czobor2011). Even then, however, only about a quarter to a third of the cognitive deficit in schizophrenia is remediated.
Aerobic exercise programs have more recently started to be examined as a means of improving cognitive deficits in schizophrenia. Early studies with older healthy adults showed not only physical fitness but cognitive benefits (Kramer, Erickson, & Colcombe, Reference Kramer, Erickson and Colcombe2006; Quigley, MacKay-Lyons, & Eskes, Reference Quigley, MacKay-Lyons and Eskes2020), leading to an interest in applications to schizophrenia. Pajonk and colleagues (Pajonk et al., Reference Pajonk, Wobrock, Gruber, Scherk, Berner, Kaizl and Falkai2010) initially demonstrated that aerobic exercise led to increased hippocampal volume in multi-episode schizophrenia, which was correlated with short-term verbal memory improvement. Kimhy and colleagues demonstrated cognitive gains with aerobic exercise programs in multi-episode schizophrenia in a well-controlled randomized controlled trial and found an association with increases in brain-derived neurotrophic factor (BDNF) (Kimhy et al., Reference Kimhy, Vakhrusheva, Bartels, Armstrong, Ballon, Khan and Sloan2015). A meta-analysis of ten early studies (Firth et al., Reference Firth, Stubbs, Rosenbaum, Vancampfort, Malchow, Schuch and Yung2017) concluded that the effect size for overall cognitive functioning was 0.33 across all studies, and 0.43 for the seven randomized clinical trials. Thus, aerobic exercise appears to have small to moderate effects on cognition in schizophrenia, in the same range as CT programs. Furthermore, the impact of aerobic exercise on functional outcome has not been systematically examined.
Given that cognitive deficits in schizophrenia are improved by CT and by aerobic exercise, but neither alone yields large effects, we have focused on whether adding aerobic exercise to CT can enhance the impact of CT on cognition and everyday functioning (Nuechterlein et al., Reference Nuechterlein, Ventura, McEwen, Gretchen-Doorly, Vinogradov and Subotnik2016). Animal models suggest that physical exercise may prime hippocampal neuroplasticity, which then results in increased neurogenesis and synaptogenesis when cognitive stimulation occurs (Fabel et al., Reference Fabel, Wolf, Ehninger, Babu, Leal-Galicia and Kempermann2009). Furthermore, exercise may increase the proliferation of neuronal precursor cells in the hippocampus, while CT promotes the survival of these cells (Kempermann et al., Reference Kempermann, Fabel, Ehninger, Babu, Leal-Galicia, Garthe and Wolf2010; Kronenberg et al., Reference Kronenberg, Reuter, Steiner, Brandt, Jessberger, Yamaguchi and Kempermann2003). Exercise-related increases in BDNF are one mechanism through which hippocampal neurogenesis, synaptic plasticity, learning and memory may be enhanced (Bekinschtein, Oomen, Saksida, & Bussey, Reference Bekinschtein, Oomen, Saksida and Bussey2011; Gomez-Pinilla, Vaynman, & Ying, Reference Gomez-Pinilla, Vaynman and Ying2008). Consistent with complementary roles, evidence from human studies has begun to suggest that combining physical exercise and CT has stronger impact than either alone (Lauenroth, Ioannidis, & Teichmann, Reference Lauenroth, Ioannidis and Teichmann2016).
While the combination of physical exercise and CT is relatively unexplored in schizophrenia, two studies of multi-episode schizophrenia patients have suggested promise for this approach. Oertel-Knöchel et al. (Reference Oertel-Knöchel, Mehler, Thiel, Steinbrecher, Malchow, Tesky and Hänsel2014) reported a 4-week, 12-session study that revealed differential improvement in working memory but not other cognitive domains in patients with schizophrenia compared to those with major depressive disorder. Malchow et al. (Reference Malchow, Keller, Hasan, Dorfler, Schneider-Axmann, Hillmer-Vogel and Falkai2015) started with endurance training and added CT twice a week after 6 weeks. Verbal learning gains occurred during the period from 6 weeks to 3 months when CT was present.
We hypothesized that the application of combined physical exercise and CT more intensively (4 h/week of CT and 3 h/week of exercise) over a longer period of time (6 months) with patients who had recently experienced a first episode of schizophrenia would provide a stronger test of the promise of this combined treatment strategy for overall cognitive improvement and everyday functioning gains. We hypothesized that adding aerobic exercise to CT would stimulate the release of BDNF, which would facilitate more rapid improvements in overall cognition, and then lead to larger gains in work/school functioning than CT alone.
Methods
Participants
First-episode patients recently diagnosed with schizophreniform disorder, schizophrenia, or schizoaffective disorder, depressed type, were recruited from multiple local public and private psychiatric hospitals and clinics. For brevity, we will refer to the participants as having schizophrenia, but we acknowledge that a minority had a schizophrenia-related psychotic disorder. Raters trained to reliability standards (Ventura, Liberman, Green, Shaner, & Mintz, Reference Ventura, Liberman, Green, Shaner and Mintz1998) on the SCID/IP, Version 2.0 (First, Spitzer, Gibbon, & Williams, Reference First, Spitzer, Gibbon and Williams2001) established each participant's diagnosis by combining the SCID direct interview with information from medical records, treatment providers, and family members. The inclusion and exclusion criteria were (1) a first episode of a psychotic illness that began within the past two years; (2) a diagnosis by DSM-IV of schizophrenia, schizoaffective disorder, mainly depressed type, or schizophreniform disorder; (3) between 18 and 45 years of age; (4) no evidence of a known neurological disorder (e.g. epilepsy) or significant head injury; (5) no evidence of alcohol or substance use disorder within the six months prior to the first episode and no evidence that substance abuse accounts for the psychotic symptoms, (6) premorbid IQ not less than 70; (7) sufficient acculturation and fluency in the English language to avoid invalidating research measures of verbal cognitive abilities; and (8) residence likely to be within commuting distance of the UCLA Aftercare Research Program. Substance use was evaluated through medical records, self-report, and family reports. The study was approved by the UCLA IRB; all participants gave written informed consent.
Treatment for all participants
All participants entered the UCLA Aftercare Research Program, an outpatient research clinic, and were provided a psychiatrist as well as a psychologist who served as a case manager and therapist. Participants received psychiatric medication managed by an Aftercare treating psychiatrist, weekly individual case management, supportive psychotherapy, and family education. Given the difficulties of public transportation for substantial distances in Los Angeles, UCLA van transportation was provided to patients who could not drive themselves to UCLA or use public transportation. To standardize medication effects, the initial antipsychotic medication was oral risperidone unless prior treatment indicated that risperidone was not effective or produced intolerable side effects. Antipsychotic dosage was individually optimized. Adjunctive medications (e.g. antidepressants, antiparkinsonian medications) were used when necessary.
Randomizaton to combined cognitive training and exercise (CT&E) v. cognitive training (CT)
After entry into the Aftercare Research Program, typically in an actively psychotic state, patients were stabilized on antipsychotic medication and provided weekly case management visits and group psychoeducation about their illness and its treatment. This process usually required about three months, followed by completion of the baseline assessments. No criterion for the remission of symptoms was required for randomization. Randomization was completed immediately following baseline assessment, using a random number table with a 1:1 ratio to CT&E v. CT, without stratification for demographics. The randomized treatment conditions lasted 6 months. The major assessment battery was repeated after 3 months and at the end of the 6-month randomized treatment period. See Fig. 1 for the recruitment and enrollment CONSORT diagram.

Fig. 1. CONSORT diagram of the progress of patients through the phases of the RCT.
Cognitive training
Both the CT and CT&E groups were provided the same systematic CT program at the Aftercare Research Program, with the first 12 weeks focused on neurocognitive training and the second 12 weeks focused on social cognitive training. Patients completed 2 h/day of computerized CT 2 days/week. Thus, each patient was provided 48 h of neurocognitive training and 48 h of social cognitive training. CT sessions were reinforced by providing $2 per session. The 1-h CT sessions involved 4–6 patients in a room on separate computers with one cognitive coach.
Neurocognitive training
Six Posit Science BrainHQ CT modules focused on improving the fundamental neurocognitive processes of auditory discrimination, speed of processing, working memory, verbal memory, and verbal reasoning. BrainHQ's auditory exercises started with basic processes involving auditory discrimination and progressed to more complex processes involving memory processing and reasoning. See Supplementary Materials for a description of the modules. The BrainHQ exercises continually adapt in difficulty level to match the performance of the participant for maximum engagement, interest, and learning. Progress was automatically tracked and immediately visible to the participant on screen and to cognitive trainers through an Internet-based Administrator Portal.
Social cognitive training
The Posit Science SocialVille modules, subsequently incorporated into the BrainHQ training package, focus on impairments in facial recognition, social perception, processing of emotion, self-referential style, and interpretation of social information. Participants began with simple face and emotion identification tasks and gradually progress to more difficult emotion discrimination and social cue interpretation tasks. Difficulty levels constantly adjusted according to the skill level of each participant for maximum engagement and reward. The more challenging exercises are designed to generalize neurocognitive skills to social situations that are known to be particularly impaired in schizophrenia. Engaging sound effects, dynamic visual stimuli, and a social reward system encouraged continued motivation.
Bridging group
Patients in both treatment groups participated in a weekly 1-h Bridging Group with other members of their own treatment group. This group was designed to aid the generalization of training to everyday life situations. Patients were helped to make direct links between the types of cognitive deficits they experienced and the neurocognitive and social cognitive skills they learned in the computerized training programs. Patients also practiced specific strategies for applying the CT skills to improve their functioning in key domains such as independent living, school/job, and social functioning. Facilitators used patient reports of work and school performance to provide structured feedback about how to improve work habits and work quality. Motivation and engagement in CT exercises were addressed by using the performance-based graphs generated by Posit Science modules. This approach incorporated peer-to-peer support in a safe, structured environment to encourage the generalization of training to major everyday life domains.
Aerobic exercise program
In addition to the CT sessions, participants randomized to the CT&E group participated in a 24-week progressive aerobic exercise program. The in-clinic exercise sessions occurred 2 sessions/week and exercise homework involved another 2 sessions/week. A certified personal trainer (Dr McEwen) designed our aerobic conditioning program based on the cognitive and physical health issues of first-episode schizophrenia patients. Most 1-h clinic exercise sessions were completed outdoors (weather permitting). The clinic exercise sessions involved a group of 5–7 participants led by a certified fitness trainer and included a dynamic warm-up (7.5 min) and a cool-down after exercise (7.5 min). The exercise sessions included 30 min of combined moderate-intensity aerobic conditioning (1-min intervals) and moderate-to-high-intensity strength and calisthenic conditioning (1-min intervals). Participants were instructed on the proper form and technique of the five different exercises at the start of each session and completed three rounds of the five sets of the aerobic and strength training intervals. The exercise dosage goal was 150 min/week of moderate aerobic activity, including two exercise sessions in the clinic (45 min duration) and two at home (30 min duration), informed by the American College of Sports Medicine and Federal guidelines (U.S. Department of Health & Human Services, 2008). The exercise intensity was individually calibrated at 60 to 80% of aerobic capacity using the gold standard heart rate calculation Karvonen formula. Each participant was given a FitBit Charge HR to capture heart rate and movement data. Participants examined their Fitbit heart rate on three occasions during the group sessions and worked with the trainer to adjust the intensity of their exercise as needed. Participants were taught to sync their FitBit with their mobile phone to remotely collect their exercise homework, allowing a measure of adherence and exercise data for home sessions. The home-based exercise was designed around the individual participant's interests and current fitness level and ranged from completing an exercise DVD, to hiking, basketball, or Zumba classes. This individualization encouraged the completion of home exercises.
A structured exercise incentives program was employed. Each participant earned $5 for every homework session and $2 for each session completed in the clinic. The greater reward for exercising at home addressed the motivational issues of first-episode patients that can be barriers to exercise (Deighton & Addington, Reference Deighton and Addington2014). In addition, an incentive reward program capitalized on in-clinic peer-to-peer interaction and friendly competition. Points were rewarded for staying in the target heart rate zone, for the perceived effort exerted during the in-clinic exercise sessions, for meeting weekly fitness goals, and for completing the homework exercise sessions. Every other month, the first place point leader received a $10 reward and the second place winner received a $5 reward. Each CT&E participant was also given an exercise text buddy, a staff member who ran their exercise group, who sent a text reminder and motivational message on the chosen exercise days.
Primary measures
The major test batteries were administered at baseline, at the 3-month point to examine the benefits of adding aerobic exercise to neurocognitive training, and at the 6-month point to examine the benefits of adding aerobic exercise to the full neurocognitive and social cognitive package.
Cognitive performance
The Overall Composite Score from the MATRICS Consensus Cognitive Battery (MCCB) (Nuechterlein et al., Reference Nuechterlein, Green, Kern, Baade, Barch, Cohen and Marder2008) was the primary index of cognitive performance. The Overall Composite Score summarizes performance across 10 tests of Speed of Processing, Attention/Vigilance, Working Memory, Verbal Learning, Visual Learning, Reasoning and Problem Solving, and Social Cognition, scaled as a T score based on community norms (Kern et al., Reference Kern, Nuechterlein, Green, Baade, Fenton, Gold and Marder2008).
Functional outcome
The 10-point Global Functioning Scale: Role (Cornblatt et al., Reference Cornblatt, Auther, Niendam, Smith, Zinberg, Bearden and Cannon2007) was the primary functional outcome measure due to its emphasis on work/school performance. The Global Functioning Scale: Social was examined to determine whether improvements in functioning generalized to social situations. Raters were trained to criterion levels of interrater reliability and used weekly contact with the participants and information from family members, employers, and teachers to rate work/school performance, but were not blind to intervention status.
Secondary measures
Brain-derived neurotrophic factor (BDNF)
BDNF is a principal growth factor known to mediate the effects of exercise in the brain (Cotman, Berchtold, & Christie, Reference Cotman, Berchtold and Christie2007). Serum BDNF was collected via venipuncture upon clinic arrival in the morning at baseline and after 2 weeks, 3 months, and 6 months. Assays were conducted by the UCLA Center for Pathology Research Services using the ELISA immunoassay kits from R&D Systems (Minneapolis, MN).
Fitness and muscular endurance testing
We examined the aerobic conditioning benefits of physical exercise (De Hert, Schreurs, Vancampfort, & Winkel, Reference De Hert, Schreurs, Vancampfort and Winkel2009) through the YMCA Fitness Test (YMCA, 2000). Cardiovascular fitness was measured as heart rate recovery in the 3-min step test, while muscular endurance was measured by the 1 min half sit-up test.
Data analyses
All analyses were completed with SPSS Statistics v26 GLMM and Correlation modules.
For the two primary outcomes we fit a generalized linear mixed model (GLMM) with Group (CT&E, CT) as the between-subjects factor, Time (baseline, 3 mos., 6 mos.) as the within-subjects factor, and a Group by Time interaction. The outcomes at 3 months and 6 months were then examined separately, as we expected that cognitive gains would be present by 3 months, with functional outcome gains requiring longer to develop. All available outcome data were examined, without regard to the number of treatment sessions that the patients completed.
Changes in BDNF, physical fitness, muscular endurance, and other secondary outcomes were analyzed in an exploratory fashion through GLMM. The relationships of BDNF change and exercise dosage to cognitive and functional outcome were examined through correlational analyses.
Data were analyzed on an intent-to-treat basis, using all available observations from all study participants regardless of the degree of participation.
Results
Sample characteristics and treatment participation
The demographic and clinical characteristics of patients in each treatment condition are provided in Table 1. The 47 participants have a mean age of 22.4 years and a mean of 12.8 years of education, are predominantly male (70%) and single (93%), and have diverse racial and ethnic backgrounds. Their first psychotic episode began an average of 8.9 months prior to project entry. At baseline, their mean MCCB Overall Composite T Score was 24.9, mean Global Functioning Scale: Role rating was 4.0 (of 10), and the mean BPRS positive and negative symptom factor scores were 2.1 and 2.7, respectively. These cognitive, role functioning, and symptom levels are typical of first-episode schizophrenia patients who are stabilized outpatients but have substantial impairment in cognition and role functioning. None of the demographic or clinical characteristics differed significantly between treatment conditions except sex, for which more females were assigned by chance to the CT&E group (χ2 = 7.38, df = 1, p < 0.01). Preliminary analyses with sex as a covariate indicated no impact on the primary treatment outcomes, so it was omitted from the main analyses.
Table 1. Demographic and clinical characteristics of patients in each treatment condition

The two treatment groups did not differ over the 6-month period in their attendance of the CT sessions (CT&E mean = 59.4 (s.d. = 29.3), CT mean = 57.2 (s.d. = 26.9), t42 = 0.26, p = 0.80). Similarly, they did not differ in their attendance at the weekly bridging group (CT&E mean = 17.9 (s.d. = 9.6), CT mean = 16.1 (s.d. = 8.4), t42 = 0.67, p = 0.51). The CT&E group completed a mean of 33.1 (s.d. = 17.5) exercise sessions in the clinic and 25.5 (s.d. = 14.2) home exercise sessions.
The rates of antidepressant use were comparable in the CT&E (33%) and CT (26%) groups (Chi-square = 0.30, p = 0.59). The rates of anticholinergic antiparkinsonian use also showed no significant difference for CT&E (29%) v. CT (13%) groups (Chi-square = 1.82, p = 0.18).
Cognition
The mixed model Group × Time analysis of the MCCB Overall Composite T Scores was completed with the baseline T score as a covariate, as some CT studies have suggested that the impact of CT differs by the severity of initial cognitive deficit. As hypothesized, the Group × Time (0, 3, and 6 months) interaction was significant (F = 3.33, p = 0.04), as shown in Fig. 2. A follow-up Group × Time (0 v. 3 mos.) contrast indicated that the source of this overall interaction is the substantially greater cognitive improvement with CT&E in the first 3 months (mean gain of 6.5 v. 2.2 T scores, t = −2.57, p = 0.012, Cohen's f = 0.43) (See Table 2).

Fig. 2. Differential cognitive gains with Cognitive Training & Exercise v. Cognitive Training (significant Group × Time interaction with baseline cognition covaried, F = 3.33, p = 0.04), due to substantially greater cognitive improvement for combined Cognitive Training & Exercise in the first 3 months (mean gain of 6.5 v. 2.2 T scores, t = −2.57, p = 0.012, Cohen's f = 0.43).
Table 2. Means and SDs for the outcome variables by group and occasion
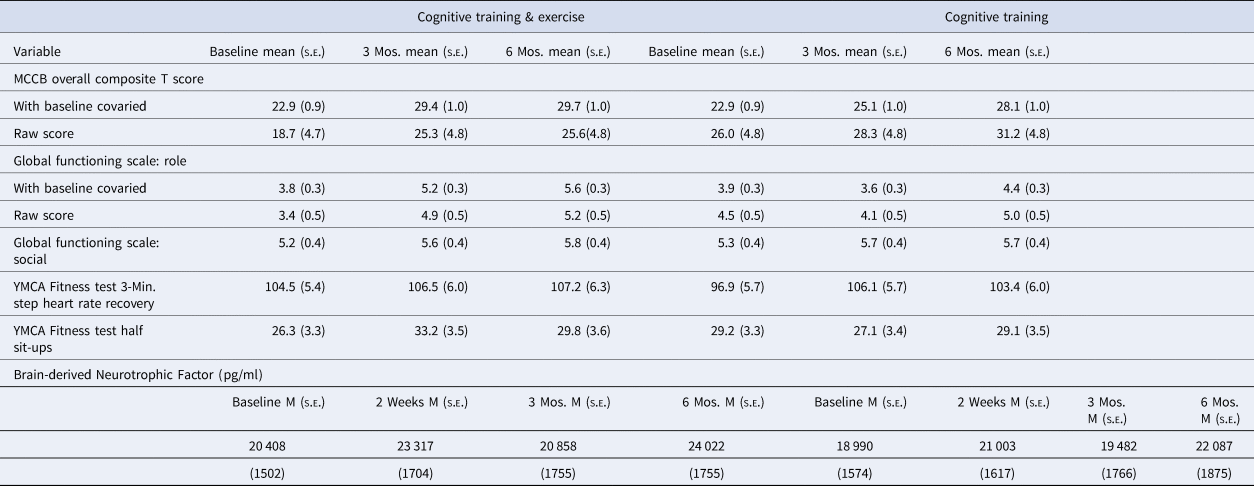
Functional outcome
Baseline Global Functioning Scale: Role was included as a covariate in the mixed model analyses due to a tendency for the groups to differ at baseline. As hypothesized, the Group × Time (0, 3, and 6 months) interaction for the Global Functioning Scale: Role rating was significant (F = 8.52, p < 0.001), as shown in Fig. 3 and Table 2. The improvement over 6 months was more than three times as large in CT&E compared to CT (mean gain of 1.8 v. 0.5, Cohen's f = 0.53). The impact of adding exercise to CT did not generalize to social functioning, as the Global Functioning Scale: Social rating showed an improvement in both groups over 6 months (F = 4.32, p < 0.02) but no Group × Time interaction (F = 0.24, p = 0.79).

Fig. 3. Differential work/school functioning gains with Cognitive Training & Exercise v. Cognitive Training (significant Group × Time interaction with baseline rating covaried, F = 8.52, p < 0.001, Cohen's f = 0.53).
BDNF
A mixed model Group × Time analysis (0, 2 weeks, 3 months, and 6 months) revealed a significant main effect of Time (F = 3.01, p < 0.04), but no Group × Time interaction. As shown in Table 2, BDNF levels in both groups increased during the six months. A subanalysis focusing on the short-term gains of beginning a regular exercise program, using baseline and 2-week data, revealed a gain with exercise that was numerically greater than with CT alone (mean gain = 2909 v. 2013 pg/ml) but again only the main effect of Time (F = 6.62, p < 0.02) but not the Group × Time interaction was significant.
The median interval between the last exercise session and the BDNF serum blood sample was 2 days, with the mean being 4.0 (s.d. = 6.2) due to CT&E participants who did not regularly complete their assigned exercises.
Because antidepressants are known to increase BDNF (Björkholm & Monteggia, Reference Björkholm and Monteggia2016), supplementary analyses were completed using antidepressant status as a factor or examining only those participants not taking antidepressants. The inclusion of antidepressant status as a factor did not reveal a significant antidepressant effect on BDNF nor did it notably change the Group, Time, or Group × Time effects. Omitting participants on antidepressants from BDNF analyses did not lead to a significant Group × Time interaction but did result in baseline to 2-week BDNF gain in the CT&E group that diverged further from that in the CT group (mean gain = 2918 v. 1108 pg/ml).
Fitness and muscular endurance
A mixed model Group × Time (0, 3, and 6 months) analysis of heart rate recovery in the 3-min step test of the YMCA Fitness Test (YMCA, 2000) did not detect significant Time or Group × Time effects. Muscular endurance measured by the 1 min half sit-up test showed a significant Group × Time interaction for baseline to 3 months (F = 4.61, p < 0.04), with the CT&E group increasing from a mean of 26.3 to 33.2 sit-ups while the CT group decreased from 29.2 to 27.1 (Table 2). This initial significant differential improvement in muscular endurance became a nonsignificant tendency over the full 6-month period (F = 2.82, p = 0.066).
The interval between the last exercise session and the fitness test had a median of 2 days but the mean was 8.5 days (s.d. = 15.7) due to CT&E participants who did not complete assigned exercises regularly. Five participants, all in the CT&E group, were on beta blockers that reduce heart rate and restrict its range. Omitting these participants from the analyses of heart rate recovery did not alter Group or Group × Time effects.
Correlations among outcomes over time
To examine the temporal ordering of early engagement of a putative intervention target on outcomes, we calculated correlations between two-week BDNF gain, three-month cognitive gain, and six-month work/school functional gain. BDNF increases in the first two weeks after randomization tended to be predictive of three-month MCCB Overall Composite scores (r = 0.31, n = 27, p = 0.11) and six-month Global Functioning Scale: Role ratings (r = 0.34, n = 22, p = 0.12) but neither correlation was significant due to small sample size. (Several patients declined the blood draws for BDNF.) The gain in the MCCB Overall Composite score from baseline to 3 months predicted the amount of improvement in the Global Functioning Scale: Role from baseline to 6 months (r = 0.35, n = 36, p < 0.04).
An exploratory analysis to examine the relationship between the physical and the cognitive effects of treatment revealed that the gain in muscular endurance over three months (number of sit-ups) tended to correlate with the three-month improvement in cognition (MCCB Overall Composite score, r = 0.28, n = 40, p < 0.08).
Dosage of exercise and cognitive training as predictors of outcomes
To examine whether it was the amount of physical exercise that was likely to be driving the improvements in cognition and work/school functioning in the CT&E treatment condition, we computed correlations between the amount of exercise completed and these outcome variables. The gain in cognitive performance over six months (MCCB Overall Composite score) was significantly associated with the proportion of assigned exercise that was completed (r = 0.56, n = 18, p < 0.02) and particularly with the number of homework exercise sessions that were completed (r = 0.61, n = 18, p < 0.01, Fig. 4). Positive but nonsignificant associations with cognitive gains were found for the number of exercise sessions completed in the clinic (r = 0.35) and the number of CT sessions completed in the clinic (r = 0.26).

Fig. 4. Scatterplot of the relationship between the amount of homework exercise sessions completed and degree of cognitive improvement over 6 months in the combined cognitive training & exercise program (r = 0.61, n = 18, p < 0.01).
For work/school functioning (Global Functioning Scale: Role), the amount of improvement within the CT&E treatment group was significantly associated with the number of exercise sessions completed (r = 0.51, n = 20, p < 0.02). It is of interest that work/school functioning gains were also significantly associated with the number of CT sessions attended (r = 0.46, n = 20, p = 0.04) and the number of bridging sessions attended (r = 0.56, n = 20, p = 0.01).
Discussion
As hypothesized, the addition of an aerobic exercise program to CT did lead to faster gains in cognition and larger improvements in work/school functioning than the same CT alone. The differential cognitive gain was evident in the first three months, when the addition of exercise produced a large effect size equivalent to Cohen's d = 0.86. (In a two-group RCT, Cohen's d equals twice Cohen's f (Cohen, Reference Cohen1988)). Thus, while both treatment groups improved over six months of CT, aerobic exercise provided a striking additional boost in the first three months. The benefit of adding aerobic exercise to CT was also clearly evident in work/school functioning, for which the differential gain over six months showed a large effect size equivalent to Cohen's d = 1.06.
These magnitudes are clearly larger than the mean effect sizes from meta-analyses for either CT or aerobic exercise alone in individuals with schizophrenia (Firth et al., Reference Firth, Stubbs, Rosenbaum, Vancampfort, Malchow, Schuch and Yung2017; Kambeitz-Ilankovic et al., Reference Kambeitz-Ilankovic, Betz, Dominke, Haas, Subramaniam, Fisher and Kambeitz2019; Vita et al., Reference Vita, Barlati, Ceraso, Nibbio, Ariu, Deste and Wykes2021; Wykes, Huddy, Cellard, McGurk, & Czobor, Reference Wykes, Huddy, Cellard, McGurk and Czobor2011), suggesting that this combination has a synergistic effect as would be predicted from animal models (Kempermann et al., Reference Kempermann, Fabel, Ehninger, Babu, Leal-Galicia, Garthe and Wolf2010; Kronenberg et al., Reference Kronenberg, Reuter, Steiner, Brandt, Jessberger, Yamaguchi and Kempermann2003) and initial work with older humans (Lauenroth et al., Reference Lauenroth, Ioannidis and Teichmann2016). In addition, our effect size for the addition of exercise to CT is larger for cognition than in the two earlier studies of combined exercise and CT with patients in later stages of schizophrenia (Malchow et al., Reference Malchow, Keller, Hasan, Dorfler, Schneider-Axmann, Hillmer-Vogel and Falkai2015; Oertel-Knöchel et al., Reference Oertel-Knöchel, Mehler, Thiel, Steinbrecher, Malchow, Tesky and Hänsel2014). The more frequent exercise and CT sessions over a longer period, plus the focus on FEP individuals, may have contributed to the larger effect size relative to the two prior studies that combined exercise and CT. FEP individuals may have higher levels of motivation to exercise and fewer comorbid physical diseases that limit exercise than individuals at later stages of schizophrenia. The fact that the randomized treatments occurred in the context of an active rehabilitation program may also facilitate larger effects on cognition and everyday functioning (Vita et al., Reference Vita, Barlati, Ceraso, Nibbio, Ariu, Deste and Wykes2021; Wykes et al., Reference Wykes, Huddy, Cellard, McGurk and Czobor2011).
The hypothesized sequence of effects from increased BDNF to cognitive gain to improved work/school functioning was partially supported. The latter link between three-month cognitive gain and six-month work/school improvement was significant (r = 0.35, n = 36, p < 0.04), but the similar correlation magnitude between two-week BDNF increases and three-month cognitive gain did not reach significance (r = 0.31, n = 27, p = 0.11). Larger sample sizes will be needed to determine whether this promising mechanism of action is clearly supported.
The view that the ‘dosage’ of exercise completed was a key factor in the cognitive gains of the CT&E group is supported by the strong correlations between six-month cognitive gains and proportion of exercise completed (r = 0.56) and the number of homework exercise sessions (r = .61). The generalization of cognitive gains to work/school functioning improvement is related to the amount of exercise completed (r = 0.51), but in addition is associated with the extent of participation in bridging groups (r = 0.56) and CT (r = 0.46). These correlations are consistent with the view that exercise is driving the enhanced cognitive gains, while additional intervention participation facilitates generalization to everyday functioning.
Several limitations of this study should be acknowledged. First, the sample size was designed for an initial RCT of the CT&E combination and is not large enough for a strong test of the hypothesized sequence of treatment effects. Second, no treatment group received aerobic exercise alone, so the size of that effect cannot be directly compared. The prior meta-analysis of the impact of exercise on cognition in schizophrenia (Firth et al., Reference Firth, Stubbs, Rosenbaum, Vancampfort, Malchow, Schuch and Yung2017) would, however, suggest that the observed effect of the CT&E combination compared to CT alone was larger than the expected effect of exercise alone. Third, the amount of clinic treatment time for the CT&E and CT groups was not equated, as the CT&E group received two clinic-based hours of exercise per week beyond the 4 h of CT that both groups received. The strong correlations between the amount of exercise actually completed and the size of cognitive and work/school functioning gains within the CT&E group, however, would imply that it was the impact of doing the exercise, including the homework, rather than treatment time per se, that is likely to be the active factor. Finally, motivation to engage in treatment and to achieve cognitive and work/school functioning improvements after a psychotic episode may be another factor in these results. The observed significant relationship between improved cognitive performance at three months and work/school functioning at six months suggests that cognitive gains partially impacted everyday functioning, but further examination of the role of motivation to engage in treatment and recover from the psychotic episode would be useful.
Conclusions
The combination of aerobic exercise and CT has cognitive and work/school functioning benefits after a first psychotic episode that go beyond CT alone. Exercise enhances initial cognitive gains and leads to larger later work/school functioning improvements. Further study and application of this combination of treatments is clearly warranted.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291722001696
Acknowledgements
We gratefully acknowledge the assistance of dedicated UCLA Aftercare Research Program staff members Yurika Sturdevant, Psy.D., Livon Ghermezi, B.A., Fe Asuan, B.A., and Lissa Portillo, statistician Gerhard Hellemann, Ph.D., and consultant Sophia Vinogradov, M.D.
Financial support
This research was supported by the NIMH (KHN, R34 MH102529, P50 MH066286, and R01 MH110544) (ClinicalTrials.gov Identifier: NCT02267070) and supplemental funding and medication by Janssen Scientific Affairs, L.L.C. (KHN, investigator-initiated study R092670SCH4005). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, the National Institutes of Health, or Janssen Scientific Affairs, L.L.C.
Conflicts of interest
Dr Nuechterlein reports medication and supplemental research grant support from Janssen Scientific Affairs, LLC., and has served as a consultant to Astellas, Genentech, Janssen, Medincell, Otsuka, Takeda, and Teva. He is an officer in the nonprofit company, MATRICS Assessment, Inc., which publishes the MCCB, but receives no financial compensation. Dr Ventura has received funding from Brain Plasticity, Inc., Genentech, Inc., and Janssen Scientific Affairs, LLC, and has served as a consultant to Boehringer-Ingelheim, GmbH, and Brain Plasticity, Inc. Dr Subotnik has received lecture honoraria from Janssen. Other authors report no potential conflicts of interest.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.









