Introduction
Venolymphatic malformations are the most common vascular malformations and a majority is present in the head and neck. Reference Fayad, Hazirolan and Bluemke1–Reference Flors, Leiva-Salinas and Maged5 They are rare benign vascular lesions present at birth but usually presents in late childhood or early adulthood. Total surgical resection of these malformations is often not possible and recurrence is frequent besides the functional and esthetic sequelae from surgical resection. For this, sclerotherapy has become the first-line therapy of these lesions with bleomycin being a sclerosing agent commonly used in the region of the head and neck.
Bleomycin, or pingyangmycin (also known as bleomycin A5), is a cytotoxic agent used for the systemic treatment of various types of malignancies including lymphoma and cutaneous squamous cell carcinoma. 6–Reference Horbach, Rigter and Smitt10 It is also injected intralesionally for the treatment of subcutaneous tumors like warts and keloids. Reference Yamamoto8,Reference Saitta, Krishnamurthy and Brown9 In venolymphatic malformation, the intralesional injection of bleomycin induces sclerosis Reference Yamamoto11,Reference Miyamoto, Sugawara and Azuma12 through endothelial damage and fibrosis. Reference Zhang, Chen and Ren13,Reference Jia, Jia and Zhao14 Bleomycin has been considered very safe for this indication with the rare complications reported with the use of bleomycin include hyperpigmentation, necrosis, and pulmonary fibrosis. Reference Adamson and Bowden15–Reference De, Guryev and LaRiviere17
Most of the published studies are smaller single-center studies, which can be used for any significant and meaningful conclusion or guidelines. Earlier systematic reviews included venolymphatic malformation anywhere in the body. Reference Horbach, Rigter and Smitt10,Reference Horbach, Lokhorst and Saeed55–Reference van der Vleuten, Kater and Wijnen57 The purpose of our study was to perform a systematic review of the published literature to synthesize the evidence on the safety and efficacy of bleomycin for the treatment of venolymphatic malformations in the head and neck region only.
Methods
We used standard systematic review methods advocated by the Cochrane Collaboration and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. Reference Liberati, Altman and Tetzlaff18–Reference Higgins, Thomas and Chandler20
Inclusion Criteria
Study type: Any prospective or retrospective case series or clinical trials.
Patient type: Studies that enrolled patients of any age with the diagnosis of head and neck venolymphatic malformation.
Intervention: Sclerotherapy with bleomycin or pingyangmycin (a bleomycin analog) alone, or in combination with other sclerosing agents or techniques.
Outcome measures: Subjectively or objectively documented lesion size reduction, major or minor complications. Subjective size reduction was defined as reduction appreciated either by the patient or the physician documenting it. Objective size reduction was defined when size reduction was documented on any cross-sectional imaging tests. Major complications were defined as extensive necrosis, oral or respiratory obstruction, and pulmonary fibrosis. Minor complications were largely self-limiting such as transient pain, edema, hematoma, ecchymosis, atrophy, hypertrophy, infection, superficial necrosis, and hyperpigmentation.
Follow-up period: Unrestricted.
Publication types: Search was unrestricted but non-English language studies were excluded from the final review. This was mainly due to the lack of resources for translation. All publications from across the world were included. Only study articles published in peer-reviewed literature were included.
Exclusion criteria: Non-English language studies and studies with less than five patients were excluded.
Search strategy: A computerized search of PubMed, Embase, and Cochrane Library was done using index terms and keywords (see Appendix 1) from January 1995 until May 2019. In addition to online database searching, reference lists of all included studies and previous reviews were also screened.
Data collection and analysis: Two researchers independently used Covidence for the primary and secondary screening (title/abstract and full text, respectively) and data extraction (Figure 1) to streamline the production of standard intervention reviews. The titles and abstracts were screened and were categorized as “Yes,” “No,” and “Maybe.” For those categorized as “Yes” and “Maybe,” a full-text review was done to assess the inclusion of the studies. Data were then extracted from the included studies using a standard extraction form with characteristics of the trials, participants, interventions, and outcomes.

Figure 1: Flowchart of selection of studies. Emabse n = 259; Medline n = 134; Scopus n = 162.
Covidence was also used for the Risk of Bias assessment using Cochrane risk of bias criteria. Cochrane risk of bias criteria included assessment of sequence generation; allocation concealment; blinding of participants and study personnel; blinding of outcome assessors; incomplete outcome data; selective outcome reporting; and other sources of bias. Data on all relevant variables were collected (Table 1).
Table 1: Summary of all included studies

* In the case bleomycin quantity was expressed in International Units (IU), conversion to milligrams (mg) was made according to the formula 1 IU = 1 mg Reference Nuruddin, Roy and Mudhar38 .
Meta-analysis: A meta-analysis was not possible because of the heterogeneity of the study designs and interventions performed.
Grading of evidence: Overall quality of evidence was graded by interpretation of the quantitative synthesis using recommendations of the GRADE working group. Reference Atkins, Best and Briss21 The risk of bias, the completeness and context of available evidence, and the size and consistency of observed effects were considered in the grading of evidence.
Result
The flow diagram of systematic literature search and study selection is depicted in Figure 1.
Trial Design
Twenty-eight retrospective cohort studies, two prospective cohort studies, one case-control, and one randomized control study were included. In the randomized control trial, the analysis was based on intention to treat. Nineteen studies reported on venolymphatic lesions of the head and neck in general, 7 on lesions located in the orbital region, and 6 on lesions in the orofacial area. Most studies used a combination of imaging modalities for the diagnosis of venolymphatic malformations – 17 studies used ultrasound, 11 studies used CT, 20 studies used MRI while no imaging test was used in 6 studies.
Trial Participants
A total of 1121 patients with venolymphatic malformations of the head and neck were included, with a mean of 35 patients per study (SD: 17 patients, range – 6–297 patients) of whom 45.1% were females. The mean patient age was 14.4 years (range – 9 days–72 years). Reported symptoms included cosmetic issues, skin discoloration, progressive increase in size, pain, hemorrhage, fever, and erythema. The orbital lesions presented with blurry vision, scotoma, proptosis, pain, diplopia, palpable mass, ecchymosis, bleeding, motility disturbance, ptosis, eyesight obstruction, and eyelid dysfunction. Oral lesions presented with swallowing and chewing difficulties, oral obstruction, hemorrhage, functional problems (breathing, suction, and speech), distorted dentition, and discomfort. Only a few studies reported the actual percentage of patients presenting with these symptoms.
Interventions
Bleomycin was used as a sclerosing agent in 562 patients and pingyangmycin in 559 patients. The operators were neuroradiologist or radiologist in 8 studies, a surgeon in 6 studies, and not specified in 18 studies. Besides sclerotherapy sessions with bleomycin/pingyangmycin 33 patients received surgical treatment, 30 patients laser therapy, 9 patients absolute ethanol sclerotherapy, and 22 patients sodium tetradecyl sulfate sclerotherapy. It is unclear how many of these patients received the additional treatment before or after undergoing treatment with bleomycin. The type of analgesia/anesthesia performed was reported in 726 patients. Local anesthesia was administered in 393 patients (54.1%), sedation in 50 patients (6.8%), and general anesthesia in 77 patients (10.6%) while some type of analgesia/anesthesia without further detail was administered in 176 patients (24.2%). In 30 patients (4.1%), sclerotherapy was administered without any anesthetic/analgesic treatment. The imaging guidance used for treatment guidance was reported in 523 patients – ultrasound in 141 patients (26.9%), fluoroscopy in 164 patients (31.3%), and in 43 patients (8.2%), the imaging used was not specified. In 175 patients (33.4%), sclerotherapy was administered without any image guidance.
The mean number of sclerotherapy sessions per patient was 3.36 (range – 1–9). The mean concentration of bleomycin/pingyangmycin used was 1.6 mg/ml (range – 0.5–4 mg/ml). The maximum dose administered per session ranged from 0.2 to 1 mg/kg. The total maximum dose administered ranged from 0.033 to 6 mg/kg with a mean of 20.5 mg (min. = 1 mg, max. = 96.5 mg). Lower doses were usually used in children.
Outcome
Bleomycin/pingyangmycin sclerotherapy achieved subjective or objective lesion size reduction in 96.3% (95% CI 94.1%–98.5%) of patients (Table 1). Minor complications were observed in 16.2% (95% CI 10.5%–21.7%) of cases. Major complications were seen in four patients (1.1%, 95% CI 0%–2.5%) of whom one was death (0.3%, 95% CI 0%–0.8%). The circumstances of this last complication could not be deduced. Reference Kim, Sung and Roh33 The other major complications included abscess in one patient and two patients needing surgical decompression after bleomycin injection for orbital lymphagiomas to improve eyelid position. Reference Porwal, Dubey and Morey39,Reference Raichura, Alam and Noronha40 The mean duration of follow-up from the last treatment was 17 months (range – 1 month to 7 years). The imaging modality on follow-up was reported only in 817 patients. Ultrasound was used in 288 patients (35.2%), MRI in 143 patients (17.5%), and in 343 patients (41.9%), the imaging modality used was not specified. In 304 patients (37.2%), no imaging was performed on follow-up.
Risk of Bias in Included Studies
The risk of bias was assessed for allocation, blinding, incomplete outcome data, selective reporting, and other sources. The risk of bias summary for each risk of bias item for each included study is shown in Figure 2. This is shown in a risk of bias graph where the judgments about each risk of bias item are presented as percentages across all included studies (Figure 3). The risk of bias was used for grading the evidence from this review.
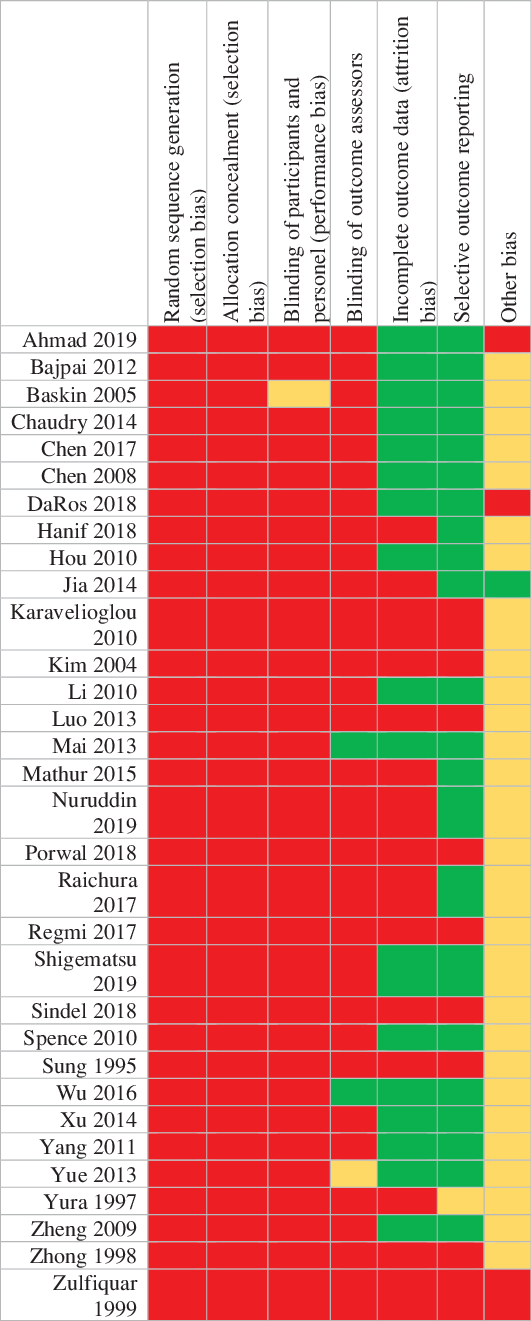
Figure 2: Judgments about each risk of bias item for each included study.

Figure 3: Risk of bias graph, judgments about each risk of bias item presented as percentages across all included studies.
Discussion
Summary of Main Results
Our systematic review assessed the efficacy and safety of bleomycin sclerotherapy for venolymphatic malformations in the head and neck region. The lesion reduction was found in 93.7% (95% CI 88.5%–98.9%) of subjective or objective lesion size making it a highly effective treatment in the head and neck regions (Table 1). No studies reported the recurrence of these lesions. Minor self-limiting complications occurred in 16.2% (95% CI 10.5%–21.7%) of patients while major complications occurred only in four patients (1.1%, 95% CI 0%–2.5%) including death of one patient (0.3%, 95% CI 0%–0.8%). However, the exact circumstances of death in this one patient were not described. Reference Sung, Chang and Choi45 Our results suggested that bleomycin/pingyangmycin treatment is relatively safe with minimal major non-self-limiting complications.
The included studies did not specify the specific location of the lesions in the head and neck region. The included studies also did not specify whether the lesions were primarily venous or lymphatic or mixed or macro- versus microcystic type. Although these will be of interest to know, our study could not shed light on these issues. It is considered that microcystic lymphangiomas tend to be more resistant to treatment than macrocystic lymphangiomas possibly because there are no obvious cysts to target within the lesion. Reference Wu, Wang and Zheng46 The therapeutic effect on large diffuse lesions was poor. Reference Wu, Wang and Zheng46
Completeness and Applicability of Evidence
This review synthesized evidence provided by 32 studies from different parts of the world published before May 2019. A high heterogeneity in the dosage, administration protocols, administration techniques, follow-up, and outcome measures after bleomycin sclerotherapy was noted among studies. This review highlights the need and may help in designing a more standardized protocol of bleomycin sclerotherapy and outcome assessment.
Quality of Evidence
The overall quality was low by GRADE approach Reference Guyatt, Oxman and Vist54 since most of the included studies suffered from a high risk of bias (Figure 3).
Potential Bias in the Review Process
Two reviewers (SF and KF) independently screened all the studies to reduce any bias during the screening process. The inclusion of only studies with full publication in peer-reviewed journals led to publication bias. This was done to keep the quality of evidence to the highest possible level. Only studies published in the English language were included due to the lack of translation service for appropriate translation of studies published in languages other than English.
How is this Different from Other Reviews?
This is the most updated review on bleomycin/pingyangmycin sclerotherapy of venolymphatic malformations. Earlier systematic reviews were assessed for venolymphatic malformation anywhere in the body. Reference Horbach, Rigter and Smitt10,Reference Horbach, Lokhorst and Saeed55–Reference van der Vleuten, Kater and Wijnen57 Our study is the first one on head and neck venolymphatic malformation. We excluded high-flow malformations in our study as they are known not to respond very well to bleomycin/pingyangmycin treatment.
Author’s Conclusion
Implication for Practice
The grade of evidence, in favor of bleomycin treatment for head and neck venolymphatic malformations, is only moderate. However, for clinical practice, a randomized clinical trial (RCT) to prove this beyond doubts is warranted.
Implications for Research
In our review, the evidence for the efficacy of bleomycin treatment for subjective or objective lesion reduction of venolymphatic malformations of the head and neck is only moderate. For a higher level of evidence, an RCT is warranted.
Conclusions
Bleomycin sclerotherapy is effective and relatively safe in the treatment of venolymphatic malformations of the head and neck region.
Conflict of Interest
The authors declare that they have no conflict of interest.
Statement of Authorship
SF – Data collection, analysis, and manuscript preparation and review; KF – Data collection and manuscript review; JL – Search and manuscript review; JJSS – Conceptualization, data collection, analysis, and manuscript preparation and review.
Ethical Approval
All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.






