Psychosocial stressors and low socioeconomic status (SES) during childhood and adolescence are associated with a greater risk of cardiovascular disease (CVD) and metabolic disorders in adulthood, with more severe and chronic stressors associated with increasing risk for adult chronic diseases (Dong et al., Reference Dong, Giles, Felitti, Dube, Williams, Chapman and Anda2004; Fuller-Thomson, Brennenstuhl, & Frank, Reference Fuller-Thomson, Brennenstuhl and Frank2010; Gilbert et al., Reference Gilbert, Breiding, Merrick, Thompson, Ford, Dhingra and Parks2015; Kittleson et al., Reference Kittleson, Meoni, Wang, Chu, Ford and Klag2006). The latest consensus of the American Heart Association (AHA) identifies adverse environments during childhood and adolescence as important determinants of obesity, Type 2 diabetes, and coronary heart disease in the American population, especially in socially vulnerable groups (Suglia et al., Reference Suglia, Koenen, Boynton-Jarrett, Chan, Clark, Danese and Isasi2018). Many studies that examine associations between childhood and adolescent stress and adult cardiometabolic diseases measure retrospective reports of early life psychosocial stress and adult cardiometabolic risk. This approach is problematic as adult reports of stress during childhood may be inaccurate due to memory limitations or biased by current levels of stress, psychopathology, health problems, or personality dimensions such as neuroticism (Baldwin, Reuben, Newbury, & Danese, Reference Baldwin, Reuben, Newbury and Danese2019). Retrospective research is particularly problematic in determining whether there are sensitive periods of development during which psychosocial stress is more strongly associated with adult cardiometabolic risk. Few studies have assessed psychosocial stress prospectively from infancy through adulthood (exceptions: Alastalo et al., Reference Alastalo, Räikkönen, Pesonen, Osmond, Barker, Heinonen and Eriksson2013a; Alastalo et al., Reference Alastalo, von Bonsdorff, Räikkönen, Pesonen, Osmond, Barker and Eriksson2013b; Johnson et al., Reference Johnson, Huelsnitz, Carlson, Roisman, Englund, Miller and Simpson2017). In the current study, we tested for sensitive periods of psychosocial risk – defined as both psychosocial stressors and socioeconomic risk – that may have the strongest associations with cardiometabolic outcomes in young adulthood.
The few prospective studies of child maltreatment have supported findings from retrospective studies demonstrating associations between greater psychosocial stress during childhood and poorer adult cardiometabolic outcomes (Johnson et al., Reference Johnson, Huelsnitz, Carlson, Roisman, Englund, Miller and Simpson2017). Maternal verbally aggressive behavior during infancy has been associated with higher child blood pressure (BP) at 5–6 years of age (Smarius, Strieder, Doreleijers, Vrijkotte, & de Rooij, Reference Smarius, Strieder, Doreleijers, Vrijkotte and de Rooij2018). Adolescents who had experienced child abuse also showed higher resting diastolic blood pressure (DBP) and lower systolic blood pressure (SBP) and DBP reactivity (Gooding, Milliren, Austin, Sheridan, & McLaughlin, Reference Gooding, Milliren, Austin, Sheridan and McLaughlin2015). Some studies have found a threshold effect, such that adolescents who experienced four or more previous adverse experiences demonstrated higher resting heart rate, waist circumference, and body mass index (BMI) (Pretty, O'Leary, Cairney, & Wade, Reference Pretty, O'Leary, Cairney and Wade2013). A previous study with the current cohort demonstrated associations between higher psychosocial risk during infancy and higher BMI z score at 10 years (Doom et al., Reference Doom, Reid, Blanco, Burrows, Lozoff and Gahagan2019b). By adolescence, greater risk for metabolic syndrome (MetS) and greater cardiometabolic risk across anthropometric, BP, and serum cardiovascular measures was observed for adolescents who experienced higher psychosocial risk in infancy (Doom et al., Reference Doom, Reid, Blanco, Burrows, Lozoff and Gahagan2019b).
Less research has examined whether particular sensitive periods are associated with adult cardiovascular risk. A prospective longitudinal birth cohort in New Zealand found that childhood maltreatment between ages 3–11 years was associated with higher levels of high-sensitivity C-reactive protein (CRP), an inflammatory marker predictive of CVD, at age 32 years (Danese, Pariante, Caspi, Taylor, & Poulton, Reference Danese, Pariante, Caspi, Taylor and Poulton2007). In a different cohort in the United States, children with documented physical abuse experiences before age 11 years matched with children with no maltreatment experiences had higher levels of CRP than those without maltreatment 30 years later (Widom, Czaja, Bentley, & Johnson, Reference Widom, Czaja, Bentley and Johnson2012). Another study found that psychosocial stressors before age 12 years were correlated with elevated chronic disease risk in adulthood (Slopen, Non, Williams, Roberts, & Albert, Reference Slopen, Non, Williams, Roberts and Albert2014). These prospective studies suggest that experiencing high psychosocial risk before age 12 years may be particularly associated with poor adult cardiometabolic outcomes. These effects may be partially mediated through early life programming of the sympathetic nervous system, hypothalamic–pituitary–adrenal (HPA) axis, immune system, and epigenetics, as well as through changes in behavior (Nusslock & Miller, Reference Nusslock and Miller2016; Reynolds, Reference Reynolds2013; Suglia et al., Reference Suglia, Campo, Brown, Stoney, Boyce, Appleton and Dunn2020).
There are multiple developmental periods within the first 12 years of life. Few prospective studies have been able to examine the relative importance of specific developmental periods. In a study of adults who were temporarily separated from their parents as children during World War II, those who were separated during early childhood (4–7 years old) were more likely to show elevated BP compared to adults not separated from their parents as children, with stronger effects for males (Alastalo et al., Reference Alastalo, Räikkönen, Pesonen, Osmond, Barker, Heinonen and Eriksson2013a). In the same cohort, male children who were separated at > 7 years of age showed the highest prevalence of CVD in adulthood compared to adult males who were not separated as children (Alastalo et al., Reference Alastalo, von Bonsdorff, Räikkönen, Pesonen, Osmond, Barker and Eriksson2013b). In a large retrospective study of adults in the United States, heart disease in adulthood was associated with psychosocial stress experienced during ages 6–10 years and 15–17 years, though the timing of stress mattered less than repeated exposure to stress (Friedman, Montez, Sheehan, Guenewald, & Seeman, Reference Friedman, Montez, Sheehan, Guenewald and Seeman2015). Still further, another study found that low SES in infancy (1–2 years of age) was predictive of higher inflammatory protein interleukin (IL)-6 during adulthood, even after controlling for adult SES (Carroll, Cohen, & Marsland, Reference Carroll, Cohen and Marsland2011). In the current cohort, lower social capital in infancy, which involved lower parental income and education, not having insurance, and household overcrowding, was associated with lower high-density lipoprotein (HDL) cholesterol in adolescence (Madewell, Blanco, Burrows, Lozoff, & Gahagan, Reference Madewell, Blanco, Burrows, Lozoff and Gahagan2019). Together, these findings suggest that there may be different sensitive periods of psychosocial risk for different cardiometabolic outcomes. However, it may be that psychosocial risk during adulthood is still more predictive of cardiometabolic health than psychosocial risk during childhood (for a review of associations between adult stress and coronary heart disease, see Wirtz & von Känel, Reference Wirtz and von Känel2017), emphasizing the need to control for adult psychosocial risk when measuring adult health outcomes (Pollitt et al., Reference Pollitt, Kaufman, Rose, Diez-Roux, Zeng and Heiss2007). There is also evidence that experiencing stressors during multiple developmental stages, rather than during any particular stage, is the most predictive of adult heart disease (Friedman et al., Reference Friedman, Montez, Sheehan, Guenewald and Seeman2015). The repeated exposure to stress may predict chronic responses and adaptations to stress that may lead to wear and tear on the cardiovascular system over time (McEwen, Reference McEwen1998), which could be more predictive of cardiovascular risk in adulthood than timing of stress. We tested the timing versus chronicity question in our study using repeated, prospective measures of psychosocial risk from infancy to young adulthood.
The current study examined various psychosocial risks during infancy, at 5 years, at 10 years, in adolescence, and at young adulthood, to determine the timing of risk that is most closely associated with young adult cardiometabolic outcomes. The study also examined whether the mean or maximum levels of psychosocial risk across the four periods from infancy through adolescence were better predictors of young adult cardiometabolic outcomes than psychosocial risk at a specific time point. The current study also examined multiple types of cardiometabolic risk factors, including BMI, waist circumference, percent body fat (%BF), and BP, rather than using a single metric of cardiometabolic health. A relatively large, prospective, longitudinal cohort in Santiago, Chile was used to test these associations. Based on research findings from this cohort and others (Carroll et al., Reference Carroll, Cohen and Marsland2011; Doom et al., Reference Doom, Reid, Blanco, Burrows, Lozoff and Gahagan2019b; East et al., Reference East, Delker, Blanco, Burrows, Lozoff and Gahagan2019; Madewell et al., Reference Madewell, Blanco, Burrows, Lozoff and Gahagan2019; Smarius et al., Reference Smarius, Strieder, Doreleijers, Vrijkotte and de Rooij2018), we hypothesized that psychosocial risk during infancy would demonstrate the strongest association with cardiometabolic outcomes in young adulthood. Due to evidence that stress before age 12 may be particularly predictive of later cardiometabolic health (Slopen et al., Reference Slopen, Non, Williams, Roberts and Albert2014), we further hypothesized that psychosocial risk at 5 years and 10 years, and mean and maximum of psychosocial risk, would also be predictive of cardiometabolic outcomes, though associations would be weaker than with psychosocial risk in infancy.
Method
Analyses used data from the Santiago Longitudinal Study, which began as an iron deficiency anemia preventive trial in infancy, with follow-up at 5 years, 10 years, adolescence, and young adulthood (Lozoff et al., Reference Lozoff, De Andraca, Castillo, Smith, Walter and Pino2003). From 1991–1996, 6-month-old infants and their mothers, who were from working-class neighborhoods, were recruited from community clinics in Santiago, Chile. Inclusion criteria for enrollment included birth weight ≥ 3.0 kg, singleton term birth, stable caregiver, vaginal delivery, and residence in four communities in Santiago. Exclusion criteria included birth complications, major congenital anomaly, phototherapy, illness, hospitalization longer than 5 days, iron therapy, another infant less than 12 months old in the household, infant in day care, or a caregiver who was illiterate or psychotic (self-reported or reported by family on a recruitment questionnaire) (Lozoff et al., Reference Lozoff, De Andraca, Castillo, Smith, Walter and Pino2003). The Institutional Review Boards at the participating institutions in the United States and Chile approved the initial and follow-up studies. Informed consent was obtained from the parent at each time point and the target child once they reached young adulthood. Child assent was obtained at age 10 years and at adolescence.
Study design
Children without iron deficiency anemia at 6 months were randomized to receive high-iron, low-iron, or no iron supplementation for 6 months (Lozoff et al., Reference Lozoff, De Andraca, Castillo, Smith, Walter and Pino2003). Children with iron deficiency anemia and the next nonanemic infant enrolled were treated with iron instead of entering the preventive trial and enrolled into additional study components, referred to as Study 2 (Roncagliolo, Garrido, Walter, Peirano, & Lozoff, Reference Roncagliolo, Garrido, Walter, Peirano and Lozoff1998). Infants in both studies were assessed for anemia again at 12 and, for some, at 18 months. Infants with iron deficiency anemia at 12 or 18 months received iron therapy and completed additional assessments as part of Study 2. All participants in Study 2 reported on psychosocial risk using the same procedures as participants in the preventive trial. These study design factors (participating in Study 2, receiving iron in the supplementation trial) were included as covariates for the analyses.
Due to limited funding at the age 5 years assessment, the no-added-iron group, the high-iron supplementation group, and Study 2 participants were invited to participate in a follow-up assessment where psychosocial risk was assessed (see details of psychosocial risk measure below). At age 10 years and in adolescence (11–17 years; M = 14.3), all infancy study participants were invited to participate in an assessment where psychosocial risk was assessed (Lozoff, Castillo, Clark, & Smith, Reference Lozoff, Castillo, Clark and Smith2012). In young adulthood (21–27 years; M = 23.0), all participants were invited to participate in a cardiometabolic risk assessment. A total of 1040 participants provided cardiometabolic data at the young adult visit and were included in the current analyses. For the dual X-ray absorptiometry (DXA) scan to assess body fat, a subset of the full sample (N = 631) participated in the assessment. This subset was chosen because they participated in the 5-year assessment and adolescent cardiovascular assessment, which would give us the most complete longitudinal data for these individuals.
Psychosocial risk
Clinical psychologists interviewed mothers about family-level psychosocial risk when the infants were 6–12 months old. Mothers reported on family-level psychosocial risk at 5 years, 10 years, and adolescence as well. Psychosocial risk was operationalized as a composite variable using the top quartile of risk in seven categories at infancy, 5 years, 10 years, and adolescence: maternal depressive symptoms (Radloff, Reference Radloff1977, Reference Radloff1991), low support for child development in the home (Bradley, Corwyn, & Whiteside-Mansell, Reference Bradley, Corwyn and Whiteside-Mansell1996; Caldwell & Bradley, Reference Caldwell and Bradley1984), family stressors ((Bradley et al., Reference Bradley, Corwyn and Whiteside-Mansell1996; Holmes & Rahe, Reference Holmes and Rahe1967), father absence, low family SES (Alvarez, Muzzo, & Ivanovic, Reference Alvarez, Muzzo and Ivanovic1985), and years of maternal and paternal education. Quartiles were defined within each age. Both higher psychosocial stress and greater socioeconomic risk during childhood have been associated with more cardiovascular risk factors in adulthood (Dong et al., Reference Dong, Giles, Felitti, Dube, Williams, Chapman and Anda2004; Kittleson et al., Reference Kittleson, Meoni, Wang, Chu, Ford and Klag2006). Thus, we chose to include both family SES and maternal and paternal education in the composite to more fully capture the socioeconomic environment of the family (Hauser, Reference Hauser1994).
Descriptive statistics were calculated for each of the seven risk variables to determine quartiles. Maternal depressive symptoms were assessed using the mother's report on the Center for Epidemiological Studies – Depression Scale (Radloff, Reference Radloff1977, Reference Radloff1991) (CES-D; infancy risk quartile ≥ 22). A trained researcher completed the Home Observation for Measurement of the Environment (HOME) Inventory (Caldwell & Bradley, Reference Caldwell and Bradley1984) through home observation in infancy. The HOME measured home support for child development, including opportunities for variety in daily stimulation, provision of play materials, organization of the environment, and the parent's responsivity and involvement with the target child (infancy risk quartile < 28). The HOME is well validated and shows sensitivity to variations in families, with validation in Latin America (Bradley et al., Reference Bradley, Corwyn and Whiteside-Mansell1996; Caldwell & Bradley, Reference Caldwell and Bradley1984). The mother also reported the number of family stressors using a modified Social Readjustment Rating Scale (Holmes & Rahe, Reference Holmes and Rahe1967) (risk quartile > 5). Father absence was reported by the mother and was assigned a value of 1 if absent and 0 if present. SES was measured with a modified Graffar index (higher scores indicate lower SES; infancy risk quartile ≥ 27 (Alvarez et al., Reference Alvarez, Muzzo and Ivanovic1985). The Graffar accounts for family-level factors such as number of people in the home, employment status of the head of household, home ownership, housing construction type and size, availability of running water, ownership of major material goods (e.g., home appliances, car), and crowding. The mother reported the number of years she attended formal education and the years of education completed by the child's father (infancy risk quartile < 9 years). At age 5 years, 10 years, and adolescence, mothers reported on the same family-level psychosocial risk measures as in the infancy assessment, with risk quartiles calculated within each specific age period. At the age 5 years and 10 years assessments, clinical psychologists interviewed mothers to answer HOME questions. At the adolescent assessment, mothers answered questions about the HOME directly.
Participants in the risk quartile for each variable were assigned a value of 1, while those in the other three quartiles were assigned a value of 0. These values were then summed (within each age period) to create a risk score that ranged from 0 to 7. For missing values, scores were prorated with nonmissing items using the mean across available items. If a participant was missing more than three risk categories, they were assigned a missing value for the composite. We have used these composites previously (Doom, Gahagan, Caballero, Encina, & Lozoff, Reference Doom, Gahagan, Caballero, Encina and Lozoff2019a; Doom et al., Reference Doom, Reid, Blanco, Burrows, Lozoff and Gahagan2019b). Cutoffs for risk quartiles at age 5 years, 10 years, and adolescence are reported in the supplemental materials. In infancy, there was a higher rate of missing data on maternal depressive symptoms (32%) and father absence (26%) than at other time points, due to sampling necessitated by budget cuts (Lozoff et al., Reference Lozoff, De Andraca, Castillo, Smith, Walter and Pino2003). Maternal depressive symptoms and father absence in infancy were imputed with SPSS 25.0 (10 imputations) using infancy control variables as well as depressive symptoms and father absence variables at ages 5 years, 10 years, and at adolescence. These imputed values were used to determine risk quartile for the variables.
This study tested whether the mean or maximum of psychosocial risk from infancy through adolescence was more closely associated with young adult cardiometabolic outcomes than psychosocial risk at a particular time point. For the mean psychosocial risk, a mean of the psychosocial risk scores at infancy, 5 years, 10 years, and adolescence was calculated. For the maximum psychosocial risk, the highest psychosocial risk score at any time point from infancy, 5 years, 10 years, and adolescence was used to assess the highest level of psychosocial risk.
In young adulthood, mothers no longer reported on family-level psychosocial risk. Instead, young adults themselves reported on perceived stress using the Perceived Stress Scale (PSS-14) (Cohen, Kamarck, & Mermelstein, Reference Cohen, Kamarck and Mermelstein1983) at a 21 year assessment preceding the young adult cardiometabolic evaluation. The 21 year visit largely targeted neuromaturation outcomes related to the aims of the original iron supplementation trial, though information about young adult psychosocial risk and mental health was also collected. At 21 years, participants reported on their years of education and depressive symptoms using the raw depression subscale of the Adult Self-Report Scale (Achenbach & Rescorla, Reference Achenbach and Rescorla2003). These variables were entered separately in the models as covariates.
Young adult anthropometric and cardiometabolic assessment
The following cardiometabolic risk factors were assessed in young adulthood (median = 22.7 years): BMI, waist circumference, fat mass index (fat mass/height2), percent fat mass (fat mass/total body mass), systolic BP (SBP) and diastolic BP (DBP), fasting plasma glucose, triglycerides (TG), and total and HDL cholesterol.
A research physician used standardized procedures to measure the height (cm) to the nearest 0.1 cm, using a Holtain stadiometer, and weight (kg) to the nearest 0.1 kg, using a scale (Seca GmbH & co. Hamburg, Germany). Waist circumference was measured with nonelastic flexible tape and recorded to 0.1 cm (Seca 201, Seca GmbH & co. Hamburg, Germany). Measurements were taken twice, with a third measurement if the difference between the first two exceeded 0.3 kg for weight, 0.5 cm for height and 1.0 cm for waist circumference. BMI was calculated as kg/m2. Total fat mass (TFM) was determined on DXA (apparatus: Lunar Prodigy Corp., Madison, WI, USA. Software: Lunar iDXA ENCORE 2011, Version 13.60.033). After 15 min at rest and before the other physical evaluations, SBP and DBP were measured three times on the nondominant arm using a standard mercury sphygmomanometer; the average value of the last two measurements was used for analyses. Fasting serum total glucose (enzymatic colorimetric test; QCA S.A., Amposta, Spain), total cholesterol, TG, and HDL-cholesterol (HDL-C) dry analytical methodology (Vitros®; Ortho Clinical Diagnostics Inc., Raritan, NJ, USA), were measured after an 8–12 hour overnight fast.
We used the International Diabetes Federation (IDF) criteria for MetS (Alberti, Zimmet, & Shaw, Reference Alberti, Zimmet and Shaw2006) to create a continuous measure of the number of MetS risk components. The following criteria were used to create five dichotomous variables that were added to create the number of MetS components variable, which ranged from 0–5: (a) waist circumference ≥ 90 cm for males and ≥ 80 cm for females, (b) TG ≥ 150 mg/dL, (c) HDL-cholesterol <40 mg/dL for males and <50 mg/dL for females, (d) SBP >130 mm Hg or DBP >85 mm Hg, and (e) fasting plasma glucose ≥100 mg/dL.
Covariates
We considered the following as covariates: age, sex, birthweight, weight increase from birth to 6 months (in grams), received iron supplementation in preventive trial (1 = yes, 0 = no), participated in Study 2 (and thus received iron treatment, 1 = yes, 0 = no), daily formula/milk consumption (mean daily formula/milk consumption [mL/day] between 6 and 12 months, obtained at weekly home visits), and the young adult's self-report of perceived stress, education level, and depressive symptoms at 21 years. We considered family history of CVD based on participant report on whether a parent had any of the following conditions (yes = 1, no = 0): heart disease before age 60, hypertension, dyslipidemia, or diabetes. The sum of these four variables was calculated to create a family history of CVD risk score ranging from 0 to 4. Multiple imputation techniques were used to impute control variables with missing values (Rubin, Reference Rubin1987) by the study biostatistician with IVEWARE in SAS (Lozoff et al., Reference Lozoff, De Andraca, Castillo, Smith, Walter and Pino2003; Raghunathan, Solenberger, & van Hoewyk, Reference Raghunathan, Solenberger and van Hoewyk2000).
Data analytic plan
Principal component analysis to create cardiometabolic outcome factors
Principal component analysis with SPSS 25.0 was used to decide how best to calculate composites of cardiometabolic outcomes, similar to those created using principal component analysis in a study by Goodman and colleagues (Goodman, Dolan, Morrison, & Daniels, Reference Goodman, Dolan, Morrison and Daniels2005). For the continuous cardiometabolic outcome variables, individual values were converted to z scores and then winsorized to 3 SD above or below the mean to handle outliers. The following cardiometabolic variables were included in the principal component analysis: BMI, waist circumference, fat mass, fat mass index, percent fat mass (fat mass/total body mass), SBP, and DBP. This analysis yielded a BP composite (systolic and diastolic; factor loadings ≥ .92; α = .83) and a BMI and waist circumference composite consisting of those two variables (factor loadings ≥ .98; α = .97). As only a subsample of participants completed DXA assessments in young adulthood, we considered the DXA variables as a separate factor composed of fat mass, fat mass index, and percent fat mass (factor loadings ≥ .96; α = .98). To account for sex differences in cardiometabolic risk variables, each of the variables in the composites was standardized within sex such that the three composites were sex-adjusted. The mean of the z-scored variables in each of the components was calculated as the value for that component. The BMI and waist circumference composite, BP composite, fat mass composite, and the number of MetS components variables were used as dependent variables in two separate analyses. As the number of MetS components variable included waist circumference and BP, which were part of the factors identified in the principal component analysis, it was analyzed separately from the individual components as a measure of composite risk.
Sensitive periods model for BMI and waist circumference, BP, and fat mass
Path analysis with bootstrapping (10,000 iterations) in Mplus 7 (Version 1.4) was conducted to test whether psychosocial risk at infancy, 5 years, 10 years, or adolescence was most predictive of cardiometabolic outcomes described above. Figure 1a shows the main paths tested without covariates displayed. We kept the dependent composites separate to test whether psychosocial risk at different time points has unique associations with the three different types of cardiometabolic outcomes: (a) BMI and waist circumference composite, (b) BP composite, and (c) fat mass composite. The across-time measurement of psychosocial risk was included by the direct paths from infancy to 5 years, 5 years to 10 years, and 10 years to adolescence, as well as direct paths from each of the four psychosocial risk timing variables to each of the three dependent variables of cardiometabolic risk. Model fit was evaluated with the comparative fit index (CFI ≥ .90), root mean square error of approximation (RMSEA < .06), and standardized root mean square residual (SRMR < .08) (Browne & Cudeck, Reference Browne, Cudeck, Bollen and Long1993; Hu & Bentler, Reference Hu and Bentler1999; Kline, Reference Kline2005). The standard maximum likelihood estimator was used to estimate the model and to handle missing data. Covariates on each of the psychosocial risk variables included sex, birthweight, receiving iron supplementation in infancy, and participating in Study 2 in infancy. Covariates on each of the cardiometabolic risk variables included sex, birthweight, weight change from 0–6 months (indexing rapid growth), receiving supplemental iron in infancy, participating in Study 2 in infancy, age, education level, perceived stress, depressive symptoms, and family cardiovascular risk. To improve model parsimony, a covariate remained in the model if it demonstrated a direct association with the variable at p < .10. Direct paths with p values greater than .10 were removed in a stepwise manner where the variable with the highest p value was removed first and the model was recomputed until all p values were less than .10 (final covariates shown in Table 3). After fitting the model for significant covariates, the same process was conducted for the paths between psychosocial risk and cardiometabolic outcomes. The final paths in the model (minus covariates) are shown in Figure 1b. A two-sided alpha level of 0.05 was used to determine statistical significance. The estimates and 95% confidence intervals (CI) were presented for all analyses (Table 3).

Figure 1. Path analysis comparing psychosocial risk at infancy, age 5 years, age 10 years, and adolescence predicting cardiometabolic outcomes in young adulthood. Figure 1a includes all the paths tested, and Figure 1b includes the final model. Values presented are standardized coefficients. Solid lines represent significant pathways (p < .05). *p < 0.05, **p < 0.01, ***p < 0.001.
Sensitive periods model for number of MetS components
The same model as above was tested except with number of MetS components as the single dependent variable instead of the three dependent variables of the BMI and waist circumference, BP, and fat mass composites. The model that was tested is shown in Figure 2a without covariates. The final paths of the model (minus covariates) is shown in Figure 2b. The final paths, including covariates, are described in Table 4.
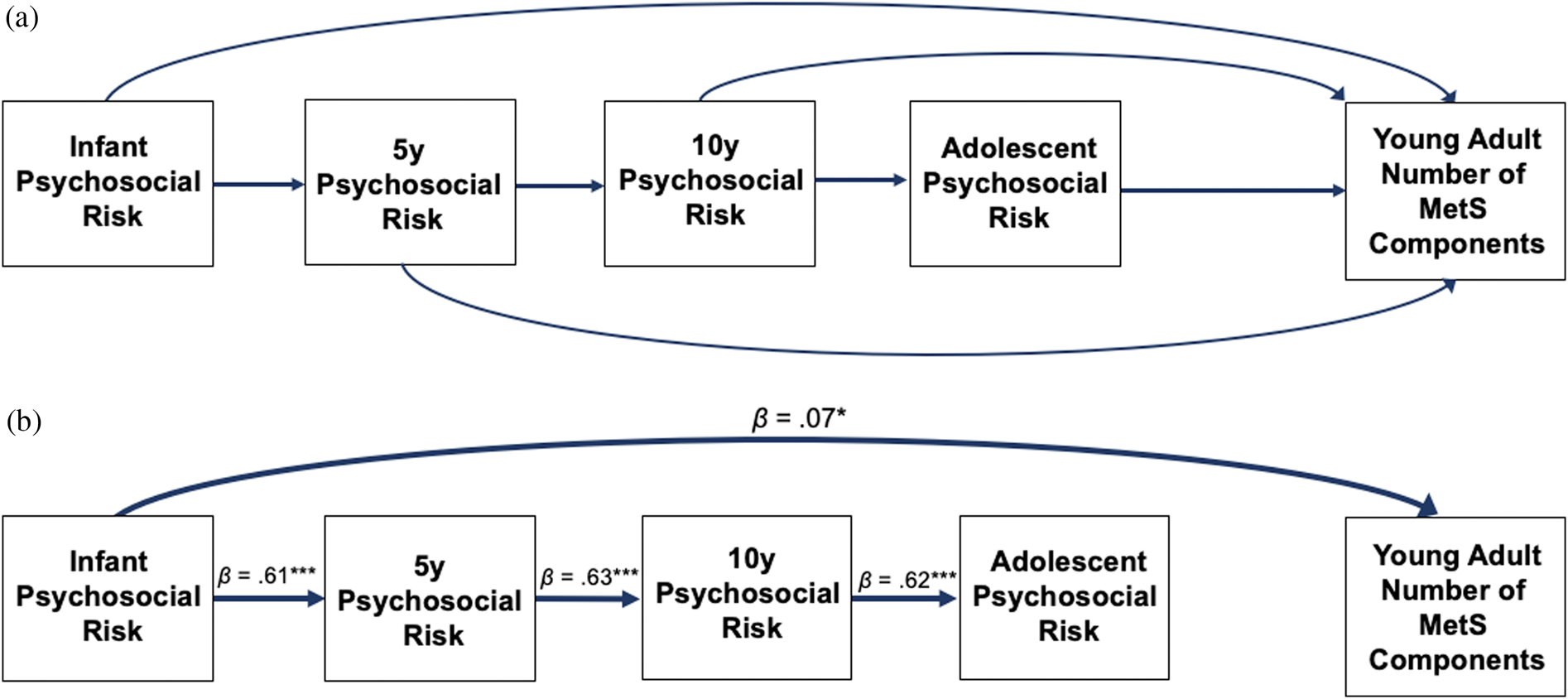
Figure 2. Path analysis comparing psychosocial risk at infancy, age 5 years, age 10 years, and adolescence predicting number of metabolic syndrome (MetS) components in young adulthood. Figure 2a includes all the paths tested, and Figure 2b includes the final model. Values presented are standardized coefficients. Solid lines represent significant pathways (p < .05). *p < 0.05, **p < 0.01, ***p < 0.001.
Mean and maximum psychosocial risk
Additional analyses were conducted to determine whether the mean psychosocial risk across the four time points or the maximum psychosocial risk at any of the four time points predicted cardiometabolic outcomes better than risk at any one time point. Four models were conducted. Two models tested mean psychosocial risk across the four time points: one model used the BMI and waist circumference, BP, and fat mass sensitive periods model, and one model used the number of MetS components sensitive periods model. Two models tested maximum level of risk across the four time points: one model used the BMI and waist circumference, BP, and fat mass sensitive periods model, and one model used the number of MetS components sensitive periods model. We conducted each model separately as our measures of mean and maximum risk were highly correlated (r = .92). To test the added predictive value of the mean psychosocial risk, we computed the final sensitive periods models (Figures 1b and 2b) and added the mean risk score as a predictor of each of the cardiometabolic risk dependent variables in the two separate models (first model with BMI and waist circumference, BP, and fat mass; second model with number of MetS components). We repeated this method, substituting the maximum psychosocial risk score variable for the mean psychosocial risk variable in the two sensitive periods models. Associations of mean and maximum risk with each of the dependent variables were compared to determine whether either was a better predictor of cardiometabolic outcomes than variables in the model that tested timing of psychosocial risk.
Results
Descriptive statistics
Mean age of participants at the young adult cardiometabolic assessment was 23.0 years (range: 21–27; SD = 1.0). Age at the adolescent assessment also spanned a range from 11–17 years (M = 14.3, SD = 1.6); the age ranges were relatively smaller at infancy, 5 years, and 10 years. All participants were Hispanic/Latinx, and over half (52.1%) were female. Descriptive statistics for participants in the young adult assessment are included in Table 1, and a correlation table of study variables is shown in Table 2.
Table 1. Descriptive statistics (N = 1040).
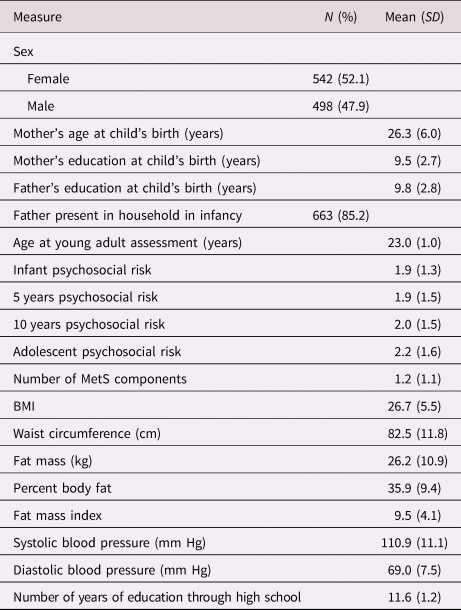
Note. Percentages are calculated using participants with non-missing data on the variable. Values on the psychosocial risk composites ranged from 0–7. MetS= metabolic syndrome
Table 2. Correlation table.
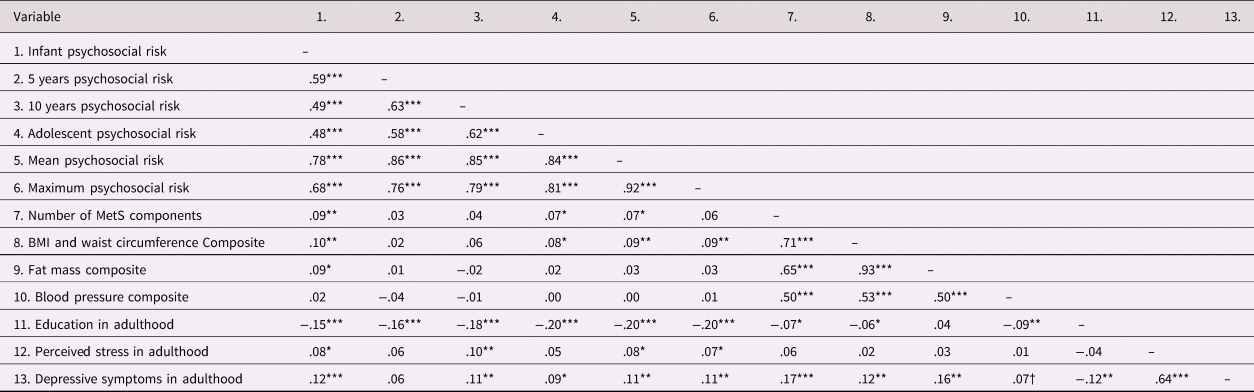
N = 1040. Correlations computed using SPSS v25. †p < 0.10, *p < .05. **p < .01. ***p < .001. MetS = metabolic syndrome.
Sensitive periods model for BMI and waist circumference, BP, and fat mass
The final model had good fit to the data (CFI = .94, RMSEA = .05, SRMR = .04). Greater infant psychosocial risk was associated with a higher BMI and waist circumference composite (β = 0.08, 95% CI: 0.03 to 0.13, p = 0.002) and higher body fat (DXA) composite (β = 0.07, 95% CI: 0.01 to 0.12, p = 0.023) (Table 3). Infant psychosocial risk was not associated with the BP composite, and this path was excluded from the final model. Figure 3 displays mean levels of BMI and waist circumference composite and body fat composite by quartiles of infant psychosocial risk. Psychosocial risk composites at 5 years, 10 years, and adolescence were not associated with any of the cardiometabolic risk variables, and these paths were excluded from the final model. BP was the only dependent variable not associated with any psychosocial risk variable. All direct associations, including standardized estimates and 95% CI, are displayed in Table 3.
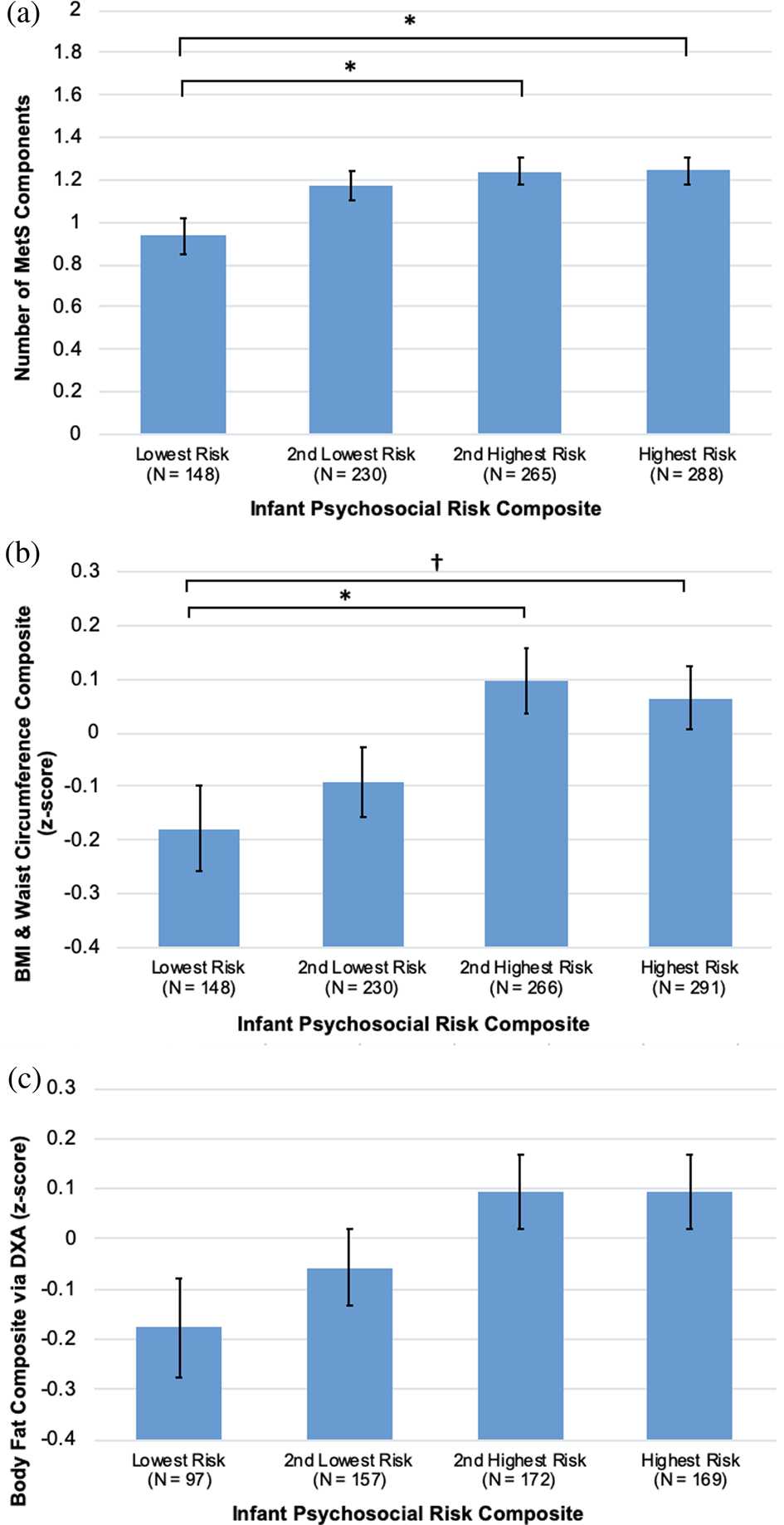
Figure 3. Infant psychosocial environment composite risk quartiles by (a) number of metabolic syndrome (MetS) components, (b) body mass index (BMI) and waist circumference composite, (c) body fat composite, all controlling for sex, young adult education level, and family cardiovascular risk. Group differences were tested in SPSS 25 using Bonferroni corrections, †p < .10, *p < .05, **p < .01.
Table 3. Estimates of direct pathways from psychosocial risk at infancy, 5 years, 10 years, and adolescence to cardiometabolic outcomes in young adulthood.
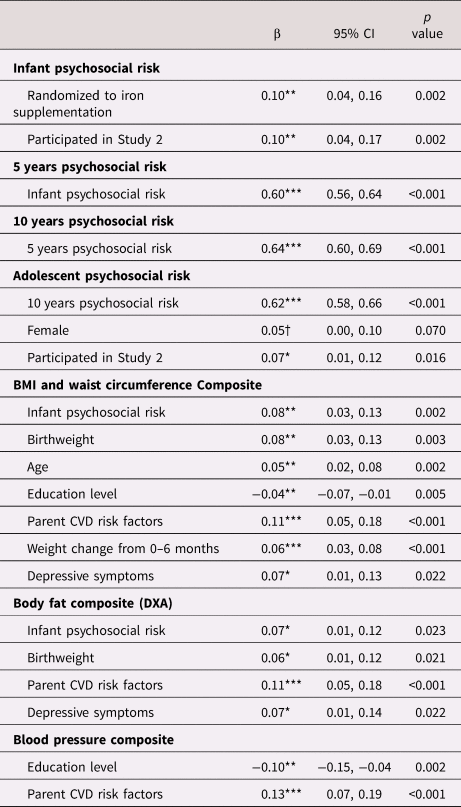
Note. All estimates reported are standardized estimates and 95% confidence intervals (CI) for each of the direct pathways to cardiometabolic outcomes. Dependent variables are in bold with independent variables and associated standardized (β) coefficients.
Note. Covariates were included in the final model if they were associated with the model variable at p < .10, and psychosocial risk variables were only included as predictors of cardiometabolic outcomes if they were associated at p < .10. †p < 0.10, *p < .05. **p < .01. ***p < .001. CVD = cardiovascular disease.
Sensitive periods model for number of MetS components
The final model fit was satisfactory (CFI = .90, RMSEA = .07, SRMR = .05). Greater infant psychosocial risk was associated with a higher number of MetS components (β = 0.07, 95% CI: 0.01 to 0.12, p = 0.020). Psychosocial risk composites at 5 years, 10 years, and adolescence were not associated with the number of MetS components, and these paths were excluded from the final model. Figure 3 displays mean levels of number of MetS components by quartiles of infant psychosocial risk. All direct associations, including standardized estimates and 95% CI, are displayed in Table 4.
Table 4. Estimates of direct pathways from psychosocial risk at infancy, 5 years, 10 years, and adolescence to number of metabolic syndrome (MetS) components in young adulthood.
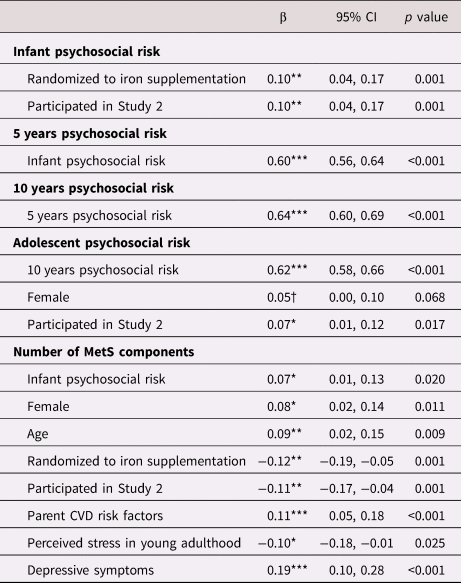
Note. All estimates reported are standardized estimates and 95% confidence intervals (CI) for each of the direct pathways to number of MetS components. Dependent variables are in bold with independent variables and associated standardized (β) coefficients.
Note. Covariates were included in the final model if they were associated with the model variable at p < .10, and psychosocial risk variables were only included as predictors of number of MetS components if they were associated at p < .10. †p < 0.10, *p < .05. **p < .01. ***p < .001. CVD = cardiovascular disease.
Mean and maximum psychosocial risk
Mean and maximum psychosocial risk were not associated with any of the cardiometabolic risk variables, p > .59 (see supplement). The strength of β values between infant psychosocial risk and each of the three cardiometabolic risk outcomes (number of MetS components, BMI and waist circumference, and body fat) remained similar to the models without mean and maximum psychosocial risk.
Discussion
Using prospective measures of psychosocial risk from infancy through adolescence, results from the current study suggest that psychosocial risk experienced during infancy has the strongest associations with young adult cardiometabolic outcomes compared to psychosocial risk at 5 years, 10 years, and adolescence. Specifically, higher infant psychosocial risk was associated with a higher number of MetS components, a higher BMI and waist circumference composite, and a higher body fat composite as measured by DXA. No associations with cardiometabolic outcomes were found for psychosocial risk measured at age 5 years, age 10 years, or adolescence. Infancy psychosocial risk showed stronger associations with young adult cardiometabolic risk than mean risk from infancy through adolescence or maximum psychosocial risk at any time point.
The finding that infant psychosocial risk was associated with greater number of MetS components, BMI and waist circumference composite, and body fat composite in young adulthood is consistent with previous research that demonstrated that low SES at ages 1–2 years is related to higher IL-6 in adulthood, independent of adult SES (Carroll et al., Reference Carroll, Cohen and Marsland2011). Childhood SES was measured retrospectively in the study by Carroll and colleagues (Reference Carroll, Cohen and Marsland2011) by looking at house and vehicle ownership, number of people living in the home and number of bedrooms. In the current study, the SES variable was similar but also included additional items such as parental employment status. We also considered psychosocial stressors such as family stressors, maternal depressive symptoms, and father absence. Our study and the Carroll et al. (Reference Carroll, Cohen and Marsland2011) study suggest that cardiometabolic outcomes in young adulthood are associated with socioeconomic and psychosocial risk in infancy. Further, this finding extends prior research from this cohort reporting that psychosocial risk in infancy is associated with cardiometabolic outcomes in adolescence (Doom et al., Reference Doom, Reid, Blanco, Burrows, Lozoff and Gahagan2019b) by demonstrating that psychosocial risk during infancy is associated cardiometabolic outcomes in young adulthood as well. These findings support the developmental origins of disease hypothesis by suggesting that infancy is an important period during which psychosocial factors may have implications for future cardiometabolic health.
We did not find associations between psychosocial risk at age 5, age 10, or adolescence and any of the cardiometabolic outcomes. This lack of association is inconsistent with previous research that related childhood stressors to adult BP (Alastalo et al., Reference Alastalo, Räikkönen, Pesonen, Osmond, Barker, Heinonen and Eriksson2013a), a predictor of cardiovascular disease. In addition, our null findings for adolescent psychosocial risk are inconsistent with a previous study that found that stress experienced at ages 15 to 17 was particularly associated with adult heart disease risk (Friedman et al., Reference Friedman, Montez, Sheehan, Guenewald and Seeman2015). These discrepancies between studies might be due to measuring different types of stress. Severe adverse childhood experiences, such as parent–child separation, were addressed in previous studies (Alastalo et al., Reference Alastalo, Räikkönen, Pesonen, Osmond, Barker, Heinonen and Eriksson2013a; Friedman et al., Reference Friedman, Montez, Sheehan, Guenewald and Seeman2015), whereas we analyzed a broader spectrum of psychosocial risk, which included family stressors, maternal depressive symptoms, father absence, and low SES. Some studies of the timing of stress were conducted in a sample of adults who were separated from their parents as children during World War II (Alastalo et al., Reference Alastalo, Räikkönen, Pesonen, Osmond, Barker, Heinonen and Eriksson2013a; Alastalo et al., Reference Alastalo, von Bonsdorff, Räikkönen, Pesonen, Osmond, Barker and Eriksson2013b), which is a very different type of stressor than having low SES or high maternal depressive symptoms. Another factor to consider in evaluating discrepant results across various studies is the age at which cardiometabolic outcomes were assessed. Previous studies found the association in older adults rather than young adults as in the current study (Felitti et al., Reference Felitti, Anda, Nordenberg, Williamson, Spitz, Edwards and Marks1998; Power, Pereira, & Li, Reference Power, Pereira and Li2015). Our findings suggest that the sensitive period of infancy is a stronger predictor of cardiometabolic outcomes in young adulthood than the other developmental periods. However, it is possible that if cardiometabolic risk is assessed in this sample in middle or later adulthood, psychosocial risk at ages 5–10 years might show associations with cardiometabolic risk at these later time points. It may be that psychosocial risk requires many years to be biologically and behaviorally embedded to lead to differences in cardiometabolic risk. For example, cardiometabolic diseases such as coronary heart disease and MetS often do not appear until later in adulthood (Berry et al., Reference Berry, Dyer, Cai, Garside, Ning, Thomas and Lloyd-Jones2012). Thus, discrepant findings may be a function of years since psychosocial risk was assessed rather than there being something unique about the developmental period of infancy. However, there is precedent for there being a unique impact of psychosocial risk during infancy on adult cardiometabolic risk considering evidence of early life programming of the sympathetic nervous system, HPA axis, immune system, metabolic system, and behavior (Barker, Reference Barker2007; Birn, Roeber, & Pollak, Reference Birn, Roeber and Pollak2017; Nusslock & Miller, Reference Nusslock and Miller2016; Reynolds, Reference Reynolds2013).
Mean and maximum psychosocial risk from infancy through adolescence were not associated with cardiometabolic outcomes when added to the model with infant psychosocial risk. These findings contrast with a previous study demonstrating that adverse childhood events experienced at multiple time points were more strongly related to CVD than events experienced at specific time periods (Friedman et al., Reference Friedman, Montez, Sheehan, Guenewald and Seeman2015). The different findings may relate to different degrees of severity and measures of psychosocial risk. Severe events were reported retrospectively (Friedman et al., Reference Friedman, Montez, Sheehan, Guenewald and Seeman2015) compared to a broad psychosocial risk measure assessed prospectively in our study. Inconsistent results may also be observed because the biological responses to the early exposure to psychosocial risk were not considered, which could shed light on early biological programming.
Chile is an important setting for studies of emerging cardiometabolic risk. The country underwent a rapid economic transition from a low- to a high-income country (Albala, Vio, Kain, & Uauy, Reference Albala, Vio, Kain and Uauy2002) but with a less dramatic social and educational transition. Chile now has rates of obesity similar to those in the United States and other high-income countries. Nearly all research on sensitive periods of psychosocial risk and cardiometabolic risk have been conducted in countries with high stability in gross domestic product throughout the lives of participants. The change in economic context for the families in this study across children's development could also have changed the types, chronicity, and severity of stressors they were experiencing. These findings suggest that the larger sociocultural context, rather than just the family context, may be an important moderator of the associations of childhood psychosocial risk with young adult cardiometabolic outcomes. The results of this study may be particularly applicable to other countries experiencing rapid economic transitions.
Study limitations and strengths
Several study limitations should be noted. Some data for a portion of the sample were missing at age 5 years and for the young adult DXA assessment due to lack of funding, which may impact the generalizability of our findings. The restriction of recruitment of families from low- to middle-income neighborhoods in Santiago, Chile, also limits the generalization of results to other socioeconomic contexts. However, it is the individuals from lower SES backgrounds that are most exposed to stressors from an early stage. Psychosocial risk measures focused on collection of parental indicators of risk such as SES, parent education, home support for child development, and father absence rather than on the target participants’ perceptions of environmental risk. We may be missing associations of perceived psychosocial risk with different measures of cardiometabolic risk, since prospective and retrospective measures of childhood stress appear to have different associations with psychiatric outcomes (Newbury et al., Reference Newbury, Arseneault, Moffitt, Caspi, Danese, Baldwin and Fisher2018). Perceptions of the environment from the child and biological measures of stress such as disrupted stress system or immune system responses could provide a fuller understanding of the environment and stress biology leading to cardiometabolic risk in young adulthood. Risk during the prenatal period was not assessed in the current study, and the reports of infant psychosocial risk by mothers in this cohort could reflect a continuation of psychosocial risk experienced in the prenatal period. Thus, cohort studies starting in pregnancy and continuing through adulthood would be well-suited to clarifying effects of early life exposures. In addition, the young adult measures of risk did not include the same variables as the measures from infancy through adolescence. Young adults may not be impacted in the same way by risk factors reported by their mother. We have included young adult measures of perceived stress, depressive symptoms, and education as covariates as these factors may be related to concurrent psychosocial risk. Unsurprisingly, there was fairly low variation in levels of psychosocial risk from infancy through adolescence (see Figures 1b and 2b, and Table 2), though there was enough variation to detect infancy as a sensitive period of psychosocial risk to predict young adult cardiometabolic outcomes when controlling for psychosocial risk at different ages. Strengths of this study include the relatively large sample, good follow-up rates, the objective measures of multiple cardiometabolic risk factors, and the prospective measurement of psychosocial risk that was assessed consistently from infancy through young adulthood. We also included multiple covariates in all analyses which strengthen the interpretation of the model results.
Future directions
The findings of this study suggest several areas for further study. First, although there is a better understanding of biological and behavioral alterations following early life stress, few prospective longitudinal studies are able to target which of these psychosocial risks may be particularly deleterious for cardiometabolic health. This future research direction is in line with Dr. Megan Gunnar's pioneering research on how psychosocial stress in early life is embedded biologically and behaviorally to influence health across development (Gunnar, Doom, & Esposito, Reference Gunnar, Doom, Esposito, Lamb and Lerner2015). In our study, psychosocial stress in infancy could have influenced the development of cardiovascular risk in adulthood through biological alterations such as changes in the HPA axis, epigenome, cardiovascular system, or immune system, or through behavioral alterations such as changes in health behaviors (Bunea, Szentágotai-Tătar, & Miu, Reference Bunea, Szentágotai-Tătar and Miu2017; Hughes et al., Reference Hughes, Bellis, Hardcastle, Sethi, Butchart, Mikton and Dunne2017; Juruena, Agustini, Cleare, & Young, Reference Juruena, Agustini, Cleare and Young2017; Reynolds, Reference Reynolds2013; Shonkoff et al., Reference Shonkoff, Garner, Siegel, Dobbins, Earls and McGuinn2012). These alterations may be adaptive for dealing with stress in the short-term but may have long-term negative effects on cardiovascular health. By closely tracking psychosocial stress experiences and potential developmental cascades of biological, psychosocial, and behavioral adaptations to stress over time, and then understanding which adaptations have the greatest impact on cardiometabolic health, we will be able to create interventions that target these mediators to improve health. However, we must also be cautious of using interventions to alter biological, psychosocial, or behavioral adaptations to stress if we are not also changing the stressful environment that produced those adaptations. For example, high levels of psychosocial stress predict greater attention bias to threat (Lakshman et al., Reference Lakshman, Murphy, Mekawi, Carter, Briscione, Bradley and Powers2020), which over time may lead to poorer cardiovascular health in a stressful environment. However, an intervention that reduces attention bias to threat may be harmful in the short term if an individual does not adequately attend to the many threats in their environment.
Second, understanding why psychosocial risk during certain developmental periods predicts cardiometabolic outcomes during young adulthood is important for understanding the biological embedding of early experiences and for creating developmentally sensitive interventions. The current study suggests that psychosocial risk during infancy is particularly predictive of young adult cardiometabolic outcomes, which leads to the question of what may be unique about infancy that it predicts later cardiometabolic outcomes. Future research should incorporate what is already known about the rapid stages of biological, brain, socioemotional, and cognitive development in the first years of life so that we can better understand why infancy may be a sensitive period during which psychosocial risk may be especially predictive of later cardiometabolic health. This work on uncovering mechanisms by which stress influences health during sensitive periods is guided by Dr. Gunnar's work with children who have experienced the extreme psychosocial stress of living in an institution during a sensitive period of development early in life (Doom, Georgieff, & Gunnar, Reference Doom, Georgieff and Gunnar2015; Gunnar, DePasquale, Reid, & Donzella, Reference Gunnar, DePasquale, Reid and Donzella2019; Gunnar & Van Dulmen, Reference Gunnar and Van Dulmen2007). Similar to her work, we need to explore how psychosocial risk at specific developmental periods influences potential mediators of later cardiometabolic outcomes and understand which factors predict who is able to recover from stress while maintaining positive health.
Third, we need to better understand resilience factors that protect individuals who have experienced high psychosocial risk early in life from developing poor cardiovascular outcomes. One important resilience factor – and a target of Dr. Gunnar's work on resilience – is high-quality social relationships (Fisher, Gunnar, Dozier, Bruce, & Pears, Reference Fisher, Gunnar, Dozier, Bruce and Pears2006; Gunnar, Reference Gunnar2017). It will be important to understand what types of relationships at specific points in development are most beneficial for reducing poor cardiometabolic outcomes following early stress. Similarly, individual-level factors such as genetics, coping skills, emotion regulation, and health behaviors will be important to consider. In addition, environmental factors, such as neighborhood social support or availability of high-quality education and support services for families who have experienced high psychosocial risk, will be important moderators to test. As not all children who have experienced high psychosocial risk go on to develop CVD, it will be important to learn who is resilient to these outcomes to understand whether we can target resilience factors to improve health.
Finally, there is a need for more randomized controlled trials (RCTs) to (a) understand causal processes in the association between psychosocial stress and cardiometabolic outcomes through RCTs that reduce psychosocial stress, and (b) to test the effectiveness of psychosocial interventions to improve health. For example, a RCT of a family- or society-level intervention to reduce psychosocial and socioeconomic risk in infancy would shed light on whether psychosocial risk during infancy causes poor cardiometabolic outcomes. Thus, the RCT would provide the strongest evidence that interventions reducing psychosocial risk predict better cardiometabolic outcomes. Similarly, conducting RCTs of interventions that target potential mediators between psychosocial risk and cardiometabolic health will allow us to test whether changing the level of the mediator improves adult cardiometabolic health. Research using causal inference models will also be important for establishing more rigorous evidence of causality. Researchers using RCTs that reduce psychosocial risk or improve hypothesized mediators or moderators of the association between psychosocial risk and psychosocial outcomes should consider collecting data on cardiometabolic outcomes, such as height, weight, waist circumference, and BP. These cardiometabolic measures are low burden and inexpensive to collect but will add a great deal to our understanding of psychosocial factors that positively impact cardiometabolic health across development. Leveraging existing interventions to enhance our understanding of how psychosocial risk influences cardiometabolic health is an important future direction that holds promise to reduce the burden of CVD, which is the number one cause of death in the United States (Benjamin, Muntner, & Bittencourt, Reference Benjamin, Muntner and Bittencourt2019). Overall, exploring mechanisms, considering sensitive periods and developmental processes, targeting resilience factors, collecting rigorous experimental evidence, and testing interventions using RCTs will have important public health implications.
Conclusion
Psychosocial risk experienced during infancy was more strongly associated with young adult cardiometabolic outcomes than psychosocial risk experienced at other time points. Infant psychosocial risk also predicted young adult cardiometabolic outcomes better than the mean or maximum level of psychosocial risk from infancy through adolescence. Our results suggest that reducing psychosocial risk during infancy could be integrated into prevention efforts to improve later cardiometabolic health. Future directions could further probe the biological and behavioral mechanisms in the association between infancy psychosocial risk and adulthood cardiometabolic outcomes by prospectively measuring psychosocial risk and intensively studying the biological and behavioral alterations at each developmental period from infancy through adulthood. In addition, future work using large longitudinal datasets with both biological and behavioral measurements could be used to uncover additional risk and protective factors across development.
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579420001248
Acknowledgments
We thank the families who have participated and continue to participate in this research. We also acknowledge the contribution of Marcela Castillo, lead psychologist involved in research coordination at INTA, University of Chile, who passed away in 2015.
Funding Statement
Funding from F32HD088029 (PI: Doom), K01HL143159 (PI: Doom), R01HD14122 (PI: Lozoff), R01HD33487 (PI: Lozoff & Gahagan), and R01HL088530 (PI: Gahagan).
Conflicts of Interest
None.










