It has been repeatedly shown that dietary protein supplementation at times of protein scarcity results in improved resistance and the expression of immunity towards gastrointestinal nematodes in periparturient mammal hosts(Reference Donaldson, Van Houtert and Sykes1–Reference Chartier, Etter and Hoste3), including laboratory rodents(Reference Houdijk, Jessop and Knox4–Reference Sakkas, Houdijk and Jones7). This may be related to the increased protein demands that the periparturient period imposes on the parasitised host, i.e. for milk production, as it has been suggested that scarce protein supply may be preferentially allocated to reproductive rather than immune functions(Reference Coop and Kyriazakis8, Reference Kyriazakis and Houdijk9). As host responses following increased supply of dietary protein would be responses to dietary essential and conditionally essential amino acids (AA), it may be expected that their reduced supply can lead to reduced expression of immunity. Both innate and acquired immune responses are dependent upon adequate provision of AA for the synthesis of antigen-presenting molecules, Ig, cytokines and acute-phase proteins as well as for the provision of energy-providing substrates either directly, or following their conversion to other AA (e.g. glutamine) or to glucose(Reference Calder10, Reference Kim, Mateo and Yin11). At the same time, requirements for protein and/or AA may also increase as a direct consequence of metabolic changes associated with inflammation and infection(Reference Le Floc'h, Melchior and Obled12) and the physiological status of the animal, such as pregnancy and lactation(Reference Clowes, Aherne and Baracos13). The fact that 30–50 % of essential AA in the food may be catabolised by the small intestine(Reference Stoll, Henry and Reeds14, Reference Wu15) is indicative of their role in gut integrity, function and local immune response(Reference Wang, Qiao and Li16, Reference Wu17).
Herein we used a laboratory model(Reference Houdijk, Jessop and Knox4) to investigate the effect of reduced supply of two essential AA on host resistance and lactational performance (dam and litter weight gain). Our expectation was that single AA scarcity would impair protein synthesis in the host, with penalties on both its resistance and resilience to parasitic infection. Although there is an abundance of literature on the effects of individual AA deficiency on food intake(Reference Gietzen, Hao and Anthony18), performance(Reference D'Mello and D'Mello19) and immune responses(Reference Li, Yin and Li20) of growing animals, to our knowledge, this is the first study on specific AA deficiency in periparturient parasitised hosts.
In the present study, we focused on leucine and methionine, which have both been implicated in host responses to parasitism. Leucine requirements are increased during parasitism(Reference Yu, Bruce and Calder21), as parasitised hosts show an increased intestinal metabolism of leucine and consequently reduced availability of leucine for other tissues. In addition, lactation increases leucine requirements, as an increased channelling of leucine from the blood flow to the mammary gland has been observed(Reference Trottier, Shipley and Easter22, Reference DeSantiago, Torres and Tovar23). In lactating rats, this increase may be up to 35 % higher than that of non-lactating rats(Reference Vina and Williamson24).
Methionine, as the only essential sulphur-containing AA (SAA) and a precursor to a number of other SAA(Reference Grimble25), plays pivotal roles in protein synthesis, maintenance of gut functions and regulation of the mucosal response to antigens(Reference Grimble25, Reference Fang, Yao and Zhang26). There is evidence suggesting that demand for SAA may be increased during gastrointestinal nematode infections(Reference Hoskin, Lobley and Coop27, Reference Liu, Smith and Briegel28), probably due to the loss of SAA-rich endogenous protein via increased sloughing of epithelial cells and especially mucin secretion(Reference Poppi, MacRae and Brewer29). Importantly, methionine supplementation of growing rats infected with Nippostrongylus brasiliensis has resulted in a reduction of worm burdens(Reference Cummins, Bolin and Duncombe30).
In the present experiment, we tested the null hypothesis that the reduced provision of either leucine or methionine, at high levels of other AA intake, will similarly penalise lactational performance and resistance to N. brasiliensis as a consequence of reduced AA availability to maintain the protective immune response.
Experimental methods
Animals, housing and feeding strategy during gestation
All experimental procedures were approved by the SAC Ethical Review Committee (ED AE 24/2007) and carried out under Home Office authorisation (PPL 60/3626). A total of seventy-two second-parity female Sprague–Dawley rats (Charles River UK Limited) were housed in a room where ambient temperature was maintained at 21°C, relative humidity ranged from 45 to 65 %, and artificial lighting was provided between 08.00 and 18.00 hours. Rats were individually housed in solid-bottomed cages with fresh sawdust provided weekly. Shredded plastic bubble wrapping for nesting material was provided 3 d before the expected parturition date. Wire-bottomed cages were used during mating and for faeces collection during the primary infection as described previously(Reference Houdijk, Jessop and Knox31). For mating, female rats were placed with a proven male breeder and mating was confirmed through the presence of a vaginal plug.
Until mating was confirmed, rats were given ad libitum access to standard rat chow (Rat and Mouse No. 3; Special Diet Services). After mating was confirmed, rats were given ad libitum access to a high-protein food, with 210 g digestible crude protein (CP) and 16·4 MJ metabolisable energy/kg DM until 10 d into gestation to allow for the establishment of pregnancy and placental development. Rats were then transferred to a low-protein food containing 60 g CP and 17·3 MJ metabolisable energy/kg DM, which was given until parturition. This feeding protocol was used to reduce body protein reserves during the second half of gestation in order to maximise the degree of protein AA scarcity during lactation when rats would be fed low-protein foods(Reference Houdijk, Jessop and Knox4, Reference Pine, Jessop and Oldham32).
Feeding treatments
Upon parturition (day 0), dams were allocated to one of four feeding treatments, i.e. low protein (LP), high protein (HP), HP with leucine levels as in low protein (HP-Leu) and HP with methionine levels as in low protein (HP-Met). The LP and HP foods were formulated to supply 150 and 250 g CP/kg, respectively. These CP levels were chosen on the basis of previous observations where lactating rats fed foods supplying 150 g CP/kg have shown significantly reduced lactational performance, elevated worm burdens and penalised expression of immunity compared with those receiving 250 g CP/kg(Reference Jones, Houdijk and Sakkas6, Reference Sakkas, Houdijk and Jones7). The protein source used for the LP food was methionine-enriched casein, and the LP food was the basis for all other foods. Casein was methionine-enriched, to overcome its natural deficiency in SAA content(Reference DeMan33), as our aim was for LP foods to be balanced in their AA profile. The HP food was prepared by adding a purified AA mixture to the LP food at the expense of starch and sucrose (Table 1) to mimic the 250 g CP/kg food(Reference Jones, Houdijk and Sakkas6, Reference Sakkas, Houdijk and Jones7). For the HP-Leu and HP-Met foods, leucine and methionine were likewise replaced in the added AA mixture with starch and sucrose. The resulting composition of the four experimental foods is presented in Table 1. Daily allowances were offered at 90 % of observed DM intake during a previous experiment where similar diets were fed ad libitum (Reference Jones, Houdijk and Knox5), using dam parturition body weight (PBW) as a scaling factor. Foods were offered in increasing amounts during lactation, reflecting the natural increase in food intake with time as observed in previous experiments using this animal–parasite model system(Reference Houdijk, Jessop and Knox4, Reference Jones, Houdijk and Knox5). Food offered averaged 21, 25, 33 and 41 g DM/d during days 1–2, 3–5, 6–8 and 9–11, respectively. Foods offered during lactation were sampled during their preparation for the analysis of DM, CP (Kjeldahl-N × 6·38), diethyl ether extract, ash, acid-detergent fibre, starch, sucrose and AA profile (Table 2).
Table 1 Composition of the experimental foods used during lactation
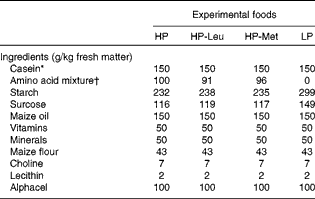
HP, high protein; HP-Leu, HP with leucine levels as in low protein; HP-Met, HP with methionine levels as in low protein; LP, low protein.
* Casein was enriched with methionine (casein:methionine ratio was 99:1).
† The amino acid mixture included in the experiment foods HP, HP-Leu and HP-Met represented methionine-enriched casein, or that without leucine, or that without methionine, respectively.
Table 2 Chemical composition of the experimental foods used during lactation
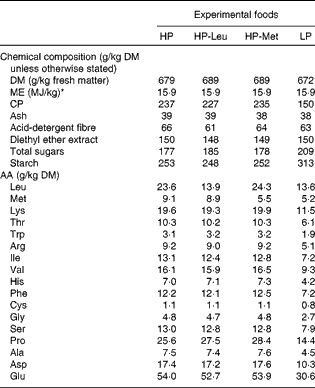
HP, high protein; HP-Leu, HP with leucine levels as in low protein; HP-Met, HP with methionine levels as in low protein; LP, low protein; ME, metabolisable energy; CP, crude protein; AA, amino acids.
* Food ME content was calculated by multiplying its contents of protein (casein+AA), digestible carbohydrates (starch, sucrose and maize flour) and fat (maize oil) with the ME contents of protein (17 MJ/kg), carbohydrates (17 MJ/kg) and fat (38 MJ/kg)(Reference Astrup, Tremblay, Gibney, Lanham-New, Cassidy and Vorster53).
Infection protocol
All rats received a primary infection of 1600 third-stage infective larvae of N. brasiliensis on day − 37 (with day 0 as mean achieved parturition date), which were suspended in 0·5 ml sterile PBS that was subcutaneously injected in the hind leg. On day 2, rats were either reinfected with 1600 third-stage infective larvae N. brasiliensis or sham-infected with PBS (primary infection only). The N. brasiliensis strain used was maintained at Moredun Research Institute through passage in growing Sprague–Dawley male rats.
Experimental design
The effects of the four feeding treatments were assessed on lactational performance and on parasitological and immunological variables on days 8 and 11 (corresponding to days 6 and 9 post-secondary infection, respectively). These two sampling time points post-secondary infection were included, as previous studies with the same model suggested that the nutritional sensitivity of host resistance and the expression of immunity in this model may vary with time(Reference Jones, Houdijk and Knox5–Reference Sakkas, Houdijk and Jones7). All rats in these eight factorial feeding treatment–endpoint combinations (four feeding treatments × two endpoints) received the secondary infection. Sham-infected control rats were also included until day 8 only (first endpoint) for each feeding treatment to assess the effect of reinfection on lactational performance and the expression of immunity. This resulted in a total of twelve experimental treatments. Rats were blocked for PBW and were randomly allocated to these twelve treatments; on the morning, parturition was observed to be complete. Total sample size aimed for was n 6 for infected rats and n 5 for control rats. However, the minimum realised sample size was n 5 for infected rats and n 4 for control rats due to infertility, miscarriage or dead pups.
Performance and parasitology
Body weight and food intake
Rats were weighed daily throughout the experiment, and daily body weights taken post-parturition were used to calculate lactational dam weight gain. The pups were counted and the whole litter weighed daily from day 0. Litter size was standardised at twelve pups on day 1 to have equal initial nutrient demands. Dam and litter weights from day 1 onwards were used to calculate litter weight gain. Any refusals observed during the lactation period were weighed to assess observed DM intake.
Faecal egg counts
A faecal egg count (FEC, in eggs/g fresh faeces) was performed 7 d after the primary infection (day − 30) to confirm establishment of infection. A second FEC was performed on day − 23 to confirm parasite expulsion. To this effect, faeces were collected through overnight housing on bottom-wired cages(Reference Houdijk, Jessop and Knox31) and FEC were performed using a modified saturated salt flotation method(Reference Christie and Jackson34).
Worm burden and nematode eggs in the colon
Rats were killed humanely through gradually increasing ambient CO2 concentration for parasitological assessment and immune responses (see below). Nematodes were harvested from the small intestine as outlined previously(Reference Houdijk, Jessop and Knox31). The resulting worms were then stored in formaldehyde for subsequent counting and assessment of sex and maturation status according to morphology. The contents of the large intestine were weighed and assessed for worm eggs as described for FEC above. This was then multiplied by the large-intestinal content weight to obtain the total number of nematode eggs in the colon (EIC).
Immunology
Inflammatory cells
A 2 cm sample of the small intestine at a 25 cm distance from the pylorus was washed with PBS to collect worms for total worm counts (see above) and then fixed in 4 % paraformaldehyde for 6 h. The fixative was then replaced by 70 % ethanol. Resulting intestinal samples were wax embedded and sections taken and stained using standard histological techniques. Sections were stained for counting three types of inflammatory cells, i.e. eosinophils (carbol chromatrope 2R), goblet cells (alcian blue and counterstaining with periodic acid-schiff) and mast cells (toluidine blue). Cells were enumerated by counting ten complete, well-orientated, villus crypt units per section and results are expressed as the number of cells/villus crypt unit.
Systemic antibodies
Terminal blood samples taken from the chest cavity following heart puncture were centrifuged at 200 g for 10 min and serum supernatants stored at − 70°C for determination of antibody levels. The concentration of parasite-specific IgA, IgG, IgG1, IgG2a, IgG2b and IgE antibodies was measured by ELISA. Briefly, ELISA plates were coated with 1 μg/ml adult somatic N. brasiliensis antigen(Reference Ball, Selkirk and Knox35) and then blocked overnight at 4°C with 10 % dried milk powder in PBS. Diluted serum samples (1:1000) were added to the plate and incubated at room temperature for 1 h. Antibody isotypes were detected by the addition of rat-specific secondary antibodies at one in 5000 dilution for anti-rat IgE, IgG, IgG1, IgG2a and IgG2b and one in 1000 dilution for anti-rat IgA. Anti-rat IgG, IgG2a and IgG2b were obtained from Sigma and anti-rat IgG1, IgA and IgE were obtained from Serotec. Horseradish peroxidase-conjugated anti-rat IgG was quantified using Sigma Fast-OPD (Sigma) substrate with the reaction being stopped by the addition of 2·5 m-H2SO4. Bound anti-rat IgE, IgG1, IgG2a and IgG2b was detected by the addition of a horseradish peroxidase-conjugated anti-mouse Ig antibody (Dako) at a dilution of one in 1000 before being quantified using Sigma Fast OPD as described previously. Antibody levels were expressed as the means of two optical density readings at 492 nm.
Calculations and statistical analysis
Inspection of the raw data showed that five out of sixteen HP-Met rats refused approximately 75 % of their already restricted allowances (four allocated to day 8 and one to day 11 endpoints). Such refusals are in agreement with other studies showing a reduced food intake that varies greatly between individual methionine-deficient foods(Reference Elshorbagy, Valdivia-Garcia and Refsum36–Reference Nkabyo, Gu and Jones38). In the present study, these were noted as soon as the food was offered, i.e. from day 0 onwards and before the secondary infection. In order to avoid bias arising from reduced food intake per se rather than from a methionine deficiency alone, the data from these five rats were omitted from all main analysis.
Data were analysed using restricted maximum likelihood, to account for differences in the achieved number of replicates per group, arising from the omission of five HP-Met rats with very high refusals and the a priori different replicates of infected and control dams for each feeding treatment. The factors used were feeding treatment (LP, HP, HP-Leu and HP-Met), infection pressure (secondary infection and sham control) and their two-way interaction on achieved feed intake, dam weight gain and litter weight gain including data from all animals until day 8. The same factors were used for the analysis of inflammatory cells and antibody responses taken on day 8 from the infected and control dams. Restricted maximum likelihood was also used to assess the effects of feeding treatment, endpoint (days 8 and 11) and their two-way interaction on worm burdens, percentage of female worms, EIC, inflammatory cells and antibody responses. Contrast statements were used to locate three a priori-defined feeding treatment comparisons, i.e. HP v. HP-Leu, HP v. HP-Met, and HP v. LP. Performance data are presented as arithmetic means, associated with standard errors or standard errors of the difference. However, because of their skewed nature, EIC, worm burdens, inflammatory cells and antibody responses were log-transformed (n+1) to normalise variance before statistical analysis. These data are reported as back-transformed means with lower and upper limits of transformed standard errors. All statistical analyses were performed using Genstat 11 for Windows release 11.1, 2008 (VSN International).
Results
Faecal egg counts and performance until parturition
FEC taken on day − 37 and day − 30 averaged 8835 (7945–9825) and 0 (95 % CI 0, 0) eggs/g, respectively. Pre-mating FEC did not differ between the lactational treatment groups (P= 0·511). Mean body weight on arrival was 374 (se 6·8) g. During the first 10 d of gestation, pregnant rats grew from 387 (se 6·4) to 425 (se 6·3) g and had an average DM intake of 21·2 (se 0·40) g/d. From then onwards and until parturition, the pregnant rats continued to grow to a mean weight of 512 (se 5·7) g and had an average DM intake of 19·9 (se 0·46) g/d, which dropped to an average of 7·3 (se 2·0) g/d just before parturition. Mean dam PBW averaged 406 (se 7·7) g, mean litter size averaged 14·4 pups (se 0·39) and mean litter body weight averaged 76·6 (se 1·8) g.
Achieved DM intake, dam weight gain and litter weight gain
Fig. 1 shows the effects of the feeding treatments on achieved mean DM intake (Fig. 1(a)), dam weight gain (Fig. 1(b)) and litter weight gain (Fig. 1(c)) in the infected and sham-infected control rats from parturition until day 8 of lactation. Achieved DM intake was not affected by feeding treatment (P= 0·65), infection (P= 0·22) or their interaction (P= 0·30; Fig. 1(a)). Relative to the HP dams, the LP (P< 0·001), HP-Leu (P= 0·05) and HP-Met dams (P= 0·001) had a lower mean body-weight gain, which was not affected by infection (P= 0·85), or the interaction between feeding treatment and infection (P= 0·11; Fig. 1(b)). Relative to the HP litters, the LP (P= 0·074) and HP-Leu litters (P= 0·085), but not the HP-Met litters (P= 0·19) tended to have a lower weight gain, which was not affected by infection (P= 0·97), or the interaction between feeding treatment and infection (P= 0·26; Fig. 1(b)).

Fig. 1 (a) Average food intake, (b) dam weight gain and (c) litter weight gain for rats either reinfected (■) with Nippostrongylus brasiliensis or sham-infected (□) on day 2 of lactation and fed restricted amounts of a high-protein food (HP, 250 g crude protein (CP)/kg DM), low-protein food (LP, 150 g CP/kg DM) or HP with similar levels of leucine (HP-Leu) or methionine (HP-Met) as in LP. Values are means, with their standard errors represented by vertical bars.
Total worm burden, eggs in the colon and worm sex ratio
Fig. 2(a)–(c) shows the effects of the feeding treatments on total worm burdens, EIC and worm burden composition for days 8 and 11 of lactation. Across the endpoints, the HP rats harboured fewer worms than the LP (P< 0·001) and HP-Leu rats (P= 0·002) but not the HP-Met rats (P= 0·513; Fig. 2(a)), while total worm burdens were not affected by endpoint (P= 0·431), and the interaction between feeding and endpoint (P= 0·466). However, EIC was lower for the HP rats than for the LP (P< 0·001), HP-Leu (P= 0·001) and HP-Met rats (P= 0·011; Fig. 2(b)), while, likewise, they were not affected by endpoint (P= 0·392) and the interaction between feeding treatment and endpoint (P= 0·588). The worm sex ratio (Fig. 2(c)) was not affected by feeding treatment (P= 0·537) or the interaction between feeding treatment and endpoint (P= 0·149), while the day 8 rats tended to harbour relatively fewer female worms than their day 11 counterparts (P= 0·068).

Fig. 2 (a) Backtransformed mean worm burdens, (b) backtransformed mean total eggs in the colon with backtransformed upper values of log-transformed mean with their standard errors and (c) percentage of female worms of lactating rats, reinfected with Nippostrongylus brasiliensis on day 2 of lactation and culled on either day 8 (■).
Gut histopathology and systemic antibody levels
Table 3 shows the effects of the feeding treatments on small-intestinal eosinophil, goblet cell and mucosal mast cell counts, respectively, in the sham-infected control rats (day 8) and infected rats (days 8 and 11). Eosinophil and mucosal mast cell numbers, but not globule leucocyte numbers, increased with infection and with time, but none was affected by the feeding treatments. Tables 4 and 5 show the effects of the feeding treatments on the serum levels of N. brasiliensis-specific total IgG, IgA and IgE (Table 4), and IgG1, IgG2a and IgG2b (Table 5) in the sham-infected control rats (day 8) and infected rats (days 8 and 11). Infection significantly increased the serum levels of all antibodies assessed (P< 0·001), but there were no significant effects of feeding treatment or interactions between feeding treatment and infection. Feeding treatment and endpoint tended to interact for IgE (P= 0·063), as IgE levels were lower in the HP-Met rats compared with the other feeding treatment groups on day 11 only. Feeding treatment, endpoint and their interaction did not significantly affect the levels of any other isotypes measured.
Table 3 Backtransformed mean number of small-intestinal mucosal inflammatory cells (number per villus crypt unit), with backtransformed range*, of rats reinfected with Nippostrongylus brasiliensis or sham-infected on day 2 of lactation and fed restricted amounts of a high-protein food (HP, 250 g crude protein (CP)/kg DM), low-protein food (LP, 150 g CP/kg DM) or HP with similar levels of leucine (HP-Leu) or methionine (HP-Met) as in LP (Mean values with their ranges)
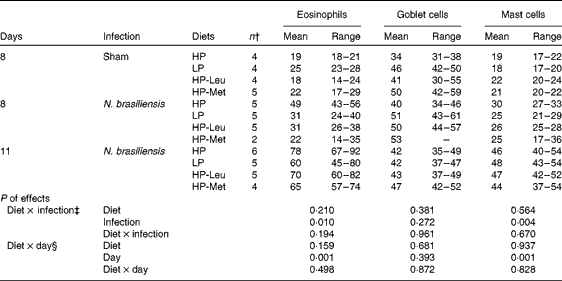
* Backtransformed values of log-transformed mean values with their standard errors.
† Number of replicates; data from five HP-Met rats were omitted due to high levels of refusals (four from day 8 and one from day 11).
‡ Using data from sham and infected rats on endpoint day 8 of lactation only.
§ Using data from endpoints day 8 and day 11 of lactation from infected rats only.
Table 4 Backtransformed mean levels of total serum IgG, IgA and IgE, with backtransformed range*, of rats reinfected with Nippostrongylus brasiliensis or sham-infected on day 2 of lactation and fed restricted amounts of a high-protein food (HP, 250 g crude protein (CP)/kg DM), low-protein food (LP, 150 g CP/kg DM) or HP with similar levels of leucine (HP-Leu) or methionine (HP-Met) as in LP (Mean values with their ranges)

* Backtransformed values of log-transformed mean with their standard errors.
† Number of replicates; data from five HP-Met rats were omitted due to high levels of refusals (four from day 8 and one from day 11).
‡ Using data from sham and infected rats on endpoint day 8 of lactation only.
§ Using data from endpoints day 8 and day 11 of lactation from infected rats only.
Table 5 Backtransformed mean levels of serum IgG1, IgG2a and IgG2b, with backtransformed range*, of rats reinfected with Nippostrongylus brasiliensis or sham-infected on day 2 of lactation and fed restricted amounts of a high-protein food (HP, 250 g crude protein (CP)/kg DM), low-protein food (LP, 150 g CP/kg DM) or HP with similar levels of leucine (HP-Leu) or methionine (HP-Met) as in LP (Mean values with their ranges)

* Backtransformed values of log-transformed mean with their standard errors.
† Number of replicates; data from five HP-Met rats were omitted due to high levels of refusals (four from day 8 and one from day 11).
‡ Using data from sham and infected rats on endpoint day 8 of lactation only.
§ Using data from endpoints day 8 and day 11 of lactation from infected rats only.
Discussion
Previous studies carried out with this rodent–nematode parasite model have shown that feeding low-CP foods results in higher worm burdens, egg excretion, increased proportion of female worms and reduced immune responses compared with feeding high-CP foods(Reference Houdijk, Jessop and Knox4–Reference Sakkas, Houdijk and Jones7). Here we hypothesised that a reduced availability of either all AA (LP) or of the essential AA leucine or methionine would result in penalties on both lactational performance and the expression of immunity to parasites, thus resulting in higher worm burdens. Our null hypothesis was that the magnitude of these effects would be similar between the two AA considered, as a single essential AA deficiency could have an impact on all (essential) AA utilisation, especially if the AA is rate limiting(Reference D'Mello and D'Mello19, Reference McDonald, Edwards and Greenhalgh39). We demonstrated that dietary scarcity of all AA (LP) or of either methionine or leucine indeed reduced lactational performance and increased parasitism in the infected host. However, in contrast to our earlier findings(Reference Jones, Houdijk and Sakkas6), these observed effects did not appear to be related to changes in mucosal immune cell populations or serum antibody levels, which were largely unaffected by our feeding treatments. The effects observed of our EAA-deficient foods are discussed below in relation to their reduced supply for the activation and maintenance of the host's bodily functions associated with the expression of immunity to N. brasiliensis and lactational performance.
Parasitic infection leads to increased protein requirements of immune hosts, mainly in order to activate, or maintain, an effective protective response, but also to repair and replace damaged tissue arising from immunopathological responses(Reference Kyriazakis and Houdijk9). Effector molecules of the immune response are highly proteinaceous, and inflammation and immune system activation are characterised by the synthesis of specific proteins that play crucial roles in the defence of the host against pathogens and modulation of the ongoing immune response(Reference Le Floc'h, Melchior and Obled12, Reference Coop and Kyriazakis40). In addition, lactation also increases protein requirements, as a consequence of increased AA uptake into the mammary gland(Reference Trottier, Shipley and Easter22, Reference Vina and Williamson24). In our experimental design, these effects were expected to be even more pronounced due to the depleted state of the labile protein reserves as a result of the consumption of a low-CP food during the second half of gestation(Reference Pine, Jessop and Oldham32).
As in our previous experiment(Reference Sakkas, Houdijk and Jones7), restricted feeding of LP foods to lactating parasitised hosts resulted in protein scarcity. The penalties of protein scarcity were evident from a reduced lactational performance (both lower dam weight gain and, to a lesser extent, litter weight gain) and higher levels of parasitism (worm burdens and EIC) in LP rats compared with HP rats. The more pronounced effects on dam weight gain compared with litter weight gain suggest that dams may mobilise body protein reserves to minimise the penalties of dietary protein scarcity on milk production(Reference Houdijk, Jessop and Kyriazakis41). In previous studies, and in contrast to the present results, the effects of CP supply on parasitological measurements have concurred with the effects on the number of inflammatory cells in the intestinal epithelium, such as mucosal mast cells, goblet cells and eosinophils, and local and circulating antibody responses(Reference Jones, Houdijk and Knox5, Reference Jones, Houdijk and Sakkas6). It should be noted that our previous studies were designed to induce a higher degree of protein scarcity than the one imposed in the present study, achieving CP supply during lactation as low as 6·6 g/d per kg PBW(Reference Jones, Houdijk and Knox5, Reference Jones, Houdijk and Sakkas6) as opposed to 13·3 g/d per kg PBW offered in the present experiment. Here, we deliberately chose to use a less severe level of CP scarcity in order to minimise the possible effect of AA imbalance per se for the essential AA-deficient foods. Consequently, the body-weight loss observed (Fig. 1) may have mobilised sufficient AA from body protein reserves to meet AA requirement for producing the elevated levels of inflammatory cells and Ig upon infection (Tables 3 and 4). However, it is also well known that parasite expulsion is mediated by a range of effector molecules(Reference Lawrence42, Reference Patel, Kreider and Urban43) and that not a single mechanism is responsible for the expulsion of the parasite population. Therefore, it cannot be excluded that our feeding treatments may have affected other than the measured immunological responses.
The present data showed that HP-Leu feeding penalised litter and dam weight gain relative to HP feeding. The uptake of AA from the mammary gland usually exceeds their quantitative excretion in milk, especially for leucine, in many mammalian species including pigs, goats and rats(Reference Trottier, Shipley and Easter22, Reference DeSantiago, Torres and Tovar23, Reference Bequette, Metcalf and Wray-Cahen44). A decrease in leucine blood concentration occurs during lactation(Reference Vina and Williamson24) when enzymes that are associated with its catabolism are induced in the mammary gland(Reference DeSantiago, Torres and Suryawan45), directing it for the synthesis of glutamine and glutamate(Reference Li, Knabe and Kim46), which are abundant AA in milk proteins(Reference Davis, Fiorotto and Reeds47). Additionally, the excess net uptake of leucine by the mammary gland is further enhanced by dietary protein supplementation(Reference Bequette, Metcalf and Wray-Cahen44). As a consequence, one would expect that leucine deficiency would lead to a penalised pup performance arising from the reduced supply of leucine to the mammary gland and/or an increased mobilisation of body protein tissue(Reference López, Sánchez and Picó48), which would be reflected in the dam body-weight gain.
Despite the relatively small effect of leucine deficiency on lactational performance as reflected in dam and litter weight gain, HP-Leu dams had significantly higher worm burdens and EIC compared with HP dams, which was not associated with differences in any of the immune measurements taken. The parasitological data indicate that the penalties induced on resistance were higher in comparison with the lactational performance under leucine deficiency. This is in agreement with the suggestion that immunity to parasites is given a lower priority than reproductive functions in periparturient hosts when nutrients are scarce(Reference Coop and Kyriazakis8). The absence of an effect on the immune parameters measured is contrary to the expectation, as cell-culture and animal feeding studies show a dose–response between leucine supply and the immune response to pathogens(Reference Calder10). Leucine is the sole branched-chain AA that can activate the mTOR pathway in intestinal epithelial cells(Reference Ban, Shigemitsu and Yamatsuji49), and it may play an important role in intestinal repair via stimulation of protein growth(Reference Naomoto, Yamatsuji and Shigemitsu50). A damaged mucosa could be associated with the higher number of worm burdens in our HP-Leu dams. Although the number of inflammatory cells in the intestinal mucosa, such as mast cells and eosinophils that are partly responsible for wound repair, did not appear to be affected by the feeding treatment, differential protein or gene expression in these cells cannot be excluded.
The present data showed that HP-Met feeding penalised dam weight gain but not litter weight gain relative to HP feeding. The importance of methionine supply in lactating rats has long been recognised(Reference Maruyema and Phillips51), and improved dam weight gain during lactation has been observed upon methionine supplementation in both low- and high-casein diets(Reference LeClerc, Chanussot and Miller52). Substantial body-weight loss of approximately 44 % has also been observed in non-lactating adult rats fed methionine-restricted foods(Reference Elshorbagy, Valdivia-Garcia and Refsum36). The absence of the effects on litter weight gain but also on worm burdens in the presence of body-weight loss suggests that the aforementioned mobilisation of body protein reserves may have supplied sufficient methonine to overcome the penalties of dietary methione scarcity on milk production and resistance. While this seems to contrast with earlier observations, where methionine supplementation of casein-based foods resulted in reduced N. brasiliensis worm burdens in growing rats(Reference Cummins, Bolin and Duncombe30), it should be noted that, in line with our observations on the intake of the HP-Met food, these results could have arisen from an increased food intake per se. Indeed, casein is limiting in SAA(Reference DeMan33), and it is for this reason that we consistently supplement casein with methionine to study the effect of CP supply on lactational resistance to parasites in the N. brasiliensis re-infection lactating rat model(Reference Houdijk, Jessop and Knox4–Reference Sakkas, Houdijk and Jones7, Reference Houdijk, Jessop and Knox31). The discrepancy between the effect of HP-Met on worm burdens and the number of worm EIC could suggest that low dietary methionine supply affects nematode fecundity. It has indeed been observed that worm egg excretion may be more sensitive to protein scarcity than worm expulsion(Reference Houdijk, Kyriazakis and Jackson2).
We omitted approximately 45 % of the HP-Met rats from the main data analysis, as they showed very high refusals, in order to avoid confounding effects of methionine supply with feed intake per se. An additional restricted maximum-likelihood analysis on the parameters measured supports the view that this was warranted. Compared with their counterparts, the infected HP-Met animals showing high refusals had lower DM intake (26·4 v. 7·1 g/d; sed 2·50 g/d; P< 0·001), dam weight gain ( − 0·58 v. − 9·02 g/d; sed 0·977; P< 0·001) and litter weight gain (16·5 v. 3·1 g/d; sed 1·66; P< 0·001). In addition, they carried higher worm burdens (127 (98–165) v. 409 (307–545); P= 0·015) and their eosinophil numbers were lower (48 (36–64) v. 20 (12–35) cells/villus crypt unit; P= 0·089). The latter observation support the aforementioned view that the absence of the effects of CP scarcity on inflammatory cells in the main dataset was probably the outcome of using a less severe level of CP scarcity than used in our earlier studies. While the number of eggs in the colon, mast cells and goblet cells of HP-Met refusers were similar to those in their counterparts, their Ig levels were significantly elevated by 39–51 % (P< 0·05). Although it cannot be excluded that the latter had partly arisen from a reduced blood volume arising from the severely reduced food intake, this further suggests that serum immunoglobulins are unlikely to play a direct role in worm expulsion from the gastrointestinal tract(Reference Jones, Houdijk and Sakkas6).
In conclusion, leucine and methionine deficiency in a high-protein food penalised lactating host resistance to a secondary infection with gastrointestinal nematodes and reduced lactational performance. From the immune indicators measured in the present study, which have previously been associated with the immunomodulatory effects of dietary protein, none was significantly affected. Consequently, further studies to elucidate the underlying mechanisms of reduced resistance following leucine and methionine scarcity should concentrate on other indicators of immunity. In light of the role of AA as immunonutrients and the strong effect that protein supply has on the periparturient host resistance to gastrointestinal nematodes, more AA could be tested using the model established here, including at different dietary levels so as to minimise the consequences of AA imbalance per se. The present results imply that AA balance and thus protein quality should be important considerations in immunonutrition strategies for livestock animals, in which periparturient breakdown of immunity to parasites causes significant economic losses.
Acknowledgements
The authors wish to thank the staff of the March Building at the University of Edinburgh for animal husbandry, Ian Nevison (Biomathematics and Statistics Scotland) for statistical advice and Meike Rademacher (Evonik, Germany) for the AA analyses of the experimental foods. This study was supported by the Biotechnology and Biology Sciences Research Council (BBSRC). The Scottish Agricultural College and the Moredun Research Institute receive support from the Scottish Government, Rural and Environmental Research and Analysis Directorate. P. S. is grateful to the Hellenic State Scholarship Foundation for the provision of a postgraduate scholarship. None of the authors has a conflict of interest in relation to this study. This study has resulted from the postgraduate studies of P. S. and is part of his PhD thesis. P. S. and L. A. J. are joint first authors. P. S., L. A. J., J. G. M. H., S. A., D. P. K. and I. K. conceived and designed the experiment. P. S. and L. A. J. performed the experiment. D. P. K. provided the infective larvae and facilities for the immunological analysis. P. S., L. A. J. and J. G. M. H. analysed the data. P. S., L. A. J., J. G. M. H., S. A., D. P. K. and I. K. contributed to data interpretation and manuscript preparation.









