Introduction
Neonatal mortality is one of the health parameters as well as a determinant of a country's health services. In 2015, it was estimated that the neonatal mortality rate in Indonesia was 14 per 1000 live births, with 35⋅5 % of it is caused by complications of preterm birth(1). Indonesia is ranked eighth out of ten countries with the highest number of neonatal deaths in the world, where there are 66 000 neonatal deaths or 2 % of all neonatal deaths in the world(1,2) . Indonesia is also the fifth country with the greatest number of preterm birth, only below India, China, Nigeria and Pakistan(2).
Etiology of preterm birth has not firmly established yet. From the clinical point of view, preterm birth can be caused by maternal, fetal and placental factors. Meanwhile, from the mechanism underlying preterm birth, there are several pathologies associated with preterm birth such as infection, multiple deliveries, genetic predisposition, environmental toxins, intra-amniotic inflammation, fetal allergies, uteroplacental ischaemia, uterine haemorrhage, oxidative stress, excessive uterine distension, immunity factors and nutritional deficiencies(Reference Buhimschi and Norman3).
An enormous amount of micro and macronutrients are essential during pregnancy, such as folic acid, iron, zinc, selenium, copper, Vitamin A, Vitamin B, Vitamin C, Vitamin D and Vitamin E(Reference Shah and Ohlsson4,Reference Bloomfield5) . Some of the nutrients were observed to have a significant correlation with preterm birth incidence, such as folic acid, zinc and Vitamin D. A study by Thota et al. showed that Vitamin D has an anti-inflammatory response to inhibit myometrial contractions in the process of preterm birth(Reference Thota, Farmer and Garfield6). Furthermore, a research conducted by Tamblyn et al. found that vitamin D has a role as an immunomodulatory and antibacterial secretion aggregator during pregnancy through the natural immune system and is obtained in pregnant women(Reference Tamblyn, Hewison and Wagner7). Another study by Irwinda et al. also showed that preterm birth mother had significantly lower micronutrients such as AtRA, manganese, copper, zinc, iron, copper, selenium and vitamin D(Reference Irwinda, Wibowo and Putri8).
Not only to transfer the nutrients but placenta also plays an important role as a link between mother and fetus by forming decidua. This decidua will act as a place for the presence of various immune cells during pregnancy, including Vitamin D(Reference Shin, Choi and Longtine9). In vitamin D metabolism, it first undergoes hydroxylation by the enzyme 25-Hydroxylase to form 25-Hydroxyvitamin D3 (25(OH)D3). Then, 25(OH)D3, which is the main form of vitamin D in the maternal circulation, will be carried by vitamin D binding protein to the kidneys and placenta. In the kidneys, with the help of the enzyme 1α-hydroxylase (CYP27B1) will form an active form of vitamin D, namely 1,25-Dihidroxyvitamin D3 (1,25(OH)2D3). In the placenta, CYP27B1 and vitamin D receptors (VDR) are expressed in order to extra-renally synthesise 1,25(OH)2D3(Reference Shin, Choi and Longtine9). This data suggest that placenta has a role in synthesising vitamin D.
In a previous systematic review of the status of Vitamin D globally in 2015, a prevalence of 54 % of pregnant women in deficiency was found, and 18 % in severe deficiency(Reference Saraf, Morton and Camargo10). Moreover, a study by Wibowo and Irwinda in Jakarta, Indonesia, also showed a deficiency of Vitamin D level during the first trimester in 99 % of the population(Reference Thota, Farmer and Garfield6). Wei et al. also conducted a systematic review and meta-analysis of the relationship between Vitamin D status and the incidence of preterm birth, and it was found that Vitamin D deficiency status was a risk factor for preterm birth(Reference Wei, Qi and Luo11).
This study aims to determine the status of Vitamin D derivate, which are 25(OH)D3, and 1,25(OH)2D3 in maternal serum and placenta, and its regulation in placenta by CYP27B1 between term and preterm birth.
Methods
This is an analytic observational study using the cross-sectional method to assess the status of 25(OH)D3, 1,25(OH)2D3 in maternal serum and placental tissue, and placental CYP27B1 enzyme between term and preterm birth. Data were taken from Cipto Mangunkusumo Hospital, Jakarta, Indonesia, from January 2017 to August 2019. Using the random sampling method, thirty normal pregnancy and thirty preterm birth samples were used in this study.
The inclusion criteria for the study were a mother with a single intrauterine pregnancy, whether having preterm or term pregnancy. Mothers with multiple pregnancy, fetal growth restriction, congenital anomaly, preterm premature rupture of membrane (PPROM) or having other systemic comorbidities were excluded in this study.
Maternal blood and placental tissue samples were directly taken after delivery. Sample with delivery of more than 1 h will not be included. In order to acquire the status of the 25(OH)D3 and 1,25(OH)2D3 level, a liquid chromatography-tandem mass spectrometry (LCMS/MS) method was used. This assay demonstrated good intra and interassay precision, with CV <10 %. An Agilent 6460 triplequad LCMS system was used to measure 25(OH)D3 and Acquity I Class Binary Solvent Manager FTN and Xevo TQXS Tandem Mass Spectrometry for 1,25(OH)2D3. Furthermore, CYP27B1 level was obtained using Microplate Reader Biorad Machine model 680 with software Microplate Manager ver. 5.2.1. and measured using the ELISA method. The level of 25(OH)D3 was classified into deficiency (<20 ng/ml) and normal (≥20 ng/ml)(Reference Qin, Lu and Yang12).
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Research Ethics Committee of Faculty of Medicine, Universitas Indonesia with ethical clearance number LB.02.01/X.2/179/2016. Written informed consent was obtained from all subjects, before the study is started.
Collected data were then analysed using SPSS for Macintosh ver. 20. Characteristics of patients in the form of sociodemographic and clinicopathologically were analysed descriptively. Comparative and correlative analysis was done using unpaired T-test and Pearson for normally distributed data, also Mann–Whitney and Spearman for non-normally distributed data. This study used 5 % error bound and 95 % confidence interval limit, power of the test considered to be 90 %.
Results
A total of sixty patients met the inclusion criteria and had been further analysed. Univariate test was performed to assess the general characteristics of the study subjects’ socio-demographic and clinicopathologic variables (Table 1).
Table 1. Clinical characteristics of subjects
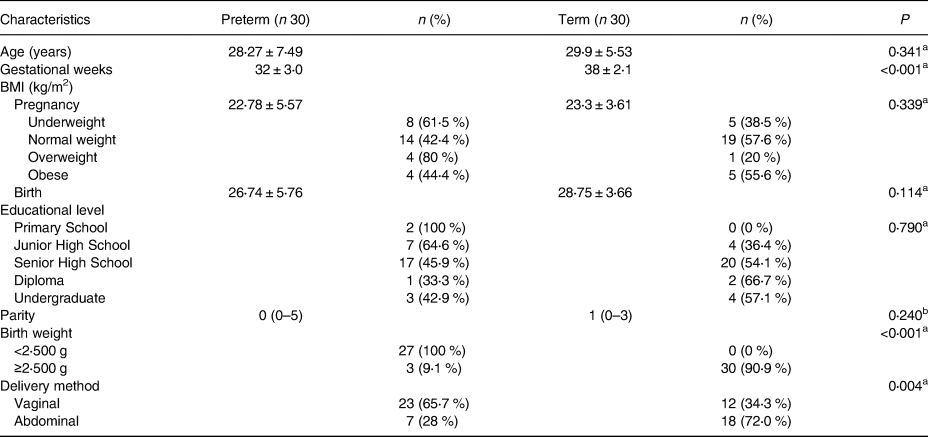
Data presented in Mean ± sd or Median (IQR).
a Unpaired T-test.
b Mann–Whitney.
Vitamin D status on preterm and term subjects were obtained and compared. Maternal serum 25(OH)D3 was classified into deficiency group (<20 ng/ml) and normal group (≥20 ng/ml). Results of this study can be found in Table 2.
Table 2. Vitamin D status of subjects
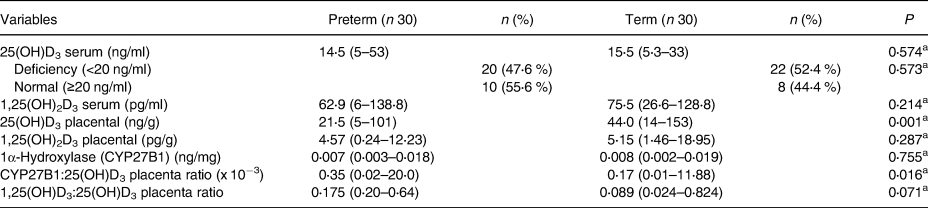
Data presented in Median (IQR).
a Mann–Whitney.
Furthermore, in order to determine the correlation between different vitamin D components, the correlation study was done to all variables. Significant correlation can be found on CYP27B1 with placental 25(OH)D3 (r −0⋅481, P < 0⋅001) and placental 1,25(OH)D3 (r −0⋅365, P = 0⋅004). Meanwhile, there was no correlation between other vitamin D components (P > 0⋅005).
Discussion
The vitamin D derivate status was suspected to be less with preterm birth, and our study depicts the same result. We found that the median of 25(OH)D3 serum level in all subjects was 15 ng/ml, with the preterm subject had 1 ng/ml less than control. These results are lower than the average obtained in previous studies in the Southeast Asian region, with a range of 20–52 ng/ml(Reference Saraf, Morton and Camargo10). Not statistically significant differences in 25(OH)D3 levels between preterm and term delivery also found in the previous study by Irwinda et al. (Reference Irwinda, Wibowo and Putri8). These findings were found in contrary with another study conducted in China in 2013, which stated that mothers with serum 25(OH)D3 levels below 25 ng/ml had a significantly higher risk of experiencing preterm birth(Reference Qin, Lu and Yang12). Another study also mentioned 25(OH)D3 serum >20 ng/ml has a protective effect against preterm birth(Reference Wagner, McNeil and Johnson13). In addition, significant differences found in placental 25(OH)D3 level (P = 0⋅001). This result is consistent with previous research in Jakarta(Reference Irwinda, Wibowo and Putri8). Another study also showed a trend of significant increases in 25(OH)D3 level per trimester(Reference Tamblyn14). This data supported the anti-inflammatory effect as well as an immune system regulator of 25(OH)D3 in maternal serum and placenta, which may prevent preterm birth and preeclampsia(Reference Thota, Farmer and Garfield6,Reference Shin, Choi and Longtine9,Reference Wagner, McNeil and Johnson13) .
There were no significant differences levels of 1,25(OH2)D3 placenta between the two groups (P > 0⋅05). These results represent that there is no difference in the active form of vitamin D in placenta between preterm and term, but the lower inactive form of vitamin D was found in preterm birth, meaning that the process of active form was already converted in preterm birth, with a low reservoir level.
Moreover, no significant differences found in the CYP27B1 level, with placental CYP27B1 and 25(OH)D3 ratio is higher in preterm birth. Furthermore, the 1,25(OH)2D3–25(OH)D3 ratio in preterm patients was clinically higher, albeit statistically insignificant (P > 0⋅05). These results showed that in preterm labour, metabolically inactive 25(OH)D3 is broken down at a higher rate than in term labour. These findings are also similar to previous studies assessing the expression of CYP27B1 mRNA in rat placenta, which found no differences between term and preterm birth groups, even after vitamin D supplementation(Reference Fu, Chen and Xu15). The previous study by Noyola-Martinez et al. also found that CYP27B1 expression would increase in the presence of some pro-inflammatory cytokines in trophoblast such as TNF-α, IFN-γ IL-6 and IL-1β(Reference Noyola-Martinez, Diaz and Zaga-Clavellina16). This study also found a significant negative correlation between CYP27B1 and 25(OH)D3 and 1,25(OH)2D3 placenta. However, a stronger negative correlation was found between CYP27B1 and 25(OH)D3, in accordance with the function of CYP27B1 to change the form of inactive vitamin D to become active.
To our knowledge, this is the first study to directly compare different vitamin D status in serum and placenta of preterm and term women in Indonesia. However, no record of dietary intake and sun exposure during their pregnancy may become the limitation as it could interfere with the result of their vitamin D status. Regarding the method used to measure the level of CYP27B1 and 25(OH)D3 and 1,25(OH)2D3, the use of DiaSorin LIAISON showed the best characteristics among others for automated 25OH-D immunoassays. However, LCMS/MS isotope dilution still can be considered as the gold standard for small molecules analytic measurement. We also followed the Vitamin D council and Institute of Medicine (IOM) for the threshold for vitamin D deficiency (<20 ng/ml). This may differ from other studies using a higher threshold for categorising vitamin D deficiency. We used this as it has been used in many associations in society, and this number believed to already have a health impact, particularly skeletal health.
In conclusion, lower placental 25(OH)D3 status and higher placental CYP27B1 and 25(OH)D3 ratio was obtained in subjects with preterm compared to term birth.
Acknowledgements
The authors would like to express sincere gratitude to all participating patients who willingly support this study. The authors would also like to extend special thanks to our parents and family for academical guidance and psychological supports.
The authors declare that there is no conflict of interest in this study.
Data from this study are to be found in Cipto Mangunkusumo National General Hospital medical records and are available upon reasonable request.
Funding for this study is fully fulfilled by authors.
R. I. designed the research concept, methodology, investigation, funding acquisition and supervision. B. A. analysed the data and wrote the paper. R. I. had primary responsibility for final content. Both authors read and approved the final manuscript.




