Glutathione S-transferases (GST), a superfamily of phase II xenobiotic-metabolising enzymes, play a key role in cellular anti-mutagen and antioxidant defence mechanisms. Seven classes of cytosolic GST are recognised in mammalian species, designated α, μ, π, σ, ζ, ω and θ. At least sixteen cytosolic GST subunits exist in humans(Reference Hayes and Strange1–Reference Brind, Hurlstone and Edrisinghe3). Individuals with very low levels of GST activity are at an increasing risk of lung, breast, oral and oesophageal squamous cell carcinoma(Reference Katoh, Kaneko and Takasawa4–Reference Casson, Zheng and Geoffrey7). Interindividual variation in the activity of GST is considered due to both environmental (e.g. diet and exposure to toxins) and genetic factors. Individual GST genes do not make a major contribution to susceptibility to diseases. Therefore, the combinations of polymorphism in different classes of GST with environmental components were suggested to contribute to increasing risk of disease.
GSTM1 and GSTT1 genes are polymorphic in humans and the null genotypes lead to the absence of enzyme function, contributing to interindividual differences in response to xenobiotics(Reference Binkova, Chvatalova and Lnenickova8). Recent epidemiological studies proposed that GSTM1 null and GSTT1 null genotypes were correlated with an increased susceptibility to diseases associated with oxidative stress, such as cancer, cardiovascular and respiratory diseases(Reference Masetti, Botto and Manfredi9–Reference Girisha, Gilmour and Mastana11).
The relationship between fruit and vegetable (F&V) intake and body antioxidant capacity has been extensively studied. The anti-oxidative vitamins (vitamin C, β-carotene) and phytochemicals such as flavonoids existing in F&V were suggested to be the functional components for the prevention of oxidative stress-related diseases(Reference Strain, Elwood and Davis12). The anticancer bioactivity of a F&V-rich diet could also be through induction of phase II metabolic enzymes; for example, consumption of Brasica vegetables and citrus fruits could induce detoxification enzymes GST(Reference Park and Pezzuto13). The induction process involved activation of certain signal transduction pathways by F&V components acting through transcription factor binding sites, which are present in the promoters of GST(Reference Chen and Kong14).
Moreover, significant interindividual variation for the body antioxidant capacity and the risk of diseases after consuming antioxidant supplements was reported, even though the subjects were in an almost similar physical condition and exposed to the same environmental components(Reference Omenn, Goodman and Thornquist15). These findings indicated that genetic susceptibility to the antioxidants (or antioxidant-rich diet) may play an important role in the different interindividuals' reactions to antioxidants or antioxidant-rich diet. In the light of the involvement of GST enzymes in antioxidant defence and metabolism of plant diet-derived anti-oxidative phytochemicals, polymorphisms in GST genes might be important for nutritional strategies aiming to up-regulate the antioxidant capacity of the body.
In the present study, a human observational study was carried out to explore the relationship of GSTM1/T1 genetic variation with recent F&V consumption and body antioxidant capacity in 190 healthy Chinese adults.
Materials and methods
Study population
Blood samples were collected from healthy Chinese volunteers (n 190, 67 males and 123 females; age range 18–23; 21·13 (sd 1·34) years). The demographics of the study population are shown in Table 1. All subjects received a general clinical and biochemical assessment before entering this study. Only those subjects with normal heart, liver and renal functions were recruited to the study. All volunteers were from Capital Medical University, Beijing, China. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethical Committee of Capital Medical University; and written informed consent was obtained from all participants.
Table 1 Profile of the study population
(Number of subjects, mean values and standard deviations)

Dietary survey
A 3-d 24-h-recall food survey was carried out with the volunteers. All participants went through face-to-face surveys by trained interviewers. A food questionnaire combined with portion amounts was used to assess the intake of F&V.
DNA isolation and genotyping study
Peripheral blood samples (6 ml venepuncture) were collected in heparinised vacutainers and stored at − 80°C. DNA was extracted from frozen peripheral heparinised blood using the Wizare genomic DNA purification kit (Promega, Madison, WI, USA). GSTT1 and GSTM1 genotypes were determined by a multiple PCR method using primers and reaction profiles as described by Zhong et al. (Reference Zhong, Wyllie and Barnes16, Reference Zhong, Zhou and Chen17), respectively. The primers for the analysed systems are: GSTM1 primers: 5′-GAA CTC CCT GAA AAG CTA AAG C-3′; 5′-GTT GGG CTC AAA TAT ACG GTG G-3′; GSTT1: 5′-TTC CTT ACT GGT CCT CAC ATC TC-3′; 5′-TCA CCG GAT CAT GGC CAG CA-3′; β-globin: 5′-GAA GAG CCA AGG ACA GGT AC-3′; 5′-CAA CTT CAT CCA CGT TCA CC-3′.
PCR were carried out in a total volume of 25 μl containing 10 ng of DNA (template), with a final concentration of 1 × reaction buffer, 1·5 mm of MgCl2, 5 % of dimethyl sulfoxide, 250 μm of deoxyribonucleoside triphosphate, 0·5 μm of each primer and 1 U of Taq DNA polymerase (Takara Biotechnology, Otsu Shiga, Japan). GST PCR products were separated by electrophoresis on a 2 % agarose gel and visualised by ethidium bromide staining. The expected sizes for GSTM1, GSTT1 and β-globin (used as an internal control) amplified products were 230, 480 and 111 bp, respectively.
GSTM1 and GSTT1 null genotypes were determined by identifying the negative band for each size (with the simultaneous presence of the positive control), while positive bands meant that the sample was homozygous or heterozygous for the indicated alleles. The genotype with homozygous deletion of the GST genes is called ‘GST−’, whereas the genotype having at least one copy of the gene is called ‘GST+’.
Plasma total antioxidant capacity assay
Plasma total antioxidant capacity (T-AOC) content was measured using commercial assay kits (Jiancheng Institute, Nanjing, China) according to the manufacturer's instructions. Briefly, Fe3+ can be restored to Fe2+, and the latter with the Philippine-solid substances to form complexes by colorimetric assay can measure T-AOC activity. Each sample was analysed in three replicates and the T-AOC level was expressed as units per ml of plasma.
Plasma vitamin C concentration determination
Plasma total vitamin C concentrations were measured spectrophotometrically after protein precipitation according to the method described by Jacques-Silva et al. (Reference Jacques-Silva, Nogueira and Broch18). Briefly, plasma was precipitated with 10 % TCA solution and centrifuged at 1000 g. The supernatant (300 μl) was treated with 2,4-dinitrophenylhydrazine (4·5 mg/ml), CuSO4 (0·075 mg/ml) and TCA 13·3 %, and incubated for 3 h at 37°C. Then 65 % (v/v) H2SO4 was added to the medium. Each sample was analysed in three replicates. The content of vitamin C was calculated using a standard curve and expressed as μg/l of plasma.
Determination of erythrocyte glutathione S-transferase activity
Erythrocyte total GST enzyme activity was measured spectrophotometrically using 1-chloro-2,4-dinitrobenzene (Sigma Aldrich, St Louis, IL, USA) as the substrate according to the method of Habig & Jakoby(Reference Habig and Jakoby19). Briefly, blood samples were collected into heparinised tubes and centrifuged at 4000 g for 10 min at 4°C to isolate erythrocytes within 2 h after sampling. The resultant erythrocytes were washed three times with an isotonic NaCl solution (1:5, v/v) and the Hb was determined. Erythrocytes were lysed with cold distilled water (1:4, v/v) and centrifuged at 14 000 g for 10 min at 4°C. The haemolysates were frozen at − 80°C until analysis. At least three independent measurements were performed for each sample and the acceptable CV had to be under 5 %. One unit of GST activity represents the formation of 1 μmol of 1-chloro-2,4-dinitrobenzene-GSH/min of incubation. GST activity was normalised per μg of Hb.
Statistical methods
Statistical package SAS (version 8.0; SAS Institute, Inc., Cary, NC, USA) was used for the data analysis. A general linear model was used to compare the mean values of plasma T-AOC, vitamin C level and erythrocyte GST activity for the different GSTM1/T1 genotypes and recent F&V consumption. This statistical method has been used widely to analyse the effects of gene polymorphism and environmental exposure on serum activity(Reference Min, Park and Park20, Reference Agachan, Yilmaz and Ergen21). Some potential confounding factors including age, sex, BMI and recent antioxidant supplementation that may interfere with these relationships were adjusted. The data are expressed as the adjusted mean and 95 % CI. We did not include tobacco smoking into the adjusted factors just because there was only one male smoker in the study population.
In the present study, the average consumption of F&V ranged from 6·67 to 1553·33 g/d. In order to categorise the subjects into low and high F&V consumption groups, the 25th and 75th percentiles of average F&V consuming amount were used. The 25th and 75th percentiles of F&V consumption were 119·0 and 384·2 g/d, respectively. The P values less than 0·05 were considered as statistically significant.
Results
Table 2 gives a comparison of plasma T-AOC, vitamin C level and the erythrocyte GST activity for different GSTM1 and GSTT1 genotypes. The polymorphism in GSTM1/T1 did not affect plasma T-AOC and vitamin C level significantly (P>0·05). GSTM1+ subjects had higher erythrocyte GST activity. Deletion of GSTM1 gene reduced GST activity when compared with the GSTM1+ genotype group (P < 0·001). The reduction of GST activity from GSTT1+ to GSTT1− was not statistically significant (P>0·05). The effects of combining genetic polymorphism of GSTM1/T1 on antioxidant parameters are presented in Table 3. No difference of plasma T-AOC and vitamin C was found among the different GSTM1/T1 genotypes (P>0·05). The GSTM1−/GSTT1+ subjects had the lowest erythrocyte GST activity compared with those in the other genotype groups (P < 0·001).
Table 2 Plasma total antioxidant capacity (T-AOC), vitamin C level and erythrocyte glutathione S-transferase (GST) activity by GSTM1 or GSTT1 genetic polymorphism*
(Number of subjects, mean values and 95 % confidence intervals)
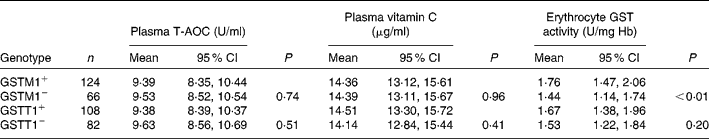
* Adjusted for age, sex and antioxidant supplement (general linear model used).
Table 3 Plasma total antioxidant capacity (T-AOC), vitamin C level and erythrocyte glutathione S-transferase (GST) activity by GSTM1/GSTT1 combining genetic polymorphism*
(Number of subjects, mean values and 95 % confidence intervals)

* Adjusted for age, sex and antioxidant supplement (general linear model used).
Table 4 indicates the association of antioxidant parameters with recent F&V consumption and GSTM1/T1 genetic polymorphisms. Plasma T-AOC in GSTM1− and GSTT1+ subjects increased with the increasing of F&V consumption (P GSTM1 − = 0·0216, P GSTT1+ = 0·0352). A similar pattern was evident for erythrocyte GST activity in GSTM1− and GSTT1+ genotypes, but the difference was not statistically significant for GSTT1+ genotypes (P GSTM1 − = 0·0009, P GSTT1+ = 0·4256). Moreover, the GSTM1− subjects had the lowest erythrocyte GST activity when compared with other GSTM1/T1 genotypes in the low F&V consumption group (P < 0·0001). No association was found among F&V consumption and GSTM1/T1 genotype and plasma vitamin C level (P>0·05). As shown in Table 5, plasma T-AOC, vitamin C level and erythrocyte GST activity tended to increase with the increasing of F&V consumption in GSTM1−/GSTT1+ or GSTM1−/GSTT1− individuals, but the difference was not statistically significant except for the GST activity in the subjects with the GSTM1−/GSTT1+ genotype (P = 0·0127). Either in the low or in the high F&V consumption group, the GSTM1−/GSTT1+ subjects had the lowest erythrocyte GST activity when compared with the other GSTM1/T1 genotype groups (P low F&V < 0·0001, P high F&V = 0·0338). No association was found between F&V consumption and antioxidant parameters in GSTM1+/GSTT1+ or GSTM1+/GSTT1− individuals.
Table 4 Plasma total antioxidant capacity (T-AOC), vitamin C level and erythrocyte glutathione S-transferase (GST) activity by fruit and vegetable (F&V)* consumption and GSTM1 or GSTT1 genetic polymorphism†
(Number of subjects, mean values and 95 % confidence intervals)

* F&V consumption was calculated with dietary records. Low F&V, less than 25th percentile (119·0 g/d); high F&V, more than 75th percentile (384·2 g/d).
† Adjusted for age, sex, BMI and antioxidant supplement (general linear model used).
‡ Mean values were significantly different in the subjects with low F&V consumption (P < 0·001).
Table 5 Plasma total antioxidant capacity (T-AOC), vitamin C level and erythrocyte glutathione S-transferase (GST) activity by recent fruit and vegetable (F&V)* consumption and GSTM1/GSTT1 combining genetic polymorphisms†
(Number of subjects, mean values and 95 % confidence intervals)

* Low F&V, less than 25th percentile (119·0 g/d); high F&V, more than 75th percentile (384·2 g/d).
† Adjusted for age, sex, BMI and antioxidant supplement (general linear model used).
‡ Mean values were significantly different in the subjects with low F&V consumption (P < 0·0001).
§ Mean values were significantly different in the subjects with high F&V consumption (P < 0·05).
Discussion
The involvement of GST in the oxidative stress defence and metabolism of exogenous antioxidants including diet-derived anti-oxidative phytochemicals suggested that the polymorphisms may be a significant determinant of individual response to F&V consumption. The GSTM1 and GSTT1 null genotypes are relatively common in Chinese(Reference Zhong, Zhou and Chen22). Therefore, in the present study, we aimed to evaluate the effects of GSTM1 and GSTT1 genetic polymorphisms and F&V consumption on body antioxidant parameters.
GST enzymes are important in the control of oxidative stress. In the study presented here, we found that GSTM1 or GSTT1 gene deletion did not influence plasma T-AOC level. Reports about the association of GSTM1/T1 polymorphisms and body T-AOC are contradictory. Tang et al. (Reference Tang, Wang and Jia23) and Mustafa et al. (Reference Mustafa, Pathak and Ahmed24) reported that individuals with either or both GSTM1/T1 gene deletion have lower T-AOC and higher oxidative stress than that in other GSTM1/T1 genotype groups. However, Martin et al. (Reference Martin, Collier and Bowen25) and Dušinská et al. (Reference Dušinská, Ficek and Horská26) reported that there was no difference among the individuals with different GSTM1/T1 genotypes in plasma T-AOC, ferric reducing/antioxidant power level and other anti-oxidative biomarkers. It is well known that F&V-rich diet contributes to the prevention of oxidative stress-related chronic diseases through the mechanism of enhancing the body antioxidant defence system. Therefore, in the present study, we further took diet (F&V consumption) into account to explore the relationship of GSTM1/T1 gene polymorphism with body antioxidant capacity. As shown in Tables 4 and 5, the lowest T-AOC level was detected in individuals with the GSTM1− or GSTM1−/GSTT1+ genotype, as well as the lowest F&V consumption. The similar trends were also observed in the subjects with the GSTT1+ or GSTM1−/GSTT1+ genotype. These results indicated that plasma T-AOC level is more sensitive to low F&V consumption in the individuals with the GSTM1− genotype. However, it was interesting to find that F&V consumption had no effects on plasma T-AOC level in the subjects with the GSTT1− genotype.
Few studies have reported the relationship of GSTM1/T1 genetic polymorphism with human plasma vitamin C level. In the present study, no difference was found in plasma vitamin C level among different GSTM1/T1 genotypes. The combination analysis of GSTM1/T1 genotypes and plasma vitamin C level indicated that the individuals who were both GSTM1 and GSTT1 null had a relatively lower plasma vitamin C level than other genotype groups, but no statistical significance. After including diet factor into the analysis, we still did not observe the relationship between GSTM1/T1 genotype with plasma vitamin C level. These results correspond with what Wilms et al. (Reference Wilms, Claughton and De Kok27) concluded upon testing the effects of H2O2 challenge in lymphocytes from individuals with different GSTM1/T1 genotypes, in which the authors did not observe the influence of GSTM1/T1 genotypes on the oxidative stress preventive effects of vitamin C in vitro. The reason contributing to the present results may be the biological redundancy in the metabolic capacity of healthy subjects. In summary, no significant difference was found between plasma vitamin C levels and GSTM1/T1 genotypes.
The effects of GSTM1/T1 polymorphisms on erythrocyte GST activity were also explored in this study. As shown in Table 2, the deletion of the GSTM1 or GSTT1 gene lowered erythrocyte GST activity, and the absence of the GSTM1 gene had a remarkable influence on erythrocyte GST activity (P = 0·0047). Combining the analysis of GSTM1/T1 polymorphism with erythrocyte GST activity indicated that when compared with the GSTM1+/GSTT1+ group, GST activity was lower in GSTM1−/GSTT1+, GSTM1+/GSTT1− and GSTM1−/GSTT1− groups. Our results are in agreement with the study of Tijhuis et al. (Reference Tijhuis, Visker and Aarts28), in which the authors detected the total GST activity in leucocytes and found that GSTM1 gene deletion decreased leucocyte total GST activity, while the absence of the GSTT1 gene had no influence on leucocyte total GST activity. Besides, erythrocyte GST activity was influenced by F&V consumption in GSTM1− or GSTM1−/GSTT1+ individuals (Tables 4 and 5). When consuming low-level F&V, the GSTM1− or GSTM1−/GSTT1+ subjects had lower erythrocyte GST activity than that in subjects with other GSTM1/T1 genotypes. The published document reported that no relationship was found between total F&V consumption with the leucocyte and rectal total GST activity(Reference Tijhuis, Visker and Aarts28). Inconsistencies were also reported by the studies focused on F&V subtypes and GST activity(Reference Nijhoff, Mulder and Verhagen29, Reference Lampe, Chen and Li30). Excepting for the study design and laboratory method, seldom taking genetic variation into account was suggested attributing to the contradiction of the data. In the present study, we found that different F&V consumption had no effects on erythrocyte GST activity (data not shown); however, when taking GSTM1/T1 genotype into account, the influences of F&V consumption on GST activity were predominant. Erythrocyte GST activity is more sensitive to F&V consumption in the subjects with the GSTM1− or GSTM1−/GSTT1+ genotype (P GSTM1 − = 0·0009, P GSTM1 − /GSTT1+ = 0·0127).
Conclusion
Together with others' reports, the results of the present study contribute to a better understanding of the combining influence of GSTM1/T1 genetic polymorphisms and F&V consumption on body antioxidant parameters. The study here presents some limitations, such as the small sample size that may temper the statistical association involving subgroup analysis testing. Moreover, the subjects chosen in the present study were Chinese, and caution should be exercised to extrapolate the data to other ethnic groups. Also, only one 3-d 24-h-recall was carried out in this study. The variation of F&V from different seasons should be considered. Thus, a combination of FFQ method with 3-d 24-h-recall dietary survey is necessary to reflect the consumption of F&V. In summary, large-scale and multiple ethnic studies are needed to evaluate the true relationship of GSTM1/T1 gene polymorphism, and consumption of F&V with body antioxidant biomarkers.
Acknowledgements
The present study was supported by grants from the National Natural Science Foundation of China (no. 30901196), Beijing Municipal Natural Science Foundation (no. 7092008) and the National High Technology Research and Development Program of China (no. 2010AA023003). The authors declare that there are no conflicts of interest. R. X. and L.-H. Y. designed the study. L.-P. M. and L.-H. Y. performed the statistical analyses and drafted the manuscript. S. L. and J.-F. F. contributed to the recruitment of volunteers. W.-W. M. and H.-L. Y. contributed to the laboratory work.







