High-yielding dairy cows are prone to oxidative stress due to intensive metabolic demands for maintenance and production. This condition can be exacerbated under certain environmental, physiological and dietary factors(Reference Bernabucci, Ronchi and Lacetera1, Reference Castillo, Hernandez and Bravo2). Although lipid supplementation of ruminant diets with n-3 PUFA is a strategy to improve the nutritional quality of dairy products, this approach could increase the risk of plasma peroxidation with deleterious consequences on animal health(Reference Gobert, Martin and Ferlay3). Peroxidation results from oxidative metabolism, which is essential for the survival of cells. However, a side effect of this phenomenon is the production of free radicals and other reactive oxygen species that can cause oxidative damage(Reference Castillo, Hernandez and Bravo2). Normally, the body is protected by a wide range of antioxidant systems working in concert with intracellular enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT), which remove superoxides and peroxides before they react with metal catalysts to form more reactive compounds(Reference Miller, Brzezinska-Slebodzinska and Madsen4).
Lactation performance and antioxidant status of cows fed oxidised fat are enhanced when antioxidants are included in the diet(Reference Vázquez-Añón, Nocek and Bowman5), which may be due to the scavenging of peroxides and the reduced peroxidation of fatty acids(6). Supplementing antioxidants such as vitamin E and sodium selenite from early summer (e.g. the period characteristic of heat stress) may improve fertility through a decrease in cortisol secretion and oxidative stress, which enhances pregnancy rates in buffalo cows(Reference Megahed, Anwar and Wasfy7). Moreover, strong positive correlations between several antioxidant enzymes (e.g. GPx) and pro-inflammatory vascular adhesion molecules suggest a protective response of antioxidants to an enhanced pro-inflammatory state in transition dairy cows(Reference Aitken, Karcher and Rezamand8). In addition, the study of Gobert et al. (Reference Gobert, Martin and Ferlay3) has shown that the association of plant polyphenols and vitamin E reduces plasma lipoperoxidation in dairy cows supplemented with a PUFA-rich diet. Antioxidants then could contribute to enhance defence mechanisms against oxidative stress with various immunity, reproductive and health benefits.
Plant lignans are natural strong antioxidants and flax (Linum usitatissimum) is known as the richest dietary source of lignans(Reference Prasad9, Reference Milder, Arts and Van De Putte10). Flax lignans are metabolised by the rumen microbiota in mammalian lignans, which are transferred in the blood, milk and urine of dairy cows(Reference Gagnon, Côrtes and Da Silva11). Prasad(Reference Prasad12) reported that millimolar concentrations of the plant lignan secoisolariciresinol diglucoside and its mammalian metabolites enterodiol and enterolactone inhibit reactive oxygen species following an in vitro incubation of venous blood. This is of particular interest as many chronic diseases are characterised by an oxidative stress component(Reference Pool-Zobel, Adlercreutz and Glei13). More recently, Côrtes et al. (Reference Côrtes, Palin and Gagnon14) have shown that flax hulls increase SOD activity and SOD1 mRNA abundance in the mammary tissue of dairy cows. These findings corroborate those of Rajesha et al. (Reference Rajesha, Murthy and Kumar15) who reported that flax antioxidants enhance rats' endogenous defence by up-regulating the expression of genes encoding for antioxidant enzymes such as SOD, CAT and GPx. As flax meal (FM) contains less than 5 % of residual oil(Reference Newkirk16), it is richer in plant lignans than in flaxseed on a DM basis, which makes it a better source of antioxidants. To our knowledge, there is no information available on the effect of FM on the activity and expression of antioxidant enzymes in dairy cows. We hypothesised that dietary FM modulates oxidative stress indicators in physiological fluids (e.g. blood, ruminal fluid and milk) and enhances those of oxidative status. We also postulated that FM contributes to extending the effect of treatment post-feeding. Therefore, the present study investigated the effects of increased concentrations of FM on the activity of SOD, CAT and GPx enzymes, 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging activity and lipid peroxidation (thiobarbituric acid-reactive substance (TBARS) production) in the blood, milk, mammary tissue and ruminal fluid of dairy cows, and the mRNA abundance of oxidative stress-related genes in mammary tissue.
Materials and methods
Animals and diets
A total of eight multiparous Holstein cows fitted with a ruminal cannula (10 cm; Bar Diamond, Inc.) were assigned to four treatments in a double 4 × 4 Latin square design with four diets and four 21 d periods balanced for residual effects. Cows averaged 686 (se 35) kg of body weight and 112 (se 21) d in milk at the start of the experiment. The cows were housed in individual stalls and had free access to water. The diets were offered in equal amounts twice daily at 08.30 and 15.30 hours for ad libitum intake (10 % refusals on an as-fed basis) and they were milked twice daily at 08.00 and 19.00 hours. The diets were isonitrogenous and isoenergetic, and they were formulated to meet requirements for cows having 657 kg of body weight and producing 37·7 kg milk with 3·8 % fat/d(17). The national guidelines for the care and use of animals were followed as recommended by the Canadian Council on Animal Care(18). The four treatments (Table 1) were control with no FM (CON) or a pre-planned inclusion of 5 % (5FM), 10 % (10FM) and 15 % (15FM) FM in DM. The FM used in the present study was prepared using the expeller meal method (i.e. mechanical extraction of meal), which leaves about 5 % of residual oil(Reference Newkirk16).
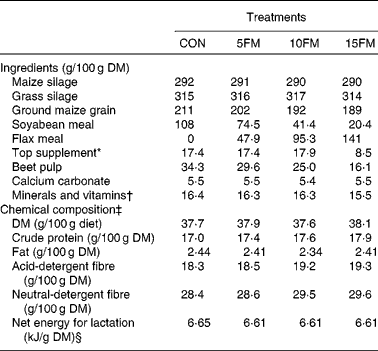
Table 1 Ingredient and nutrient composition of the total mixed diets of Holstein cows fed no flax meal (CON) or 5 % flax meal (5FM), 10 % FM (10FM) and 15 % FM (15FM)

* Contained 20 % of rapeseed meal, 30 % of maize gluten meal, 20 % of soyabean meal and 30 % of brewer's maize.
† The premix contained (per kg of premix): Ca 92 g; P 47·9 g; Mg 47·8 g; S 15·2 g; Na 137·2 g; K 13·7 g; Se 19·5 mg; I 23 mg; Fe 2013 mg; Cu 1068 mg; Mn 1796 mg; Zn 2657 mg; Co 57 mg; F 265 mg; vitamin A 442 000 IU (463·1 μmol/l); vitamin D3 56 670 IU (3 536 208 nmol/l); vitamin E 2630 IU (40 986 μmol/l).
‡ Mean of four samples that were prepared by compositing three samples collected once per week and pooled within period.
§ Calculated using the published values of feed ingredients(17).
Experimental procedures
Feed intake and milk yield were measured daily throughout the experiment, and data were averaged over the 7 d of the 4th week of each period and subjected to ANOVA. Samples of the diet were taken once weekly and pooled within period. All samples were frozen at − 20°C for subsequent drying at 55°C and further analysis. On day 21, milk samples were taken from two consecutive milkings, sampled in proportion to milk yield and pooled together. Of the two samples, one was stored at 4°C with a preservative (bronopol-B2; DNF Company) until analysed for fat, lactose, protein and urea N. Another sample was taken and 0·02 % (w/w) of sodium azide were added and kept frozen at − 80°C for further analysis of TBARS and DPPH.
On day 20 of each period, blood samples (60 ml) were collected immediately before the morning meal and 3 h post-feeding from the caudal vein into vacutainer tubes (Becton Dickinson and Cie) containing K3-EDTA (0·47 mol/l)(Reference Gobert, Martin and Ferlay3). Plasma was isolated from the blood by centrifugation at 3000 g for 12 min at 4°C and stored at − 80°C to determine enzyme activities, TBARS and DPPH. The remaining erythrocytes were stored at − 80°C for subsequent analysis of enzyme activity. Biopsies of the mammary gland were taken on day 21 of each period using the method of Farr et al. (Reference Farr, Stelwagen and Cate19) and alternating between the left and right hindquarters. Although inflammation was restricted to a very small area and disappeared within 2 d, a site at least 10 cm apart from the first one was chosen when a quarter was used for the second time. Tissue obtained from the biopsies was rinsed in sterile saline solution to remove all traces of blood, and then cut into two parts: one was immediately frozen in liquid N2 and stored at − 80°C for gene expression analyses. The other half was ground immediately with a rotor–stator homogeniser and stored at − 80°C for further analysis of antioxidant enzyme activity.
On day 20, ruminal contents were collected at 0 (immediately before the distribution of the meal), 2, 4 and 6 h after the morning meal from different locations within the rumen (anterior dorsal, anterior ventral, medium ventral, posterior dorsal and posterior ventral locations) to obtain representative samples. The ruminal contents were then strained through four layers of cheesecloth, and the filtered ruminal fluid of each sampling time was stored at − 80°C for further chemical analysis. Ruminal liquid was thawed later and one portion was used to determine TBARS production. Another portion was centrifuged at 800 g for 10 min at 4°C to remove protozoa. The supernatant was taken and centrifuged at 13 700 g for 25 min at 4°C to remove the debris of bacteria (Sorvall RC-6 Plus Superspeed Centrifuge; Thermo Scientific) and was used directly to analyse the activity of antioxidant enzymes. For DPPH analysis, 75 μl of the supernatant were mixed with methanol (2·55 ml) during 30 s and centrifuged at 9809 g for 15 min, and the upper layer was used to perform DPPH analysis.
Chemical analysis
Concentrations of DM, diethyl ether extract, acid-detergent fibre, neutral-detergent fibre and total N in the diets were analysed according to the procedures described by Côrtes et al. (Reference Côrtes, Palin and Gagnon14). Fat, lactose, protein and urea N concentrations in the milk samples were analysed by IR spectrophotometry (System 4000 Milkoscan; Foss Electric of Hillerod) following procedure 972.16 of AOAC (1990). Somatic cells were counted using an optical somatic cell counter (Fossomatic 90; Foss Electric of Hillerod).
The activity of GPx (EC 1.11.1.9), SOD (EC 1.15.1.1) and CAT (EC 1.11.1.6) in plasma, erythrocytes, ruminal fluid and mammary tissue was determined enzymatically. Activities of SOD, CAT and GPx were analysed using commercial assay kits (Cayman Chemical) according to the manufacturer's instructions. Details of these analyses have been described previously(Reference Côrtes, Palin and Gagnon14). The maximum intra- and inter-assay CV for SOD and GPx analyses were 10 and 10 %, respectively, while the maximum intra- and inter-assay coefficients of CAT were 12·5 and 10·1 %. Total protein concentration in plasma, erythrocytes, ruminal fluid and mammary tissue was determined with a bicinchoninic acid protein assay (Sigma-Aldrich).
Determination of DPPH in plasma, ruminal fluid and milk was done according to the procedures of Brand-Williams et al. (Reference Brand-Williams, Cuvelier and Berset20) and Martinez et al. (Reference Martinez, Valek and Resetic21) using a stable free-radical DPPH, with some modifications. Concentration of the DPPH (Sigma-Aldrich D9132) solution was 200 μm in methanol, prepared 1 h before use, and samples were read in polypropylene ninety-six-well plates. Concentration of the DPPH solution was the same for plasma, milk and ruminal fluid. Plasma samples were prepared according to Martinez et al. (Reference Martinez, Valek and Resetic21). Milk extracts were prepared by mixing 0·75 ml milk and 3 ml methanol. The samples were then vortex mixed for 15 s at high speed and kept at − 20°C for 48 h. The milk extract was then centrifuged at 2000 g for 15 min at 4°C, and the supernatant was used to perform the DPPH analysis. For each sample, five different volumes of the milk extract (50, 100, 125, 250 and 500 μl) were added to 500 μl of the DPPH solution, and dilutions were done in duplicate. An aliquot of 250 μl of each dilution was placed in a ninety-six-well plate as well as 250 μl of the DPPH solution (control) and 250 μl methanol (blank). The reduction in DPPH was determined at 515 nm after 30 min for plasma and milk, and after 25 min for ruminal fluid. The assay was performed in triplicate. Antioxidant capacity was calculated according to the method of Li et al. (Reference Li, Hosseinian and Tsopmo22). A linear relationship was obtained between antioxidant capacity and the volume dilution of samples as described by Smet et al. (Reference Smet, Raes and De Block23), and the 50 % effective concentration (EC50) was calculated. Lipid peroxidation was assessed in plasma, ruminal fluid and milk in the original samples using a commercially available TBARS assay kit (OXI-TEK TBARS Assay Kit; Zepto Metrix Company) according to the manufacturer's instructions.
Quantitative real-time RT-PCR amplifications of the studied genes
Total RNA was extracted from the mammary tissue and complementary DNA synthesis was performed as described previously(Reference Labrecque, Beaudry and Mayhue24). Integrity of the extracted RNA was assessed by verifying the presence of 18S and 28S RNA bands after electrophoresis on a 1 % agarose gel. Purity of the extracted RNA was assessed by measuring the 260/280 absorbance ratio with a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Inc.). The average 260/280 ratio of the extracted RNA samples was 2·01 (1·97–2·04), thus confirming RNA purity of our samples. The relative mRNA abundance of the studied genes was determined using quantitative real-time PCR amplification. Quantitative PCR amplification, detection and data analyses were performed using an ABI 7500 Fast Real-time PCR System (PE Applied Biosystems). Primer pairs were designed using Primer Express software 3.0 (PE Applied Biosystems). A detailed description of primer sequences, GenBank accession numbers and amplified product size of the CAT, SOD1, SOD2, SOD3, GPx1 and GPx3 genes has been published previously(Reference Côrtes, Palin and Gagnon14). Forward 5′-GTACCCCTGGAAATGTCAAACAG-3′ and reverse 5′-TGTGATGACGACAAAGGTTGGA-3′ primers (NM_001011678, 88 bp amplicon) were used for the nuclear factor (erythroid-derived 2)-like 2 (NFE2L2) gene, and the primer pair forward 5′-CTCAAAGCAGCAGGAGCAGA-3′ and reverse 5′-CGGTACGACCCCTTCATCC-3′ (NM_001076409, 102 bp amplicon) was used for the nuclear factor of κ light polypeptide gene enhancer in B-cells (NFKB) gene. To minimise non-specific amplification, primer optimisation tests were first performed for each gene to determine the minimum primer concentration needed for having the lowest threshold cycle and the maximum change in fluorescence (normalised reporter signal; ΔRn). Quantitative PCR amplifications were carried out in a 10 μl reaction volume containing primers (final concentrations ranging from 150 to 900 nm), 5 μl of 2 × Power SYBR Green Master Mix (PE Applied Biosystems), 3 μl of 15 × diluted complementary DNA and 0·05 μl of AmpErase (PE Applied Biosystems). Cycling conditions were 2 min at 50°C, followed by 10 min at 95°C and forty cycles of 3 s at 95°C and 30 s at 60°C. Specificity of amplified fragments was determined for all genes using the melting curve (dissociation curve) analysis. Amplification of the reference genes glyceraldehyde-3-phosphate dehydrogenase (GAPDH), peptidylpropyl isomerase A (PPIA), actin β (ACTB)(Reference Côrtes, Palin and Gagnon14) and polyubiquitin (forward 5′-TGGAGCCCAGTGACACCAT-3′ and reverse 5′-GGCCATCTTCCAGCTGCTT-3′ primers, NM_174133, 111 bp amplicon) was also performed, and the NormFinder algorithm(Reference Andersen, Jensen and Orntoft25) was then used to identify the least affected by the treatments. Polyubiquitin was identified as the best reference gene for normalisation of datasets in the present study. Quantitative PCR amplifications were performed in triplicate and standard curves were established in duplicate for each gene. Standard curves were composed of serial dilutions of complementary DNA pools(Reference Labrecque, Beaudry and Mayhue24), and were used to obtain the relative mRNA abundance of the studied genes using the standard curve method as described by Applied Biosystems(26).
Statistical analysis
All data were analysed as a 4 × 4 double Latin square design using the MIXED procedure of SAS, release 9.2 (SAS 2002; SAS Institute) according to the model:
where Y ijkl is the response variable; μ is the overall mean; T i is the global effect of treatment (i= CON, 5FM, 10FM and 15FM); P j is the fixed effect of period (j= 1–4); Q k is the fixed effect of square (k= 1, 2); A/Q kl is the random effect of cow within square; e ijkl is the residual error. The treatments were compared by contrasts in order to test the polynomial effects (linear, quadratic and cubic) of FM. Data on TBARS, DPPH and enzyme activities in plasma, ruminal fluid, erythrocytes and milk were analysed as repeated measurements and the compound symmetry was used as the covariance structure. The Shapiro–Wilk test was used to check normality between the data obtained at different sampling times. When a tendency was observed for an interaction (P≤ 0·10) between treatment and time, the effect of treatment was examined within each time group, and then the treatment effects were compared at the relevant time. Similar approaches were performed by Barret et al. (Reference Barret, Dadds and Rapee27). Differences were declared significant when P≤ 0·05, and a trend when 0·05 < P≤ 0·10.
Results
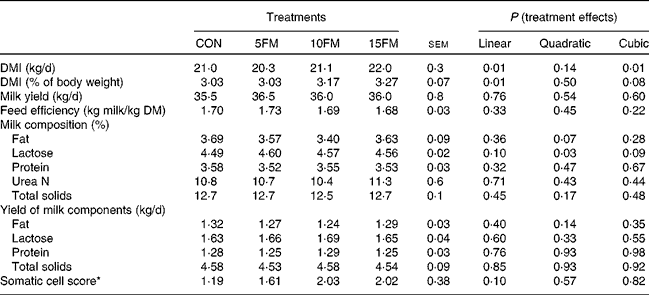
There was a linear effect of treatment on DM intake as a result of higher intake with an increased level of FM in the diet (Table 2). The concentration of FM in the diet had no effect on milk production, the composition and yield of milk components and feed efficiency (Table 2). The only exception was the proportion of lactose in milk that showed linear (P= 0·10), quadratic (P= 0·03) and cubic (P= 0·09) effects with an increasing level of FM in the diet.
Table 2 DM intake (DMI), milk yield and milk composition of Holstein cows fed no flax meal (CON) or 5 % flax meal (5FM), 10 % FM (10FM) and 15 % FM (15FM)

* Log10 (somatic cell count).
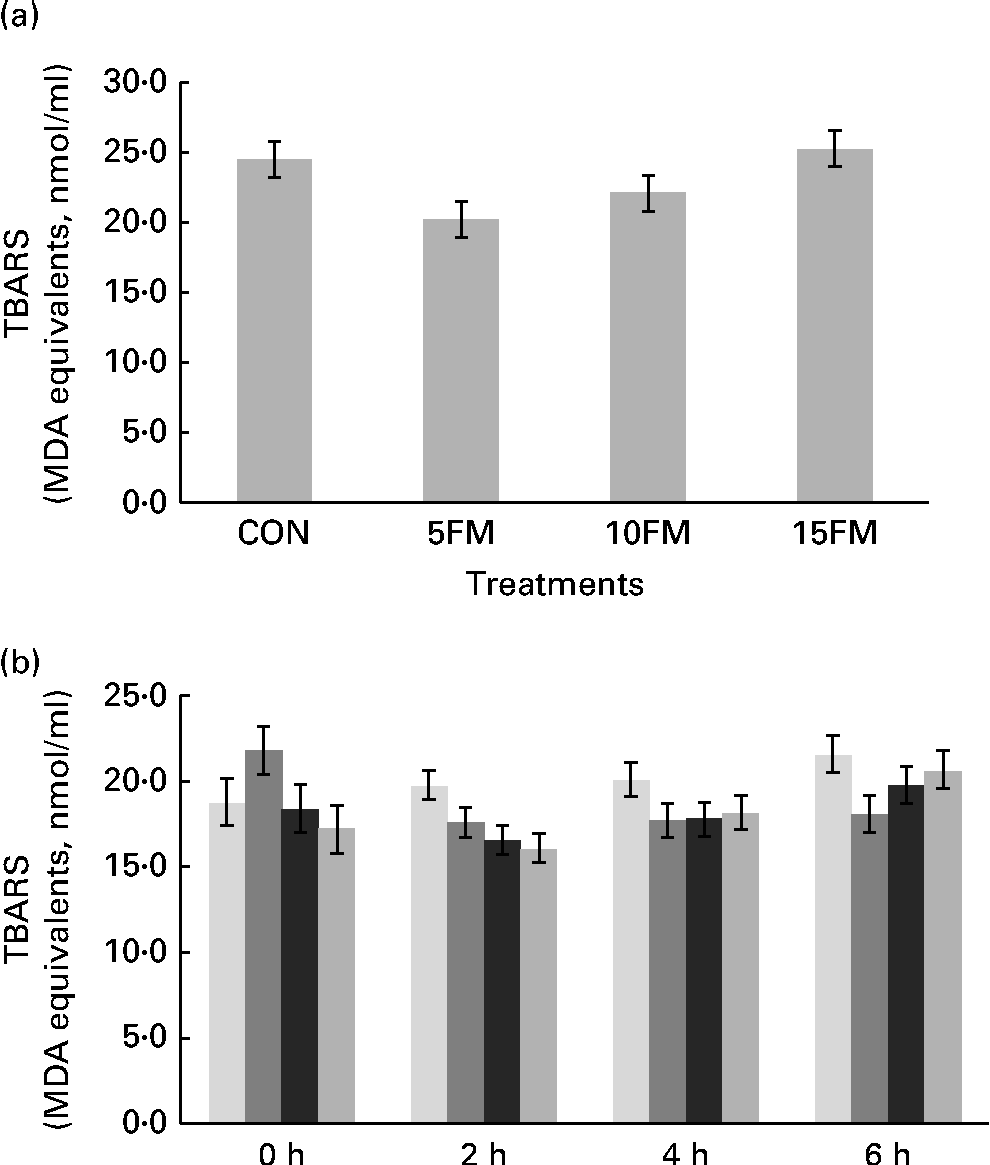
The production of TBARS, expressed in terms of malondialdehyde equivalents (nmol/ml), was lower in the milk of cows fed the 5FM and 10FM diets than in the milk of those fed the CON and 15FM diets, as shown by quadratic (P= 0·009) and cubic (P= 0·006) effects of treatment (Fig. 1(a)).

Fig. 1 Thiobarbituric acid-reactive substance (TBARS) production (malondialdehyde (MDA) equivalents) in (a) the milk and (b) ruminal fluid of Holstein cows fed no flax meal (CON, ![]() ), or 5 % flax meal (5FM,
), or 5 % flax meal (5FM, ![]() ), 10 % FM (10FM,
), 10 % FM (10FM, ![]() ) and 15 % FM (15FM,
) and 15 % FM (15FM, ![]() ) in the diet. Values are means, with their standard errors represented by vertical bars. Standard errors were 1·28 for milk. Standard errors were 1·39, 0·85, 0·99 and 1·10 for 0, 2, 4 and 6 h after feeding, respectively, for ruminal liquid. Production of TBARS was lower in the milk of cows fed 5FM and 10FM than in the milk of those fed the CON and 15FM diets as a result of quadratic (P= 0·009) and cubic (P= 0·006) effects of treatment. There was an interaction (P= 0·01) between time and treatment for TBARS production in ruminal fluid.
) in the diet. Values are means, with their standard errors represented by vertical bars. Standard errors were 1·28 for milk. Standard errors were 1·39, 0·85, 0·99 and 1·10 for 0, 2, 4 and 6 h after feeding, respectively, for ruminal liquid. Production of TBARS was lower in the milk of cows fed 5FM and 10FM than in the milk of those fed the CON and 15FM diets as a result of quadratic (P= 0·009) and cubic (P= 0·006) effects of treatment. There was an interaction (P= 0·01) between time and treatment for TBARS production in ruminal fluid.
FM supplementation had no effect on TBARS production in plasma (P= 0·43), with average values of 3·60, 4·05, 3·90 and 4·05 (se 0·28) nmol/ml for the CON, 5FM, 10FM and 15FM diets, respectively. However, the concentration of TBARS in plasma was reduced (P= 0·04) at 3 h after feeding, regardless of FM supplementation, with mean values of 4·1 and 3·7 nmol/ml at 0 and 3 h, respectively.
There was an interaction (P= 0·01) between time and treatment for TBARS measurement in ruminal fluid. When cows were supplemented with FM, there was a linear (P= 0·01) reduction in TBARS at 2 h after feeding, and there were no treatment effects at 0, 4 and 6 h after feeding (Fig. 1(b)).
Radical-scavenging activities determined in milk, plasma and ruminal fluid by the DPPH assay were not affected by FM supplementation. The values of EC50 were similar among the diets and averaged 26·70, 46·50 and 8·67 μl/ml, respectively, in milk, plasma and ruminal fluid. Regardless of the treatment, there was a time effect on DPPH in ruminal fluid (P< 0·001). The mean values of EC50 in ruminal fluid at 0, 2, 4 and 6 h after feeding were 11·1, 10·8, 10·6 and 10·8 μl/ml, respectively.
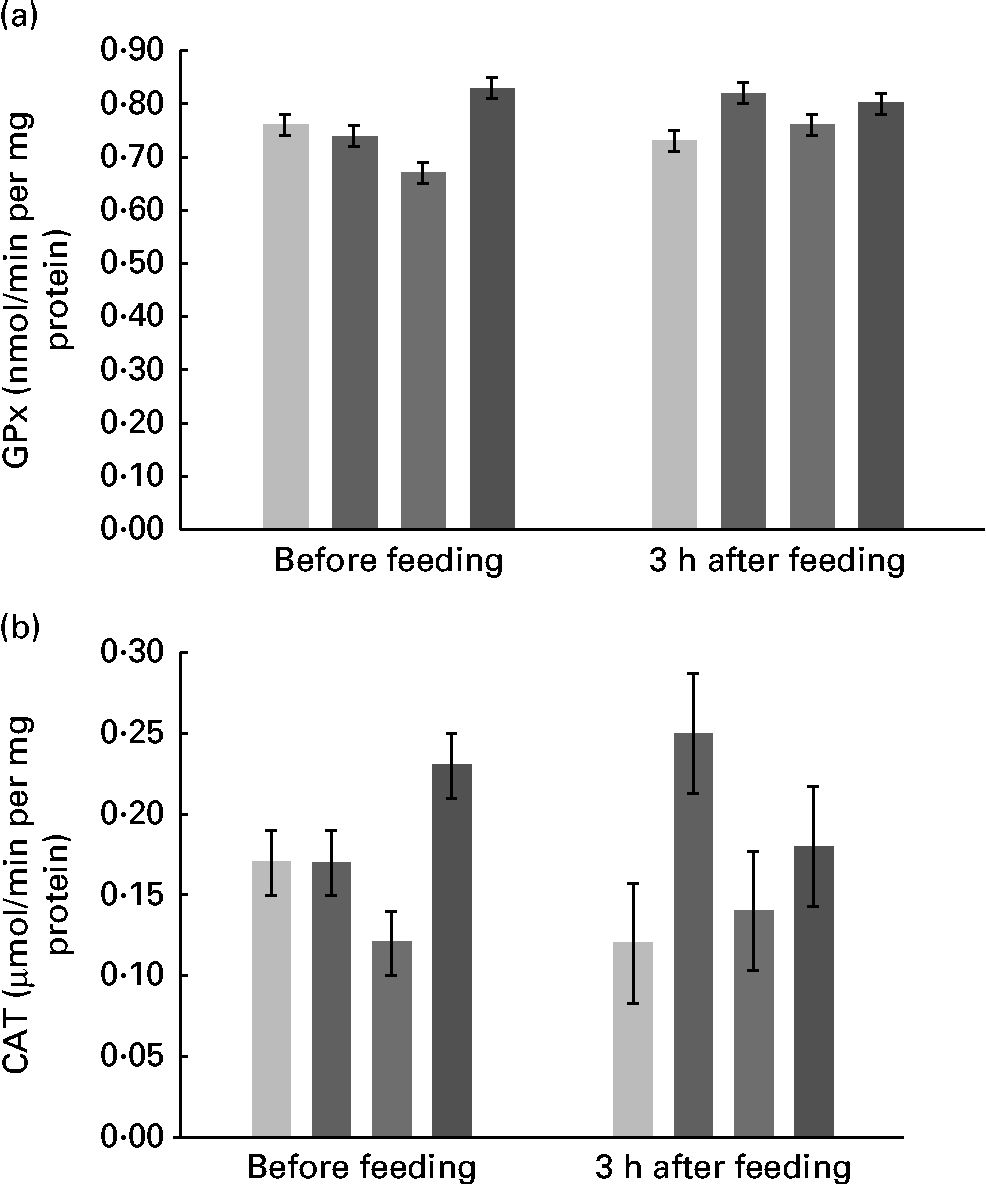
Supplementation with FM had no effect on the activity of SOD in plasma, erythrocytes, ruminal fluid and mammary tissue, with values that averaged, respectively, 0·13, 16·33, 11·58 and 33·71 μmol/min per mg protein. The values of GPx activity were similar among the diets and averaged 33·68 and 1·18 nmol/min per mg protein in mammary tissue and erythrocytes, respectively. However, there was an interaction (P= 0·03) between treatment and time (Fig. 2(a)) for plasma GPx activity. There were quadratic (P= 0·002) and cubic (P= 0·007) effects of FM supplementation at 0 h and contrasts were not significant at 3 h after feeding.

Fig. 2 Activity of (a) glutathione peroxidase (GPx) and (b) catalase (CAT) in the plasma of Holstein cows fed no flax meal (CON, ![]() ) or 5 % flax meal (5FM,
) or 5 % flax meal (5FM, ![]() ), 10 % FM (10FM,
), 10 % FM (10FM, ![]() ) and 15 % FM (15FM,
) and 15 % FM (15FM, ![]() ) in the diet. Values are means, with their standard errors represented by vertical bars. There was an interaction between treatment and time for plasma GPx and CAT activities (P= 0·03 and 0·04, respectively).
) in the diet. Values are means, with their standard errors represented by vertical bars. There was an interaction between treatment and time for plasma GPx and CAT activities (P= 0·03 and 0·04, respectively).
The activity of CAT was not altered by the treatments in mammary tissue and erythrocytes, and values averaged 47·9 and 207·9 μmol/min per mg, respectively. There was an interaction (P= 0·04) between treatment and time for CAT activity in plasma (Fig. 2(b)). Immediately before feeding, there were quadratic (P= 0·01) and cubic (P= 0·01) effects of FM supplementation, but no effect of FM supplementation (P>0·10) at 3 h post-feeding.
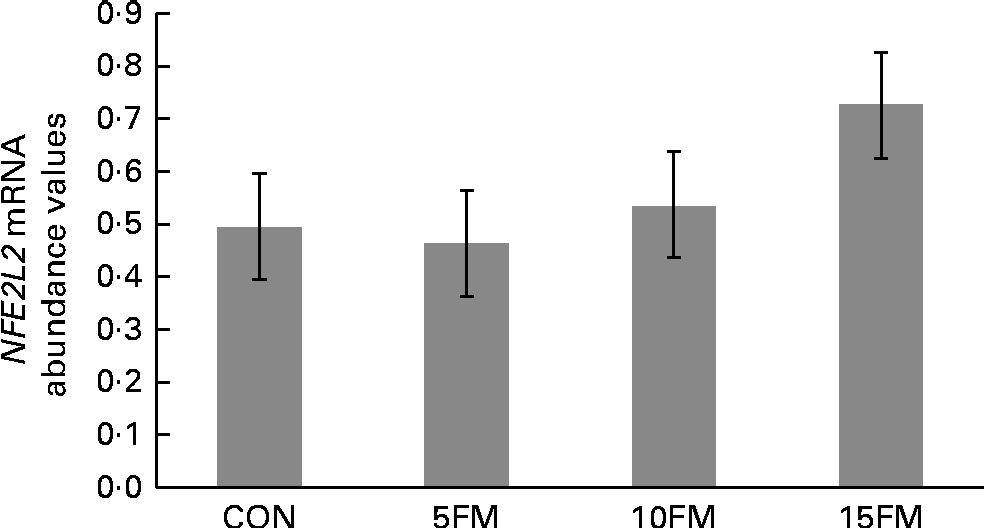
There was a trend for an overall treatment effect (P= 0·10) for NFE2L2 mRNA abundance in mammary tissue, with a linear (P= 0·03) increase with FM supplementation (Fig. 3). A linear tendency was also observed for the CAT gene, with increasing mRNA abundance values observed with higher concentrations of FM (P= 0·09; data not shown). The mRNA abundance of the CAT, GPx1, GPx3, SOD1, SOD2, SOD3 and NFKB genes was not affected by the treatment.

Fig. 3 Relative mRNA abundance of the nuclear factor (erythroid-derived 2)-like 2 (NFE2L2) gene in the mammary tissue of Holstein cows fed no flax meal (CON) or 5 % flax meal (5FM), 10 % FM (10FM) and 15 % FM (15FM) in the diet. Values are least-squares means of seven animals from the analysis performed in triplicate, with their standard errors represented by vertical bars. There was a tendency for an overall effect of FM on NFE2L2 mRNA abundance (P= 0·10; linear effect of treatment at P= 0·03).
Discussion
The oxidative status of dairy cows was monitored in the present study by the production of TBARS, which represents the peroxidation of lipids. Malondialdehyde production quantified through the TBARS assay is one of the biomarkers of damage caused in the body by reactive oxygen and nitrogen substances(Reference Vasconcelos, Goulart and Moura28). FM-supplemented cows presented a linear reduction in the TBARS of ruminal fluid 2 h post-feeding, but the treatments had no effect after 2 h of feeding. According to Sadeghian & Kojouri(Reference Sadeghian and Kojouri29), a greater period of time required to reach basal levels of malondialdehyde depends upon antioxidant supplementation and could indicate enhanced antioxidant activity. Therefore, this could suggest that within the first few hours (i.e. < 4 h) of feed consumption, antioxidants present in FM may have contributed to the protection of dietary lipids against oxidation in the rumen as suggested by the linear decrease in TBARS with the inclusion of FM in the diet. Indeed, FM supplementation increases concentrations of the mammalian lignan enterolactone in the rumen, milk and urine(Reference Gagnon, Côrtes and Da Silva11), and previous results have shown that antioxidant activity of mammalian lignans is greater than that of vitamin E(Reference Prasad12).
The production of TBARS was lower in the milk of cows fed the 5FM and 10FM diets than in the milk of those fed the CON and 15FM diets, and there was no difference among the treatments for TBARS production in plasma. However, all diets resulted in lower plasma TBARS production after feeding, which can be associated with the consumption of certain feed ingredients. Indeed, many feedstuffs contain glutathione, tocopherols, ascorbate, uric acid and β-carotene, which may improve plasma non-enzymatic antioxidant indicators(Reference Vasconcelos, Goulart and Moura28). Moreover, the greatest proportion of vitamins in the diet of cows fed 15FM probably increased the consumption of vitamin E, which is a strong antioxidant and may have influenced TBARS production.
Radical-scavenging activity was assessed by the DPPH assay, which measures modifications in antioxidant activity(Reference Brand-Williams, Cuvelier and Berset20). Supplementation with FM had no effect on DPPH determined in milk, plasma and ruminal fluid, although there was a time effect in ruminal fluid. According to the method used in the present study and described previously by Smet et al. (Reference Smet, Raes and De Block23), the lower the EC50 value, the higher the content of antioxidants. To our knowledge, only two studies have previously observed an increase in the antioxidant activity of ruminal fluid measured by the DPPH method 2 h after feeding. The first one is that of Gizi et al. (Reference Gizi, Papassotiriou and Apostolakou30) who compared faunated v. defaunated cattle (protozoa-free) and the second one is that of Aoki et al. (Reference Aoki, Furukawa and Sato31) who supplemented cows with trehalose and cellobiose, two disaccharides with and without antioxidant activity, respectively. According to Gizi et al. (Reference Gizi, Papassotiriou and Apostolakou30), the level of antioxidants in ruminal fluid increases due to the supply of lipids or fat-soluble compounds that contain carotenoids and vitamins, and/or to antioxidants obtained from the metabolism of ruminal micro-organisms. In the present study, a similar increase in antioxidant activity was observed in ruminal fluid after feeding, although this occurred regardless of the treatment, thus corroborating the results of Gizi et al. (Reference Gizi, Papassotiriou and Apostolakou30) that distinct nutrients and the microbial production of antioxidants may be responsible for increased antioxidant activity in ruminal fluid.
SOD is considered the first intracellular defence against reactive oxygen molecules(Reference Adela, Zinveliu and Pop32). This enzyme causes a dismutation of the superoxide anion radical to H2O2(Reference Vasconcelos, Goulart and Moura28), which is further degraded by CAT and peroxidase actions. In the present study, the addition of FM had no effect on the activity of SOD. These results then may suggest that the same amount of H2O2 was produced in the body fluids and tissues of animals fed or not FM. According to Hosada et al. (Reference Hosada, Miyaji and Matsuyama33), a lack of alteration in antioxidant enzymes could be due to a sufficient pool of non-enzymatic antioxidant substances probably present in the defence system of ruminants. The activity of SOD in the plasma of dairy cows has been shown to increase with antioxidant supplementation, but the effect was dependent on the type of fat fed, with the highest activity being observed when oxidised fat was added to the diet(Reference Vázquez-Añón, Nocek and Bowman5). This is corroborated with the fact that supplementation of non-oxidised lipids in the form of flax oil infusion in the abomasum has no effect on SOD activity in the plasma, erythrocytes and mammary tissue of dairy cows(Reference Côrtes, Palin and Gagnon14).
The interaction between treatment and time for plasma GPx activity before feeding was probably due to lower activity when cows were fed the 10FM diet and higher activity for those fed the 15FM diet. The higher values of GPx activity in plasma maintained with the 15FM diet may suggest that a proportion of 15FM in the diet may provide enough antioxidants to improve the oxidative status of cows. An increase in the plasma GPx activity of dairy cows has also been observed by Vázquez-Añón et al. (Reference Vázquez-Añón, Nocek and Bowman5) who fed diets containing antioxidants and oxidised or non-oxidised soyabean oil. Wullepit et al. (Reference Wullepit, Hostens and Ginnenberge34) fed marine algae (source of n-3 fatty acids) to cows, and they have attributed the increase in plasma GPx activity to the PUFA supply from algae and its induction of lipoperoxidation. On the other hand, Côrtes et al. (Reference Côrtes, Palin and Gagnon14) observed a decrease in plasma GPx activity when they fed n-3 PUFA to lactating dairy cows. Taken altogether, these results have suggested that the ingestion of antioxidant compounds may act directly on the GPx enzyme system, where they can protect against plasma lipoperoxidation even when PUFA are supplied in the diet.
Similar responses of treatment over time were observed for both plasma GPx and CAT activities, which are enzymes degrading H2O2. According to Celi(Reference Celi35), when SOD activity increases the production of H2O2, a protection from reactive oxygen substances would only be provided by a simultaneous increase in CAT and GPx activities and the availability of glutathione.
Supplementation with FM had no effect on SOD, CAT and GPx activities in mammary tissue and erythrocytes. Although erythrocytes are one rich source of antioxidant enzymes, they are also a significant source of superoxide generation in biological systems(Reference Vasconcelos, Goulart and Moura28). As the production of superoxide ions and oxygen peroxides occurs under oxidative stress(Reference Gizi, Papassotiriou and Apostolakou30), this may suggest that the lack of a treatment effect on enzyme activities was a result of the cows that were in good health, mid-lactation and positive energy balance. Changes in the activity of erythrocyte antioxidant enzymes in dairy cattle have been reported only when animals were in the transition period, which may result from cows that are under oxidative stress(Reference Bernabucci, Ronchi and Lacetera1).
In the present study, FM supplementation increased mammary NFE2L2 mRNA abundance, and had no effect on the mRNA abundance of the GPx1, GPx3, SOD1, SOD2, SOD3 and NFKB genes. The NFE2L2 gene, also known as nuclear factor-E2-related factor 2 (Nrf2), encodes for a transcription factor that plays essential roles in cellular defence against oxidative stress(Reference Nguyen, Nioi and Pickett36). This transcription factor activates the expression of a series of genes having an antioxidant response element in their promoters, including antioxidant proteins and phase 2 detoxifying enzymes(Reference Kang, Lee and Kim37, Reference Zhu, Itoh and Yamamoto38). Of interest, flax lignans are composed of polyphenolic compounds present in plants as glycoside conjugates(Reference Hu, Yuan and Kitts39), and many natural polyphenol-containing compounds have the ability to modulate Nrf2-mediated cellular events(Reference Na and Surh40, Reference Martin, Ramos and Granado-Serrano41). The reason for the lack of the overall treatment effect of FM on the GPx1, GPx3, CAT, SOD1, SOD2 and SOD3 genes remains to be determined since SOD1, CAT and GPx are all considered as established Nrf2-regulated genes(Reference Chen and Kunch42). In a previous study, we have reported that cows fed 9·88 % flax hulls, a rich source of the plant lignan secoisolariciresinol diglucoside, have higher levels of CAT, GPx1 and SOD1 mRNA in mammary tissue and lower mRNA abundance of GPx3, SOD2 and SOD3 than cows fed no flax hulls(Reference Côrtes, Palin and Gagnon14). These discrepancies may be explained by the higher oil content found in flax hulls (30 % of diethyl ether extract in DM) compared with FM ( < 5 % of residual oil content in DM)(Reference Newkirk16). Since we have shown that flax oil modulates antioxidant enzyme mRNA abundance in the cow's mammary tissue, differences in the lipid content between flax hulls and FM may indeed account for some of the observed discrepancies between our data and those of Côrtes et al. (Reference Côrtes, Palin and Gagnon14).
In conclusion, the present study shows that FM supplementation can improve the oxidative status of Holstein cows in mid to late lactation, as suggested by decreased TBARS production in ruminal fluid 2 h post-feeding and increased NFE2L2/Nrf2 mRNA abundance in mammary tissue. Further studies are required to clarify the role of FM on the oxidative status of cows during the transition and early lactation periods as cows are more prone to oxidative stress during these periods. Any beneficial effects of FM on the oxidative status of cows could lead to a prophylactic strategy against diseases affecting the health status of dairy cattle.
Acknowledgements
A. L. B. S. was recipient of a studentship from the National Council of Technological and Scientific Development (CNPq), Brazil. The authors express their gratitude to the staff of the Agriculture and Agri-Food Canada for their contribution to the present study, especially L. Veilleux, D. Beaudry and N. Gagnon for technical assistance and S. Méthot for his help in statistical analysis. The present study was sponsored by the Agriculture and Agri-Food Canada. The authors' contributions were as follows: A. L. B. S., M.-F. P. and H. V. P. drafted the manuscript; H. V. P. and M.-F. P. conceived and directed the study; A. L. B. S. coordinated the study, was in charge of collecting the animal data and performed the laboratory work on enzyme and antioxidant activities; G. T. d. S. participated in the design of the study; C. B. contributed to the conception and design of the experiment; P. L. performed the mammary biopsies. All authors critically revised the paper and approved the final version of the manuscript. None of the authors had a personal or professional conflict of interest.