INTRODUCTION
Gastroenteritis is usually described as a mild disease [Reference Kirk, Roberts and Horvath1, Reference Koopmans2] but is associated with important economic issues and morbidity concerns in developed countries [Reference de Wit3]. In The Netherlands, its burden in the general population is high, as over 25% of the population have at least one episode of gastroenteritis annually [Reference de Wit3]. Although the majority of cases are sporadic [Reference Musher and Musher4], several pathogens may cause outbreaks, to which people in closed settings are especially susceptible due to close contacts [Reference Kirk, Roberts and Horvath1, Reference Verhoef5]. The majority of gastroenteritis outbreaks in The Netherlands can be ascribed to norovirus [Reference van Duynhoven6]. In recent years, noroviruses belonging to the genotype II.4 have dominated, causing dramatic increases in the number of outbreaks [Reference Verhoef5, Reference Lopman7, Reference Widdowson8]. Norovirus has a great potential for person-to-person transmission as no more than 10–100 particles are required for infection and particles are shed in very high quantities [Reference Dolin9, Reference Ludwig10]. A recent study has estimated norovirus as the most infectious virus so far described, with the probability of developing symptoms once infected to be dose-dependent [Reference Teunis11].
The most common symptoms associated with norovirus disease are vomiting and diarrhoea, occurring after a short incubation period of 8–72 h and usually lasting for 1–3 days. Although norovirus-related disease has often been described as self-limiting in the general population [Reference Dolin9], severe and prolonged illness have been reported in frail patients [Reference Lopman12–Reference Siebenga14]. Previous studies have described living in an institution to be a risk factor for fatal outcome of gastroenteritis [Reference Wu15–Reference Frenzen18]. In addition, a recent study investigating the causes of intestinal fatal diseases showed that mortality due to norovirus was underestimated [Reference Harris19]. More in-depth analyses of the causes of fatal norovirus cases is needed to formulate public health recommendations that are easy to apply and allow the identification of patients needing specific attention in order to prevent mortality during norovirus outbreaks.
In October 2008, an outbreak of norovirus following a pilgrimage to Lourdes [Reference Verhoef20] occurred in a psychiatric institution in The Netherlands. This institution houses 285 residents in 68 homes or apartments shared by independent residents, eight medium-care buildings and one high-care building (HC building) for people with somatic diseases besides their psychiatric disorders. A total of 183 healthcare workers (HCWs) are employed at the institution. Between 25 September and 2 October 2008, 14 residents and 10 HCWs visited Lourdes on a pilgrimage. The first case was a HCW who exhibited symptoms of gastroenteritis at his hotel on 30 September. On 1 October, one of the residents showed similar symptoms during the return train journey to the institution in The Netherlands. Norovirus was determined to be the causative agent of the subsequent outbreak affecting 150 persons including four deaths between 2 October and 3 November [Reference Verhoef20]. With the source of the outbreak being Lourdes and the unusual amount of deaths reported in the institution, the outbreak resulted in large media attention in The Netherlands as well as in the international press (www.timesonline.co.uk; www.nrc.nl). The apparent severity of the outbreak led to an in-depth investigation of the event. The objective of this norovirus outbreak investigation was to retrospectively study the risk factors for norovirus disease and assess excess mortality in residents of this institution.
METHODS
Definitions
A norovirus case was defined as a person presenting ⩾2 episodes of loose stools in a 24-h period or ⩾1 episodes of unexplained vomiting and a physician diagnosis of acute gastroenteritis between 30 September and 3 November 2008. To discriminate between infections possibly contracted in Lourdes, and those contracted within the institution, an early norovirus case was defined as a norovirus case who had participated in the pilgrimage to Lourdes between 25 September and 2 October 2008 and who developed norovirus disease either during the pilgrimage or within the 72-h period thereafter. A late case was defined as a norovirus case who developed norovirus disease after the 72-h period following the return from the pilgrimage, or who did not travel.
Laboratory investigation
Throughout the outbreak period, acute phase stool samples were collected for standard diagnosis and confirmation of the pathogen causing the outbreak. Samples were tested according to a diagnostic protocol for Clostridium difficile toxins A and B (EIA, Premier C. difficile toxin A&B, Meridian Diagnostics Inc., USA), Salmonella enterica, Shigella spp., Escherichia coli, Campylobacter jejuni, astrovirus, adenovirus, rotavirus and norovirus. Testing for S. enterica was performed for locus ttrRSBCA, as described by Malorny et al. [Reference Malorny21]; for C. jejuni for the mapA gene as described by Best et al. [Reference Best22] with probes adjusted to MGB format; for Shigella spp. and E. coli for the ipaH gene as described by Vu et al. [Reference Vu23]. Testing for viruses was performed as described by Svraka et al. [Reference Svraka24].
In addition to the diagnostic protocol, testing for C. difficile was performed according to van den Berg et al. [Reference van den Berg25]. When samples tested positive for norovirus, RT–PCR and subsequent sequencing of a part of the polymerase gene (region A) and the complete VP1 gene was performed for genotyping and comparison of strains as previously described [Reference Siebenga26].
Epidemiological investigation
Study design
A retrospective cohort study was performed, including residents only, and using data available from the institution. The analysis was performed at two levels:
• Cohort 1: the complete institution including 285 residents.
• Cohort 2: the HC building including 59 residents.
For cohort 1, the following data items were collected: date of birth, gender, place of residence, and if applicable, dates of onset of disease, symptoms, date of death if this occurred between 30 September and 3 November 2008. Death certificates were requested from Statistics Netherlands (CBS) by the reporting physicians. Control measures implemented in the institution during the outbreak were extracted from a log file kept by the staff.
For cohort 2, the following additional data items were collected: somatic medical history, current use of medication as recorded by the nursing staff on 1 October 2008, treatment of symptoms of gastroenteritis during the outbreak period, smoking status, level of the residents' dependency on HCW, type of toilet facility (private or shared).
The medical records were reviewed in order to estimate the Charlson comorbidity (CC) index score [Reference Charlson27]. The CC index is a validated score combining age and a range of 16 underlying conditions, weighted according to their severity. The score was used as a measure for underlying illnesses [Reference Charlson27]. In addition, the current medication was screened for statins (hydroxymethylglutaryl-CoA reductase inhibitors), immunosuppressive drugs, gastric-acid inhibitors and diuretics used prior to the outbreak period. Finally, medication was screened for gastrointestinal side-effects in order to account for potential misclassification. The level of the residents' dependency on HCWs was assessed using the validated Barthel score [Reference Mahoney and Barthel28] (modified 20-point Barthel score). This is a score of dependence assessing the daily activities that the residents can do without assistance, with lower scores indicating greater dependence on HCWs. This score was used for ranking of the level of contact between residents and HCWs. The Barthel score was retrospectively assessed based on patients' normal conditions before the outbreak.
In addition, monthly numbers of all-cause deaths in the HC building from January 2006 to August 2009 were requested from the hospital administration service in order to investigate any excess mortality during the outbreak period. All data were collected and analysed anonymously. No medical ethics review was required.
Statistical analysis
Univariate and multivariate analyses were performed in order to identify risk factors for norovirus disease. In univariate analysis, crude relative risks (RR) and 95% confidence intervals (CI) were calculated using χ2, with P value<0·05 indicating significance and 0·05<P value<0·10 borderline significance. Variables were included in multivariate analysis if their P value was <0·20 during univariate analysis. A multivariate logistic regression model was used to obtain adjusted odds ratios (aOR). The variables remained in the multivariate model if P values were <0·10, while using the backward selection procedure to establish the most important explanatory variables. To investigate risk factors for mortality, univariate analysis was performed as described above, and a median test was used for pairwise comparison of CC scores between early cases and other residents. Analyses were performed for cohorts 1 and 2 separately, using Stata v. 10.0 software (StataCorp, USA).
In order to investigate a possible excess mortality, monthly mortality during the outbreak period was compared to the baseline data, i.e. monthly numbers of all-cause deaths in the HC building during the periods January 2006–September 2008, and November 2008–August 2009. A likelihood ratio test was used, comparing binomial probabilities of dying before or after to during the outbreak, with equal mortalities as a null hypothesis using Mathematica® version 6 [29].
RESULTS
Laboratory investigation
During the outbreak, 26 acute phase stool samples from 25 individuals were sent to the diagnostic laboratory for diagnosis. For two persons the diagnosis remained inconclusive due to insufficient material and inhibition of the PCR assay. Of the remaining 23 samples, 12 tested positive for norovirus and negative for all other pathogens, one tested positive for C. difficile and negative for all other pathogens, and 10 tested negative for all pathogens. All 12 samples tested for C. difficile toxins A and B with EIA were negative. With 12/23 samples positive for norovirus, the outbreak was attributed to norovirus as the causative agent [Reference Duizer30]. Sequencing of the PCR products showed that the strain involved was typed as genogroup II.4-2006b, as shown in the phylogenetic tree (Fig. 1). Comparison of the full capsid genes shows that one strain was involved throughout the outbreak, with only one nucleotide mutation during the outbreak period. The sequence of the outbreak strain was identical to the sequence of the strain detected in Lourdes in September 2008 (Fig. 1, strains A and E), and the strains detected in four Dutch Lourdes pilgrims unrelated to the psychiatric institution (Fig. 1, strains L, M, N, O). One non-institutionalized resident, who was therefore not part of our two cohorts, was symptomatic with norovirus, but the virus detected in this patient (Fig. 1, strain P) was genetically distinct from the outbreak strain. Additionally, hospital patients from a nearby hospital with an indicated epidemiological link to Lourdes tested positive for GII.4-2006b variant norovirus, but the sequences obtained from these patients differed considerably, implying that these patients had been infected elsewhere (Fig. 1, strains R, S, T).
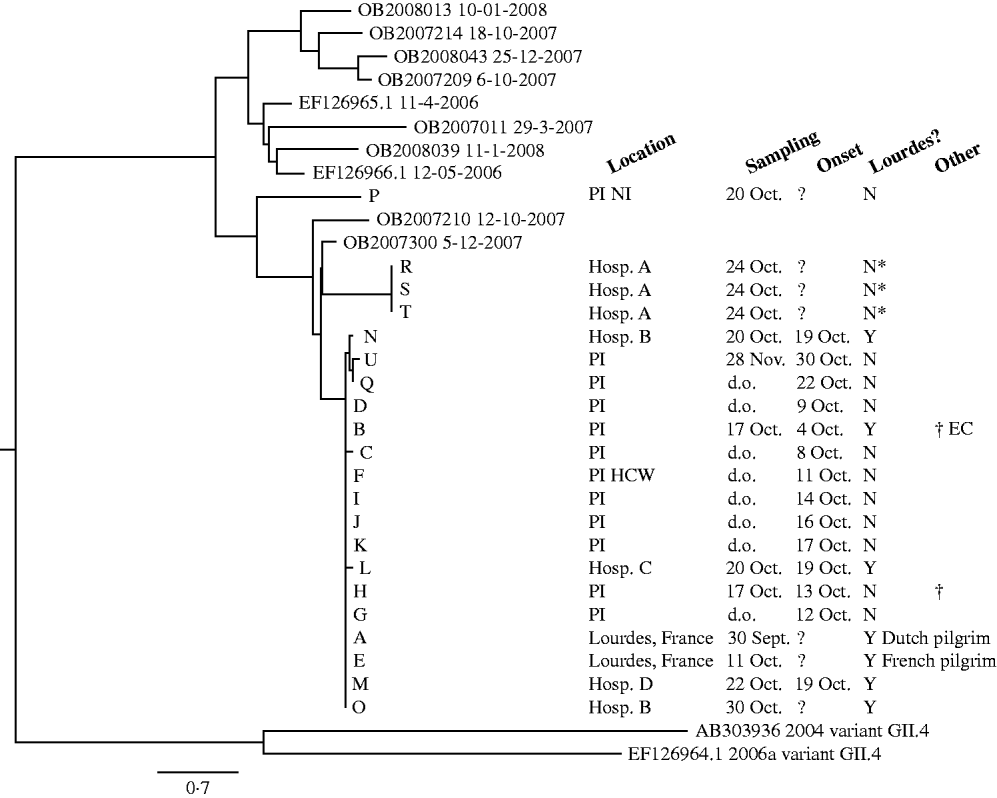
Fig. 1. Neighbour joining tree of full capsid sequences (1623 nucleotides) of norovirus samples detected during the outbreak period. Reference sequences of GII.4-2006b detected in The Netherlands were included with detection dates; the outlier group contains a GII.4-2006a and a GII.4-2004 sequence. PI, Psychiatric institution; PI HCW, psychiatric institution healthcare worker; Hosp, hospital; Y, visited Lourdes; N, did not visit Lourdes; N*, suspected link with Lourdes; d.o., during outbreak; EC, early case; ?, unknown.
Epidemiological investigation
Between 30 September and 3 November 2008, 104/285 residents of the institution (attack rate 36%) and 46/183 HCW (attack rate 25%) met the norovirus case definition. The symptoms, recorded for 25 residents, included diarrhoea (88%), vomiting (48%) and fever ⩾38°C (20%), and lasted on average 3 days (range 1–16 days). Figure 2 a shows the epidemic curve within the institution, discriminating the early and late cases as well as cases in HCWs and residents. The outbreak started and ended with HCW cases. Six residents met the early case definition, three of whom died.
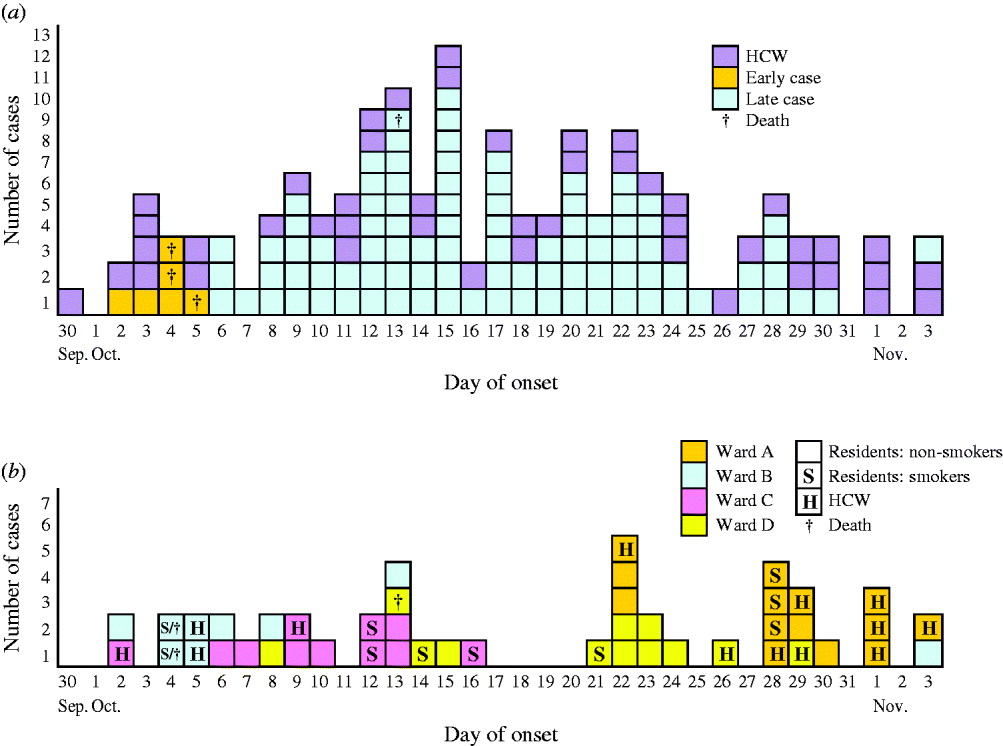
Fig. 2. Cases of norovirus infections by date of onset in a psychiatric institution, The Netherlands, 30 September to 3 November 2008. (a) All residents and healthcare workers (HCWs), differentiating between early and late patient-cases and indicating fatal outcomes (patients who died within 2 months following the date of onset of their symptoms), n=144. (b) Cases that occurred in the high-care building in HCWs and residents, differentiating the smoking status of the residents and the ward of residence/work of all the cases, n=44.
Figure 2 b shows the outbreak evolution in the HC building, with ward-to-ward spread over time. On 11 October control measures, such as wearing gloves, were implemented in the four wards of the HC building. On 28 October, wards B, C and D were thoroughly disinfected and isolated from ward A which experienced the last outbreak wave. During the outbreak period, social activities were cancelled and the staff were asked to limit cross-contact between wards. As smoking could not be prohibited, smoking rooms were still used, enabling potential further person-to-person transmission. Wards A and D had their own smoking rooms, whereas wards B and C shared one. In Figure 2 b, some clusters of smokers can be seen.
Risk factors for norovirus disease in residents
In cohort 1 (Table 1), cases, compared to non-cases, were more likely to be aged >70 years, female and a Lourdes pilgrim. In addition, cases were more frequently sharing their housing and living in the HC building. Multivariate analysis showed that higher age and participation in the Lourdes pilgrimage were independently associated with norovirus disease.
Table 1. Factors associated with acquiring norovirus disease, in patients during a norovirus outbreak in a psychiatric institution, The Netherlands, 2 October–3 November 2008
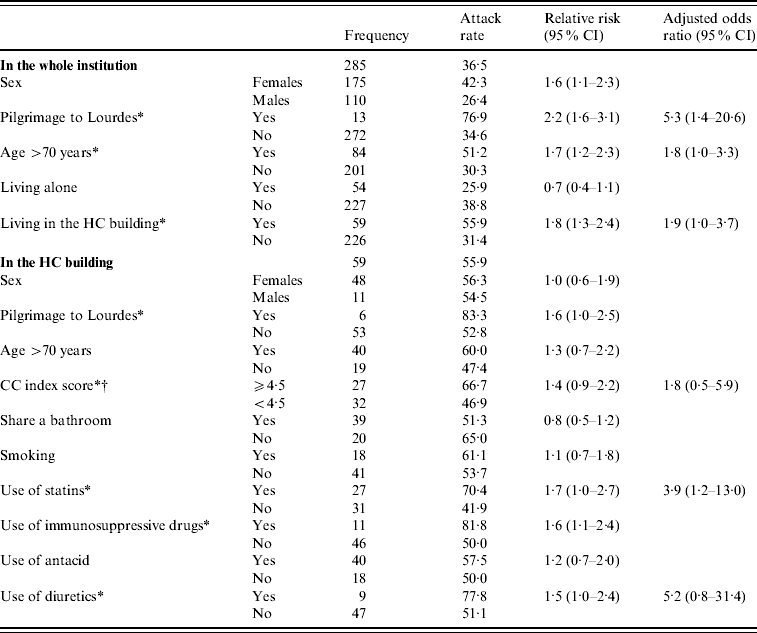
CI, Confidence interval; HC, high care.
* Variables included in the initial multivariate model.
† Charlson comorbidity (CC) index score forced into the model.
In cohort 2, compared to cohort 1, the proportion of women (81% vs. 56%, P<0·01), Lourdes pilgrims (10% vs. 3%, P=0·02) and older residents (median age 75 vs. 53 years, P<0·01) was higher.
Within cohort 2, in univariate analyses, factors significantly associated with an increased risk of being a case (see Table 1) were the pilgrimage to Lourdes and the use of statins, diuretics or immunosuppressors. In multivariate analysis, CC score being a plausible biological confounder in the association between medication and norovirus disease, it was forced into the model. As a result (Table 1), the use of statins and diuretics remained in the model as independent risk factors. While studying Barthel scores, the risk for being a case did not differ between low and high dependent residents (P=0·91). However, five highly dependent patients with Barthel scores ranging from 1 to 3 (mostly bed-bound), were infected.
Risk factors for mortality in residents
Table 2 shows the mortality rates in cohort 1 stratified for potential risk factors. In this cohort, the mortality rate was significantly higher in the early cases as well as the HC building residents, compared to the other residents of the institution.
Table 2. Factors associated with mortality in patients during a norovirus outbreak and within the 2 months thereafter, The Netherlands, 2 October–3 November 2008
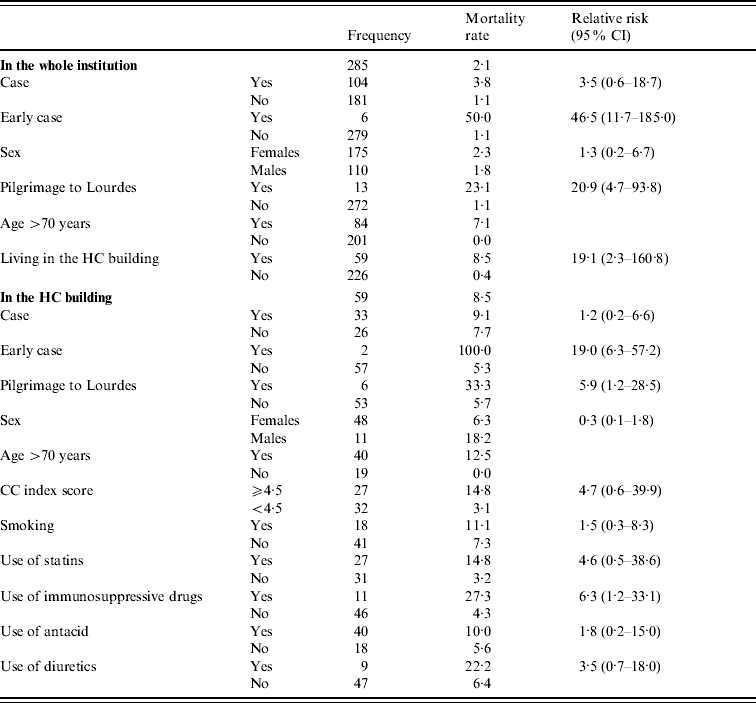
CI, confidence interval; HC, high care; CC, Charlson comorbidity.
In cohort 2, univariate analysis showed a significant increase of mortality in early cases, residents with high CC scores, and immunosuppressive drugs users. Median CC scores did not significantly differ between early cases compared to the other residents in cohort 2 (5·8 and 4·1, P=0·15) when using the median test, and no significant difference could be seen in the use of immunosuppressive drugs between early cases and other residents (Fisher's exact test=0·352). Moreover, in this cohort, no specific underlying disease was associated with mortality.
Death certificates of the six residents who died during the outbreak period are summarized in Table 3. Four of them were norovirus cases; they died on average 4·75 days after onset of symptoms. Causes of death listed gastrointestinal disorders for three of them. In addition, acute gastrointestinal bleeding and acute diverticulitis were reported for the two non-case residents who died during this period.
Table 3. Causes of death reported in death certificates of the residents that died during a norovirus outbreak in a psychiatric institution, The Netherlands, 2 October–3 November 2008
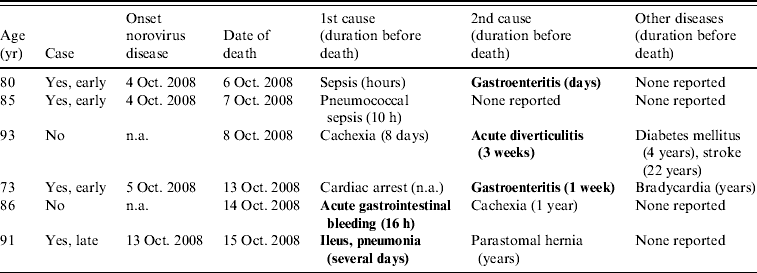
n.a., Not available.
Bold text indicates gastrointestinal-related disorders.
Risk factors for mortality in the cases
Mortality in early cases was 16 times higher compared to late cases (50% vs. 3%, P<0·001) in cohort 1. In cohort 2, the median test showed no significant difference in CC score between early and late cases (respectively 5·8 and 4·5, P=0·13) while the mortality rates were 100% (2/2) and 10% (3/31), respectively.
Monthly mortality rates
The study of monthly mortality in the HC building from January 2006 to August 2009 (Fig. 3) showed that the mortality was 12·1 times higher (95% CI 3·9–28·6) during the month of the outbreak compared to the rest of the period of observation, i.e. 83·3 vs. 6·6 deaths per 1000 patient-months.
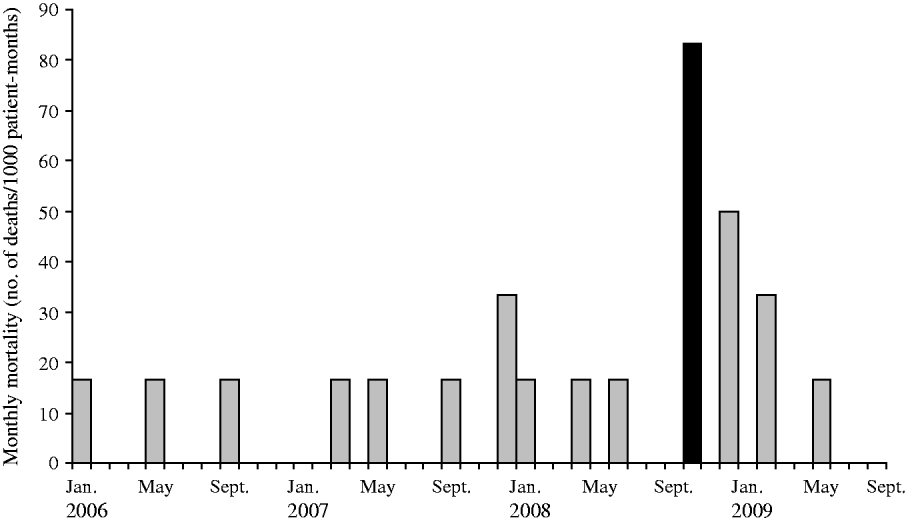
Fig. 3. Monthly mortality in the high-care building of a psychiatric institution, The Netherlands, 1 January 2006 to 31 August 2009. The outbreak period is indicated by the black bar (▪).
DISCUSSION
Thorough retrospective investigation of an extensive norovirus outbreak in a healthcare setting showed time-associated excess mortality. In addition, the use of statins and diuretics were newly identified as potential risk factors for acquiring symptomatic norovirus infection. Furthermore, the use of quantitative measures to adjust for comorbidity and evaluation of medication are relatively novel approaches in the setting of norovirus outbreaks and represent potentially useful contributions to current knowledge.
The outbreak strain detected in Lourdes in September 2008 was imported into a psychiatric institution through the pilgrimage. Attendance at the pilgrimage was strongly associated with an increased incidence of norovirus disease, as 77% of the pilgrims were symptomatic. One of the cases vomited in the presence of other residents in the train during the return journey. As previously demonstrated [Reference Marks31], there is an inverse relationship between distance from a vomiting incident and the risk of infection. Therefore, a high incidence of norovirus disease in the pilgrim group was to be expected. Age and living in a HC building were also associated with an increased risk for norovirus disease. These findings can be explained by the lower immunity and more frequent underlying conditions in elderly [Reference High32], and the greater frailty of the residents living in this specific building.
Once back in the institution, the (potentially) infected residents were placed in their accommodations, enabling simultaneous spread of the virus within several locations of the institution. Despite no statistical evidence, the epidemic curves suggest a potential role of the HCW as a vector, with some very dependent residents being infected in the HC building and subsequent ward-to-ward spread. In an outbreak setting with high attack rates in HCWs, the compliance with control measures is known to be complicated due to sick-leave of HCWs and an increased need for care of patients [Reference Friesema33]. Smoking was another interesting hypothesis to explain the spread of the virus in residents. According to staff, it was impossible to avoid exposure to smoking during the outbreak, with the consequential possibility of viral spread due to shared smoking rooms. The transmission chain of this outbreak needs further investigation, including smokers and HCWs as possible vectors.
Of residents of the HC building, for whom we were able to collect extensive data, use of statins and diuretics were the only (borderline) significant risk factors for symptomatic norovirus infection, when adjusted for underlying conditions. To our knowledge, these risk factors had never been described before. While greater severity due to higher risk of dehydration can be expected from use of diuretics [Reference Ferry34], no biologically plausible explanation was found for their role in susceptibility to infection. However, biologically plausible pathways are described for cholesterol-lowering drugs, i.e. statins. A recent study highlights the importance of the cholesterol pathway for norovirus replication and more specifically demonstrates that the inhibition of cholesterol biosynthesis using statins significantly increases the level of norovirus proteins and RNA [Reference Chang35]. These in vitro findings are in consistency with our observational study. Further investigations in that field are warranted. Statins are widely used in the industrialized world. In 2003, an estimated number of 25 million people worldwide were treated with statins amounting to an estimated 22 billion US dollars worldwide [Reference Mitka36]. Additionally, despite lack of statistical significance, it is worth mentioning that 4/5 (80%) of fatal cases were using statins, compared to 15/27 (56%) of the non-fatal cases. Finally, information on the impact of stopping statins when subjects became ill was not collected but would be interesting to examine further.
During the outbreak period six residents, including four cases, died. Gastrointestinal disorders were listed on 3/4 certificates of the deceased cases, who died on average 4·75 days after onset of symptoms. This duration is consistent with findings from a recent study suggesting that norovirus outbreaks preceded unexplained gastroenteritis deaths by 1 week [Reference Van Asten37]. The combination of the death certificate screening and the statistical analysis, together with the excess mortality observed during the outbreak in the HC building, allow us to highlight the potential severity of norovirus disease. Moreover, of interest is the excess fatality observed in early cases. All but one became symptomatic once back at the institution, excluding a potential lack of care related to travelling. The mean age of the residents infected during pilgrimage was 68 years. Elderly travellers have previously been described to be at higher risk of developing severe outcome of infection [Reference McIntosh and Lockie38] and travelling can be considered a stress factor. Moreover, presence at the initial place of the outbreak and proximity of a symptomatic case in the train may have resulted in a higher viral load. These factors might have been responsible for higher morbidity, as has been previously shown for Campylobacter [Reference Teunis39]. Additional studies are needed to understand this phenomenon and test this hypothesis.
This outbreak investigation highlights several new findings useful for targeting the intervention towards specific groups of patients during norovirus outbreaks in healthcare settings. Nevertheless, some limitations can be discussed: the case definition used in the retrospective cohort study relied on nurses' reports, and laboratory confirmation was only present for a subset of residents. Given the previously described proportions of asymptomatic norovirus shedders, it is likely that 30% [Reference Gallimore40] of the non-cases were shedding the virus. The use of this case definition may, therefore, have resulted in an underestimation of the attack rate. In addition, a symptom-based definition may have resulted in misclassification of residents, especially those using laxative medication for treatment of the side-effect constipation, which is often seen in medication used to treat psychiatric disorders. Nevertheless, analysis of the laxative medication used for residents in the HC building showed no difference between cases and non-cases (P=0·820), making misclassification unlikely. This is possibly due to the use of a case definition based on reports from the nurses who work with the residents on a daily basis and, who therefore, may be able to make the distinction between chronic diarrhoea and norovirus disease symptoms. Another limitation comes from the possibility of collecting extensive data in only one building, housing older and frailer residents compared to the rest of the institution. Results from this cohort are therefore applicable to this specific population group. Finally, detection of risk factors explaining fatal outcomes was only possible for small sample sizes. Even though we found several statistically significant associations, we may have lacked power to detect additional causes of fatal outcomes. On the other hand, the availability of in-depth medical information gave us the opportunity to use relevant and validated scores to disentangle risk factors from the effect of underlying diseases.
In conclusion, this investigation showed excess mortality associated with a norovirus outbreak, confirming the potential severity of norovirus disease in the elderly, and especially elderly travellers. For the latter, a review of currently used medication could assist in identifying patients needing specific care during a norovirus outbreak. Given the relatively high occurrence of norovirus outbreaks onboard cruise ships in the elderly, this recommendation could be of interest for cruise liners. The potential impact of statins on the susceptibility to norovirus disease requires confirmation in a prospective study. Given the frequency that statins are used, a catalysing effect on symptomatic norovirus infection would result in significant morbidity as well as financial burden in the ageing developed world.
ACKNOWLEDGEMENTS
We thank the management team of the psychiatric institution for allowing us to perform this study. We express our thanks to Françoise van Heiningen, Anke de Ruiter-van Balkom and Annelies Reuvers for their help and cooperation. We thank epidemiologists and statisticians Doris Radun, Manuel Dehnert, Harold Noel and Marianne van der Sande for their advice and microbiologists and virologists Ed Kuijper, Bas van der Veer and Sabine Dittrich for their support with the laboratory work as well as in the writing. We thank Pierre Pothier and Katia Ambert-Balay at the National Reference Centre for Enteric Viruses, Laboratory of Virology, University Hospital of Dijon, France, for sharing the samples they obtained from gastroenteritis patients in Lourdes, France, for sequencing purposes. We are grateful to the treating physicians and Jan Kardaun for their kind cooperation concerning the death certificates. This work was supported by the National Institute for Public Health and the Environment (RIVM). Marc Rondy, M.Sc., is an EPIET Fellow in the Epidemiology Department of the National Institute for Public Health and the Environment of The Netherlands.
DECLARATION OF INTEREST
None.








